Oxygen Reserve Index as a Tool to Monitor Four Techniques of Oxygen Supplementation at Different Flow Rates in Dogs Sedated with Dexmedetomidine and an Opioid
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Protocol
2.3. Measurements
2.4. Statistical Analysis
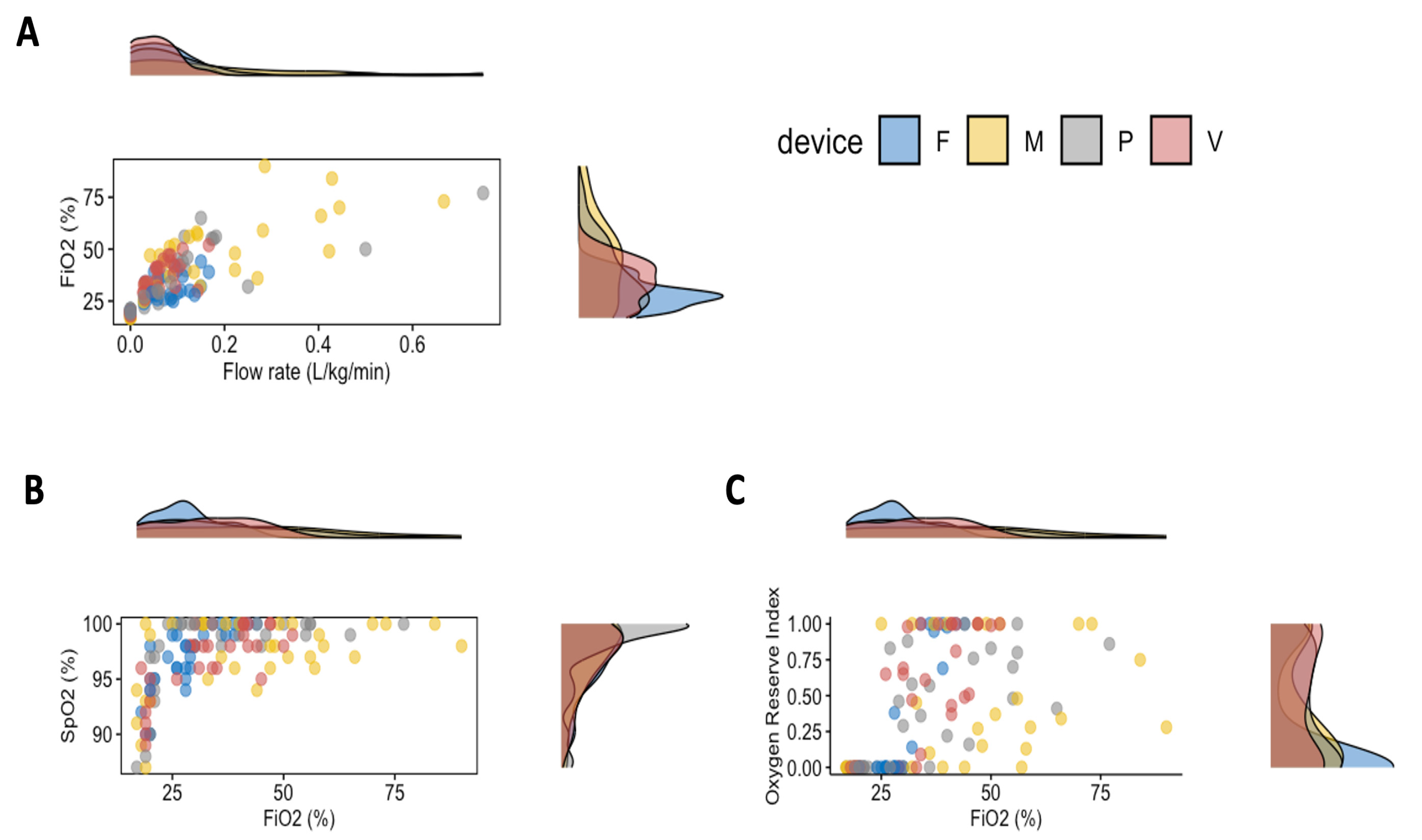
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bach, J. Oxygen delivery systems. In Veterinary Anesthetic and Monitoring Equipment, 1st ed.; Cooley, K.G., Johnson, R.A., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 193–198. [Google Scholar] [CrossRef]
- Mazzaferro, E.M. Oxygen therapy. In Small Animal Critical Care Medicine, 2nd ed.; Silverstein, D.C., Hopper, K., Eds.; Elsevier: St. Louis, MO, USA, 2015; pp. 77–80. [Google Scholar] [CrossRef]
- Dunphy, E.D.; Mann, F.A.; Dodam, J.R.; Branson, K.R.; Wagner-Mann, C.C.; Johnson, P.A.; Brady, M.A. Comparison of unilateral versus bilateral nasal catheters for oxygen administration in dogs. J. Vet. Emerg. Crit. Care 2002, 12, 245–251. [Google Scholar] [CrossRef]
- Loukopoulos, P.; Reynolds, W. Comparative evaluation of oxygen therapy techniques in anaesthetised dogs: Intranasal catheter and Elizabethan collar canopy. Aust. Vet. Pract. 1996, 26, 199–205. [Google Scholar]
- Loukopoulos, P.; Reynolds, W. Comparative evaluation of oxygen therapy techniques in anaesthetised dogs: Face mask and flow-by technique. Aust. Vet. Pract. 1997, 27, 34–39. [Google Scholar]
- Wong, A.M.; Uquillas, E.; Hall, E.; Dart, C.M.; Dart, A.J. Comparison of the effect of oxygen supplementation using flow-by or a face mask on the partial pressure of arterial oxygen in sedated dogs. N. Z. Vet. J. 2019, 67, 36–39. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, B.R.; Howard, L.S.; Earis, J.; Mak, V. British Thoracic Society Guideline for oxygen use in adults in healthcare and emergency settings. BMJ Open Respir. Res. 2017, 4, e000170. [Google Scholar] [CrossRef]
- Lellouche, F.; L’Her, E. Usual and Advanced Monitoring in Patients Receiving Oxygen Therapy. Respir. Care 2020, 65, 1591–1600. [Google Scholar] [CrossRef]
- Barletta, M.; Almondia, D.; Williams, J.; Crochik, S.; Hofmeister, E. Radiographic evaluation of positional atelectasis in sedated dogs breathing room air versus 100% oxygen. Can. Vet. J. 2014, 55, 985–991. [Google Scholar]
- Farrell, K.S.; Hopper, K.; Cagle, L.A.; Epstein, S.E. Evaluation of pulse oximetry as a surrogate for PaO2 in awake dogs breathing room air and anesthetized dogs on mechanical ventilation. J. Vet. Emerg. Crit. Care. 2019, 29, 622–629. [Google Scholar] [CrossRef]
