Effects of Artificial Light at Night on Fitness-Related Traits of Sea Urchin (Heliocidaris crassispina)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sea Urchin Materials
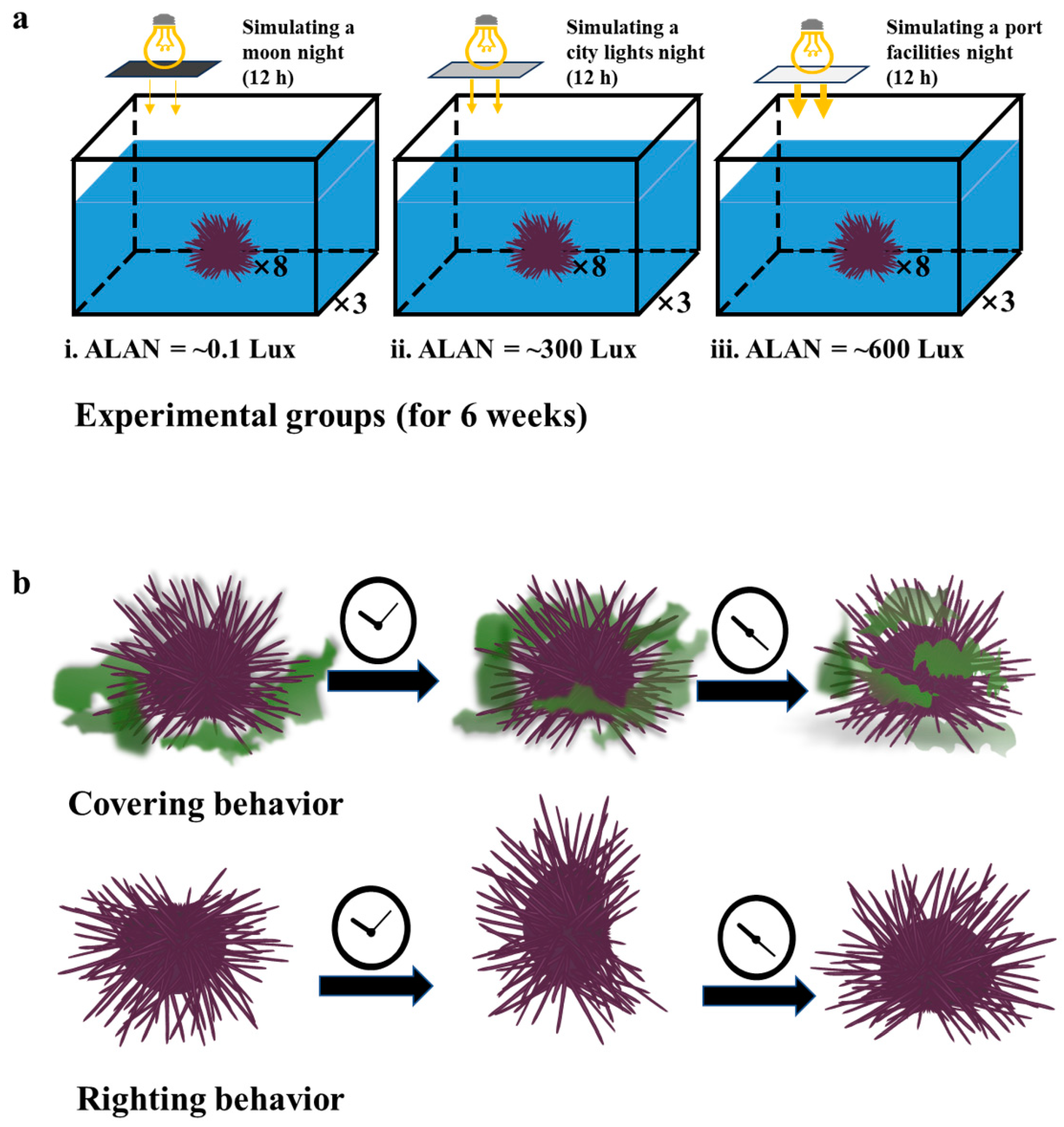
2.2. Experimental Design
2.3. Behavioral Analysis
2.4. Sampling Protocol
2.5. Physiological Analysis
2.6. Data Statistics
3. Results
3.1. Survival
3.2. Righting and Covering Behavior
3.3. Food Consumption
3.4. Body Size
3.5. Lantern Weight and Length
3.6. Gonad and Gut-Weight
3.7. 5-HIAA/5-HT Ratio
3.8. Pax6 Gene Expression
4. Discussion
4.1. Effects of ALAN on Behavioral Responses
4.2. Effects of ALAN on Growth
4.3. Effects of ALAN on Reproductive Performance
4.4. Effects of ALAN on Pax6 Gene Expression
4.5. Sea Urchins in the Wild Light Environment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaston, K.J.; Bennie, J.; Davies, T.W.; Hopkins, J. The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biol. Rev. 2013, 88, 912–927. [Google Scholar] [CrossRef] [PubMed]
- Zapata, M.J.; Sullivan, S.M.P.; Sullivan, S.M. Artificial lighting at night in estuaries implications from individuals to ecosystems. Estuar. Coast. 2019, 42, 309–330. [Google Scholar] [CrossRef]
- Bassi, A.; Love, O.P.; Cooke, S.J.; Warriner, T.R.; Harris, C.M.; Madliger, C.L. Effects of artificial light at night on fishes: A synthesis with future research priorities. Fish Fish. 2022, 23, 631–647. [Google Scholar] [CrossRef]
- Davies, T.W.; Duffy, J.P.; Bennie, J.; Gaston, K.J. Stemming the tide of light pollution encroaching into marine protected areas. Conserv. Lett. 2016, 9, 164–171. [Google Scholar] [CrossRef]
- Davies, T.W.; Smyth, T. Why artificial light at night should be a focus for global change research in the 21st century. Global Change Biol. 2018, 24, 872–882. [Google Scholar] [CrossRef]
- Marangoni, L.F.B.; Davies, T.; Smyth, T.; Rodríguez, A.; Hamann, M.; Duarte, C.; Pendoley, K.; Berge, J.; Maggi, E.; Levy, O. Impacts of artificial light at night in marine ecosystems-A review. Global Change Biol. 2022, 28, 5346–5367. [Google Scholar] [CrossRef]
- Hussein, A.A.A.; Bloem, E.; Fodor, I.; El-Sayed, B.; Tadros, M.M.; Soliman, M.F.M.; El-Shenawy, N.S.; Koene, J.M. Slowly seeing the light: An integrative review on ecological light pollution as a potential threat for mollusks. Environ. Sci. Pollut. Res. Int. 2021, 28, 5036–5048. [Google Scholar] [CrossRef]
- Davies, T.W.; Duffy, J.P.; Bennie, J.; Gaston, K.J. The nature, extent, and ecological implications of marine light pollution. Front. Ecol. Environ. 2014, 12, 347–355. [Google Scholar] [CrossRef]
- Russart, K.L.G.; Nelson, R.J. Artificial light at night alters behavior in laboratory and wild animals. J. Exp. Zool. Part A 2018, 329, 401–408. [Google Scholar] [CrossRef]