- Scheeren, T.W.L.; Belda, F.J.; Perel, A. The oxygen reserve index (ORI): A new tool to monitor oxygen therapy. J. Clin. Monit. Comput. 2018, 32, 379–389. [Google Scholar] [CrossRef]
- Applegate, R.L., 2nd; Dorotta, I.L.; Wells, B.; Juma, D.; Applegate, P.M. The Relationship between Oxygen Reserve Index and Arterial Partial Pressure of Oxygen During Surgery. Anesth. Analg. 2016, 123, 626–633. [Google Scholar] [CrossRef]
- Yoshida, K.; Isosu, T.; Noji, Y.; Ebana, H.; Honda, J.; Sanbe, N.; Obara, S.; Murakawa, M. Adjustment of oxygen reserve index (ORi™) to avoid excessive hyperoxia during general anesthesia. J. Clin. Monit. Comput. 2020, 34, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Szmuk, P.; Steiner, J.W.; Olomu, P.N.; Ploski, R.P.; Sessler, D.I.; Ezri, T. Oxygen Reserve Index: A Novel Noninvasive Measure of Oxygen Reserve—A Pilot Study. Anesthesiology 2016, 124, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, M.; Kurihara, H.; Ishida, K.; Komatsu, H.; Suzuki, K. The Oxygen Reserve Index as a determinant of the necessary amount of postoperative supplemental oxygen. Minerva Anestesiol. 2021, 87, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Bellini, L.; Dzikiti, B.T.; De Benedictis, G.M.; Algarin Sepulveda, F.R.; Maney, J.K. Oxygen reserve index as a noninvasive indicator of arterial partial pressure of oxygen in anaesthetized donkeys: A preliminary study. Vet. Anaesth. Analg. 2021, 48, 388–392. [Google Scholar] [CrossRef]
- Zanusso, F.; Zemko, P.; De Benedictis, G.M.; Bellini, L. Oxygen reserve index to predict oxygen status in anaesthetized dogs. In Proceedings of the 14th World Congress of Veterinary Anaesthesia, Sydney, Australia, 27–30 March 2023. [Google Scholar]
- Ambros, B.; Carrozzo, M.V.; Jones, T. Desaturation times between dogs preoxygenated via face mask or flow-by technique before induction of anesthesia. Vet. Anaesth. Analg. 2018, 45, 452–458. [Google Scholar] [CrossRef]
- Ko, J.C.; Weil, A.B.; Kitao, T.; Payton, M.E.; Inoue, T. Oxygenation in medetomidine-sedated dogs with and without 100% oxygen insufflation. Vet. Ther. 2007, 8, 51–60. [Google Scholar]
- Rozanski, E.A.; Bedenice, D.; Lofgren, J.; Abrams, J.; Bach, J.; Hoffman, A.M. The effect of body position, sedation, and thoracic bandaging on functional residual capacity in healthy deep-chested dogs. Can. J. Vet. Res. 2010, 74, 34–39. [Google Scholar]