- Ayalon, I.; Rosenberg, Y.; Benichou, J.I.C.; Campos, C.L.D.; Sayco, S.L.G.; Nada, M.A.L.; Baquiran, J.I.P.; Ligson, C.A.; Avisar, D.; Conaco, C.; et al. Coral gametogenesis collapse under artificial light pollution. Curr. Biol. 2021, 31, 413–419. [Google Scholar] [CrossRef]
- Colman, L.P.; Lara, P.H.; Bennie, J.; Broderick, A.C.; de Freitas, J.R.; Marcondes, A.; Witt, M.J.; Godley, B.J. Assessing coastal artificial light and potential exposure of wildlife at a national scale: The case of marine turtles in Brazil. Biodivers. Conserv. 2020, 29, 1135–1152. [Google Scholar] [CrossRef]
- Shimada, T.; Meekan, M.G.; Baldwin, R.; Al-Suwailem, A.M.; Clarke, C.; Santillan, A.S.; Duarte, C.M. Distribution and temporal trends in the abundance of nesting sea turtles in the Red Sea. Biol. Conserv. 2021, 261, 109235. [Google Scholar] [CrossRef]
- Ronconi, R.A.; Allard, K.A.; Taylor, P.D. Bird interactions with offshore oil and gas platforms: A review of impacts and monitoring techniques. J. Environ. Manag. 2015, 147, 34–45. [Google Scholar] [CrossRef]
- Maboloc, E.A.; Fang, J.K.H. Tissue regeneration of the purple sea urchin Heliocidaris crassispina. Bull. Mar. Sci. 2023, 99, 19–20. [Google Scholar] [CrossRef]
- Kanamoto, Y.; Nakamura, K. Age and growth analyses of the purple sea urchin Heliocidaris crassispina inhabiting different feeding environments in the Shimane Peninsula, Japan. Reg. Stud. Mar. Sci. 2023, 65, 103096. [Google Scholar] [CrossRef]
- Kadota, T.; Kiyomoto, S.; Masuda, Y.; Miyano, T.; Yoshimura, T. Restoration of a small-sized macroalgal bed through the removal of sea urchins in Kashiyama, Nagasaki Prefecture. Nippon. Suisan Gakk. 2022, 88, 49–57. [Google Scholar] [CrossRef]
- Feng, W.P.; Nakabayashi, N.; Inomata, E.; Inomata, E.; Aoki, M.N.; Agatsuma, Y. Sexually unbalanced gonad development and nutrition of the newly range-extended sea urchin Heliocidaris crassispina in the northeastern Honshu, Japan. Estuar. Coast. Shelf S. 2021, 249, 107120. [Google Scholar] [CrossRef]
- Ishii, M.; Unuma, T.; Masadate, A.; Hoshikawa, H.; Takahashi, K.; Kosaka, S.; Masuda, A.; Murakami, K. Accelerated photoperiod promotes gonadal maturation in the sea urchin Strongylocentrotus intermedius. Fish. Sci. 2022, 88, 299–310. [Google Scholar] [CrossRef]
- Feng, W.P.; Nakabayashi, N.; Inomata, E.; Aoki, M.N.; Agatsuma, Y. Impacts of water temperature on the physiology and behaviours of the sea urchins Heliocidaris crassispina and Mesocentrotus nudus that reflect their range extension and disappearance in the Oga Peninsula, northern Honshu, Japan. Can. J. Fish. Aquat. Sci. 2020, 78, 580–588. [Google Scholar] [CrossRef]
- Wang, X.B.; Li, X.S.; Xiong, D.Q.; Chen, H.S.; Ren, H. Effects of stranded heavy fuel oil subacute exposure on the fitness-related traits of sea urchin Strongylocentrotus intermedius. Mar. Freshwater Res. 2022, 73, 754–761. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, L.L.; Shi, D.T.; Chi, X.M.; Yin, D.H.; Sun, J.N.; Ding, J.Y.; Yang, M.F.; Chang, Y.Q. Carryover effects of short-term UV-B radiation on fitness related traits of the sea urchin Strongylocentrotus intermedius. Ecotox. Environ. Safe. 2018, 164, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, L.S.; Zhao, C.; Feng, W.P.; Sun, P.; Chang, Y.Q. Correlation analyses of covering and righting behaviors to fitness related traits of the sea urchin Glyptocidaris crenularis in different environmental conditions. Chin. J. Oceanol. Limn. 2016, 34, 1183–1190. [Google Scholar] [CrossRef]
- Verling, E.; Crook, A.; Barnes, D. Covering behaviour in Paracentrotus lividus: Is light important? Mar. Biol. 2002, 140, 391–396. [Google Scholar] [CrossRef]
- Chi, X.; Shi, D.; Ma, Z.; Hu, F.Y.; Sun, J.N.; Huang, X.Y.; Zhang, L.S.; Chang, Y.Q.; Zhao, C. Carryover effects of long-term high water temperatures on fitness-related traits of the offspring of the sea urchin Strongylocentrotus intermedius. Mar. Environ. Res. 2021, 169, 105371. [Google Scholar] [CrossRef] [PubMed]
- Pawson, D.L.; Pawson, D.J. Bathyal sea urchins of the Bahamas, with notes on covering behavior in deep sea echinoids (Echinodermata: Echinoidea). Deep Sea Res. Part II 2013, 92, 207–213. [Google Scholar] [CrossRef]
- Backström, T.; Winberg, S. Serotonin coordinates responses to social stress-what we can learn from fish. Front. Neurosci. 2017, 11, 595. [Google Scholar] [CrossRef]
- Zhao, C.; Ji, N.J.; Tian, X.F.; Feng, W.P.; Sun, P.; Wei, J. Opsin4, Opsin5, and Pax6 significantly increase their expression in recently settled juveniles of the sea urchin Strongylocentrotus intermedius (Echinodermata: Echinoidea). Inver. Reprod. Dev. 2015, 5, 119–123. [Google Scholar] [CrossRef]
- Belleza, D.F.C.; Kawabata, Y.; Toda, T.; Gregory, N.N. Effects of dead conspecifics, hunger states, and seasons on the foraging behavior of the purple urchin Heliocidaris crassispina. Mar. Ecol. Prog. Ser. 2020, 664, 133–148. [Google Scholar] [CrossRef]
- Vowles, A.S.; Kemp, P.S. Artificial light at night (ALAN) affects the downstream movement behaviour of the critically endangered European eel, Anguilla anguilla. Environ. Pollut. 2021, 274, 116585. [Google Scholar] [CrossRef]
- Pulgar, J.; Manríquez, P.H.; Widdicombe, S.; García-Huidobro, R.; Quijón, P.A.; Carter, M.; Aldana, M.; Quintanilla-Ahumada, D.; Duarte, C. Artificial Light at Night (ALAN) causes size-dependent effects on intertidal fish decision-making. Mar. Pollut. Bull. 2023, 193, 115190. [Google Scholar] [CrossRef]
- Fobert, E.K.; Burke da Silva, K.; Swearer, S.E. Artificial light at night causes reproductive failure in clownfish. Biol. Lett. 2019, 15, 20190272. [Google Scholar] [CrossRef] [PubMed]