- Canfrán, S.; Bustamante, R.; González, P.; Cediel, R.; Re, M.; de Segura, I.A. Comparison of sedation scores and propofol induction doses in dogs after intramuscular administration of dexmedetomidine alone or in combination with methadone, midazolam, or methadone plus midazolam. Vet. J. 2016, 210, 56–60. [Google Scholar] [CrossRef]
- Raekallio, M.R.; Räihä, M.P.; Alanen, M.H.; Sarén, N.M.; Tuovio, T.A. Effects of medetomidine, L-methadone, and their combination on arterial blood gases in dogs. Vet. Anaesth. Analg. 2009, 36, 158–161. [Google Scholar] [CrossRef]
- Trimble, T.; Bhalla, R.J.; Leece, E.A. Comparison of sedation in dogs: Methadone or butorphanol in combination with dexmedetomidine intravenously. Vet. Anaesth. Analg. 2018, 45, 597–603. [Google Scholar] [CrossRef]
- Pleyers, T.; Levionnois, O.; Siegenthaler, J.; Spadavecchia, C.; Raillard, M. Investigation of selected respiratory effects of (dex)medetomidine in healthy Beagles. Vet. Anaesth. Analg. 2020, 47, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.E.; Hodgson, D.S.; Bello, N.M. Effects of oxygen insufflation rate, respiratory rate, and tidal volume on fraction of inspired oxygen in cadaveric canine heads attached to a lung model. Am. J. Vet. Res. 2013, 74, 1247–1251. [Google Scholar] [CrossRef] [PubMed]

| Flow-by | Nasal Prongs | Vented Mask | Venturi | p-Value | |
|---|---|---|---|---|---|
| Weight (kg) | 24.8 ± 6.2 | 21.3 ± 10.2 | 16.4 ± 12.4 | 30.0 ± 7.0 * | 0.045 |
| BCS | 5 (4–6) | 6 (5–7) | 5 (3–6) | 5 (4–6) | 0.222 |
| Age (months) | 13 (5–97) | 80 (18–149) | 50 (10–180) | 24 (7–98) | 0.055 |
| Sex (m:f) | 4:4 | 5:3 | 5:3 | 2:6 | 0.392 |
| Temperature (°C) | 38.0 ± 1.0 | 37.6 ± 1.1 | 37.7 ± 1.0 | 38.6 ± 0.6 | 0.158 |
| Lateral recumbency (left:right) | 7:1 | 5:2 | 2:6 | 4:4 | 0.067 |
| Methadone vs butorphanol | 3:5 | 3:5 | 7:1 | 2:6 | 0.060 |
| EtCO2 (mmHg) | RR (Breath/min) | PR (Beat/min) | MAP (mmHg) | |
|---|---|---|---|---|
| Flow-by | 52 ± 5 | 15 (7–27) | 60 ± 21 | 92 ± 13 |
| Vented mask | 46 ± 4 | 14 (5–50) | 56 ± 14 | 93 ± 16 |
| Nasal prongs | 52 ± 5 | 14 (6–37) | 48 ± 13 | 91 ± 21 |
| Venturi | 49 ± 6 | 15 (6–49) | 64 ± 20 | 95 ± 12 |
| Flow rate | Method | Flow rate × Method | ||||
| F-stat | p-value | F-stat | p-value | F-stat | p-value | |
| FiO2 | 162.2 | <0.001 | 13.5 | <0.001 | 3.1 | 0.030 |
| FiO2 | Method | FiO2 × Method | ||||
| F-stat | p-value | F-stat | p-value | F-stat | p-value | |
| EtCO2 | 2.0 | 0.157 | 13.0 | <0.001 | 3.1 | 0.030 |
| SpO2 | 59.9 | <0.001 | 0.9 | 0.424 | 5.5 | 0.002 |
| ORi | 70.9 | <0.001 | 3.3 | 0.024 | 11.8 | <0.001 |
| Opioid | Method | Opioid × Method | ||||