- Kupprat, F.; Kloas, W.; Krüger, A.; Schmalsch, C.; Hölker, F. Misbalance of thyroid hormones after two weeks of exposure to artificial light at night in Eurasian perch Perca fluviatilis. Conserv. Physiol. 2021, 9, coaa124. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.C.; Ellis, T.; Davison, P.I.; Ives, M.J.; Thomas, R.J.; Griffiths, S.W.; Riley, W.D. Using novel methodologies to examine the impact of artificial light at night on the cortisol stress response in dispersing Atlantic salmon (Salmo salar L.) fry. Conserv. Physiol. 2015, 3, cov051. [Google Scholar] [CrossRef]
- Wang, H.; Ding, J.; Ding, S.; Chang, Y. Transcriptome analysis to characterize the genes related to gonad growth and fatty acid metabolism in the sea urchin Strongylocentrotus intermedius. Genes Genom. 2019, 41, 1397–1415. [Google Scholar] [CrossRef] [PubMed]
- Terwilliger, D.P.; Buckley, K.M.; Brockton, V.; Ritter, N.J.; Smith, L.C. Distinctive expression patterns of 185/333 genes in the purple sea urchin, Strongylocentrotus purpuratus: An unexpectedly diverse family of transcripts in response to LPS, beta-1,3-glucan, and dsRNA. BMC Mol. Biol. 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Z.; Guo, H.; Qin, J.; Zhang, X. Changes in aggressive behavior, cortisol and brain monoamines during the formation of social hierarchy in black rockfish (Sebastes schlegelii). Animals 2020, 10, 2357. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, X.; Bai, Q.; Zhang, Y.; Qin, J.; Zhang, X. Molecular identification of an androgen receptor and the influence of long-term aggressive interaction on hypothalamic genes expression in black rockfish (Sebastes schlegelii). J. Comp. Physiol. A 2021, 207, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guo, H.; Zhang, Z.; Wang, Y.; Qin, J.; Zhang, X. Impact of pre-aggressive experience on behavior and physiology of black rockfish (Sebastes schlegelii). Aquaculture 2021, 536, 736416. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, Q.; Xu, X.; Zhang, X. Effects of the dominance hierarchy on social interactions, cortisol level, HPG-axis activities and reproductive success in the golden cuttlefish Sepia esculent. Aquaculture 2020, 533, 736059. [Google Scholar] [CrossRef]
- Yang, M.; Yu, Y.; Ding, J.; Sun, J.; Hu, F.; Chang, Y.; Zhao, C. Effects of light intensity on Opsin4, Opsin5, and Pax6 expressions of the sea urchin Strongylocentrotus intermedius. Mar. Ecol. 2020, 41, e12593. [Google Scholar] [CrossRef]
- Parra, M.; Rubilar, T.; Latorre, M.P.; Parra, M.; Rubilar, T.; Latorre, M.P.; Ephedra, L.; Gil, D.G.; Díaz de Vivar, M.E. Nutrient allocation in the gonads of the sea urchin Arbacia dufresnii in different stages of gonadal development. Invertebr. Reprod. Dev. 2015, 59, 26–36. [Google Scholar] [CrossRef]
- Machado, A.M.; Fernández-Boo, S.; Nande, M.; Pinto, R.; Costas, B.; Castro, L.F. The male and female gonad transcriptome of the edible sea urchin, Paracentrotus lividus: Identification of sex-related and lipid biosynthesis genes. Aquacult. Rep. 2022, 22, 100936. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, J.W.; Han, T.; Huang, D.X.; Zhao, Z.H.; Feng, J.Q.; Zhou, N.M.; Xie, H.Q.; Wang, T.M. Identification and characterization of a novel 5-hydroxytryptamine receptor in the sea cucumber Apostichopus japonicus (Selenka). J. Exp. Zool. Part A 2021, 335, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Tuxbury, S.M.; Salmon, M. Competitive interactions between artificial lighting and natural cues during seafinding by hatchling marine turtles. Biol. Conserv. 2005, 121, 311–316. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Fink, D.; Butler, J.J.; Farnsworth, A.; Cabrera-Cruz, S.A. Seasonal associations with urban light pollution for nocturnally migrating bird populations. Glob. Change Biol. 2017, 23, 4609–4619. [Google Scholar] [CrossRef]
- Van Doren, B.M.; Horton, K.G.; Dokter, A.M.; Klinck, H.; Elbin, S.B.; Farnsworth, A. High-intensity urban light installation dramatically alters nocturnal bird migration. Proc. Natl. Acad. Sci. USA 2017, 114, 11175–11180. [Google Scholar] [CrossRef]
- Burt, C.S.; Kelly, J.F.; Fox, A.S.; Jenkins-Smith, H.C.; Leon-Corwin, M.; Khalighifar, A.; Trankina, G.E.; Silva, C.L.; Horton, K.G. Can ecological forecasting lead to convergence on sustainable lighting policies? Conserv. Sci. Pract. 2023, 5, e12920. [Google Scholar] [CrossRef]
- Orvain, O.; Sauriau, P.G. Environmental and behavioural factors affecting activity in the intertidal gastropod Hydrobia ulvae. J. Exp. Mar. Biol. Ecol. 2002, 272, 191–216. [Google Scholar] [CrossRef]
- Morandini, L.; Ramallo, M.R.; Scaia, M.F.; Höcht, C.; Somoza, G.M.; Pandolfi, M. Dietary L-tryptophan modulates agonistic behavior and brain serotonin in male dyadic contests of a cichlid fish. J. Comp. Physiol. A 2019, 205, 867–880. [Google Scholar] [CrossRef]
- Luarte, T.; Bonta, C.C.; Silva-Rodriguez, E.A.; Quijón, P.A.; Miranda, C.; Fariasm, A.A.; Duarte, C. Light pollution reduces activity, food consumption and growth rates in a sandy beach invertebrate. Environ. Pollut. 2016, 218, 1147–1153. [Google Scholar] [CrossRef]
- Hussein, A.A.A.; Baz, E.S.; Mariën, J.; Tadros, M.M.; El-Shenawy, N.S.; Koene, J.M. Effect of photoperiod and light intensity on learning ability and memory formation of the pond snail Lymnaea stagnalis. Invert. Neurosci. 2020, 20, 18. [Google Scholar] [CrossRef]
- Boldogh, S.; Dobrosi, D.; Samu, P. The effects of the illumination of buildings on house-dwelling bats and its conservation consequences. Acta. Chiropterol. 2007, 9, 527–534. [Google Scholar] [CrossRef]
- Silvia, L.; Manuel, T.S. Dissecting the roles of gonadotropin-inhibitory hormone in mammals: Studies using pharmacological tools and genetically modified mouse models. Front. Endocrinol. 2015, 6, 189. [Google Scholar] [CrossRef]