| F-stat | p-value | F-stat | p-value | F-stat | p-value | |
| SpO2 | 1.9 | 0.171 | 0.6 | 0.616 | 1.1 | 0.352 |
| ORi | 2.1 | 0.150 | 1.8 | 0.143 | 1.9 | 0.127 |
| Recumbency | Method | Recumbency × Method | ||||
| F-stat | p-value | F-stat | p-value | F-stat | p-value | |
| SpO2 | 1.1 | 0.287 | 0.4 | 0.776 | 0.1 | 0.953 |
| ORi | 0.9 | 0.357 | 1.8 | 0.154 | 0.5 | 0.654 |
| Flow Rate (L/kg/min) | FiO2 (%) | |
|---|---|---|
| Flow-by | ||
| 0 L/min | - | 19.8 ± 0.9 |
| 1 L/min | 0.04 (0.03–0.06) | 29.5 ± 4.8 |
| 2 L/min | 0.09 (0.06–0.11) | 30.9 ± 5.9 |
| 3 L/min | 0.13 (0.09–0.17) | 33.9 ± 6.8 |
| Vented mask | ||
| 0 L/min | - | 18.6 ± 1.2 |
| 1 L/min | 0.11 (0.03–0.22) | 43.9 ± 11.3 |
| 2 L/min | 0.21 (0.05–0.44) | 52.1 ± 20.4 |
| 3 L/min | 0.31 (0.08–0.66) | 57.1 ± 16.2 |
| Nasal prongs | ||
| 0 L/min | - | 20.0 ± 1.4 |
| 1 L/min | 0.05 (0.03–0.24) | 30.4 ± 4.6 |
| 2 L/min | 0.11 (0.05–0.50) | 41.0 ± 10.5 |
| 3 L/min | 0.16 (0.09–0.75) | 51.6 ± 16.2 |
| Venturi | ||
| 0 L/min | - | 19.2 ± 0.7 |
| 1 L/min | 0.03 (0.03–0.06) | 31.2 ± 3.2 |
| 2 L/min | 0.06 (0.05–0.11) | 40.2 ± 6.3 |
| 3 L/min | 0.09 (0.08–0.17) | 42.8 ± 6.7 |
| Administration Methods | r2 | Derived Equation | p-Value |
|---|---|---|---|
| Flow-by | 0.36 | ORi = 3.18 × Flow rate | <0.001 |
| Vented mask | 0.26 | ORi = 1.49 × Flow rate | 0.001 |
| Nasal prongs | 0.67 | ORi = 4.04 × Flow rate | <0.001 |
| Venturi | 0.78 | ORi = 7.81 × Flow rate | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellini, L.; De Benedictis, G.M. Oxygen Reserve Index as a Tool to Monitor Four Techniques of Oxygen Supplementation at Different Flow Rates in Dogs Sedated with Dexmedetomidine and an Opioid. Animals 2023, 13, 3077. https://doi.org/10.3390/ani13193077
Bellini L, De Benedictis GM. Oxygen Reserve Index as a Tool to Monitor Four Techniques of Oxygen Supplementation at Different Flow Rates in Dogs Sedated with Dexmedetomidine and an Opioid. Animals. 2023; 13(19):3077. https://doi.org/10.3390/ani13193077
Chicago/Turabian StyleBellini, Luca, and Giulia Maria De Benedictis. 2023. "Oxygen Reserve Index as a Tool to Monitor Four Techniques of Oxygen Supplementation at Different Flow Rates in Dogs Sedated with Dexmedetomidine and an Opioid" Animals 13, no. 19: 3077. https://doi.org/10.3390/ani13193077
APA StyleBellini, L., & De Benedictis, G. M. (2023). Oxygen Reserve Index as a Tool to Monitor Four Techniques of Oxygen Supplementation at Different Flow Rates in Dogs Sedated with Dexmedetomidine and an Opioid. Animals, 13(19), 3077. https://doi.org/10.3390/ani13193077