- Brüning, A.; Hölker, F.; Franke, S.; Kleiner, W.; Kloas, W. Impact of different colours of artificial light at night on melatonin rhythm and gene expression of gonadotropins in European perch. Sci. Total. Environ. 2016, 543, 214–222. [Google Scholar] [CrossRef]
- Brüning, A.; Kloas, W.; Preuer, T.; Hölker, F. Influence of artificially induced light pollution on the hormone system of two common fish species, perch and roach, in a rural habitat. Conserv. Physiol. 2018, 6, coy016. [Google Scholar] [CrossRef]
- Raible, F.; Tessmar-Raible, K.; Arboleda, E.; Kaller, T.; Bork, P.; Arendt, D.; Arnone, M.I. Opsins and clusters of sensory G-protein-coupled receptors in the sea urchin genome. Dev. Biol. 2006, 300, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Ullrich-Luter, E.M.; Dupont, S.; Arboleda, E.; Hausen, H.; Arnone, M.I. Unique system of photoreceptors in sea urchin tube feet. Proc. Natl. Acad. Sci. USA 2011, 108, 8367–8372. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ji, N.; Sun, P.; Feng, W.; Wei, J.; Chang, Y. Effects of light and covering behavior on PAX6 expression in the sea urchin Strongylocentrotus intermedius. PLoS ONE 2014, 9, e110895. [Google Scholar] [CrossRef] [PubMed]
- Pulgar, J.; Zeballos, D.; Vargas, J.; Aldana, M.; Manriquez, P.H.; Manriquez, K.; Quijón, P.A.; Widdicombe, S.; Anguita, C.; Quintanilla, D.; et al. Endogenous cycles, activity patterns and energy expenditure of an intertidal fish is modified by artificial light pollution at night (ALAN). Environ. Pollut. 2019, 244, 361–366. [Google Scholar] [CrossRef]







| Primer Names | Sequence (5′→3′) | Application | AT (°C) |
|---|---|---|---|
| Pax6-F | AAGGCTGAAGATGATGAAGA | qPCR of Pax6 gene | 58 |
| Pax6-R | GGAATGATTGGAAGACTGAC | ||
| 18S-F | ACGAAGGAGAAGACAAGG | qPCR of 18S rRNA gene | 56 |
| 18S-R | AAGCCACAAACGACAGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Wang, Z.; Jin, X.; Ding, K.; Yang, J.; Wang, T. Effects of Artificial Light at Night on Fitness-Related Traits of Sea Urchin (Heliocidaris crassispina). Animals 2023, 13, 3035. https://doi.org/10.3390/ani13193035
Xu X, Wang Z, Jin X, Ding K, Yang J, Wang T. Effects of Artificial Light at Night on Fitness-Related Traits of Sea Urchin (Heliocidaris crassispina). Animals. 2023; 13(19):3035. https://doi.org/10.3390/ani13193035
Chicago/Turabian StyleXu, Xiuwen, Zexianghua Wang, Xiuqi Jin, Keying Ding, Jingwen Yang, and Tianming Wang. 2023. "Effects of Artificial Light at Night on Fitness-Related Traits of Sea Urchin (Heliocidaris crassispina)" Animals 13, no. 19: 3035. https://doi.org/10.3390/ani13193035
APA StyleXu, X., Wang, Z., Jin, X., Ding, K., Yang, J., & Wang, T. (2023). Effects of Artificial Light at Night on Fitness-Related Traits of Sea Urchin (Heliocidaris crassispina). Animals, 13(19), 3035. https://doi.org/10.3390/ani13193035







