Guanidinoacetic Acid Attenuates Adipogenesis through Regulation of miR-133a in Sheep
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Treatments
2.2. Stromal Vascular Fraction Cells Isolation and Adipogenic Induction
2.3. Oil Red O Staining
2.4. Wound Healing Assay
2.5. MTT Assay
2.6. 5-Ethynyl-2′-deoxyuridine (EdU) Staining
2.7. RNA Preparation, Library Construction, and Sequencing
2.8. Bioinformatics Analysis
2.9. Western Blotting
2.10. Quantitative Real-Time PCR Analysis
2.11. Cell Transfection
2.12. Dual Luciferase Assay
2.13. Triglyceride Measurement
2.14. Statistical Analysis
3. Results
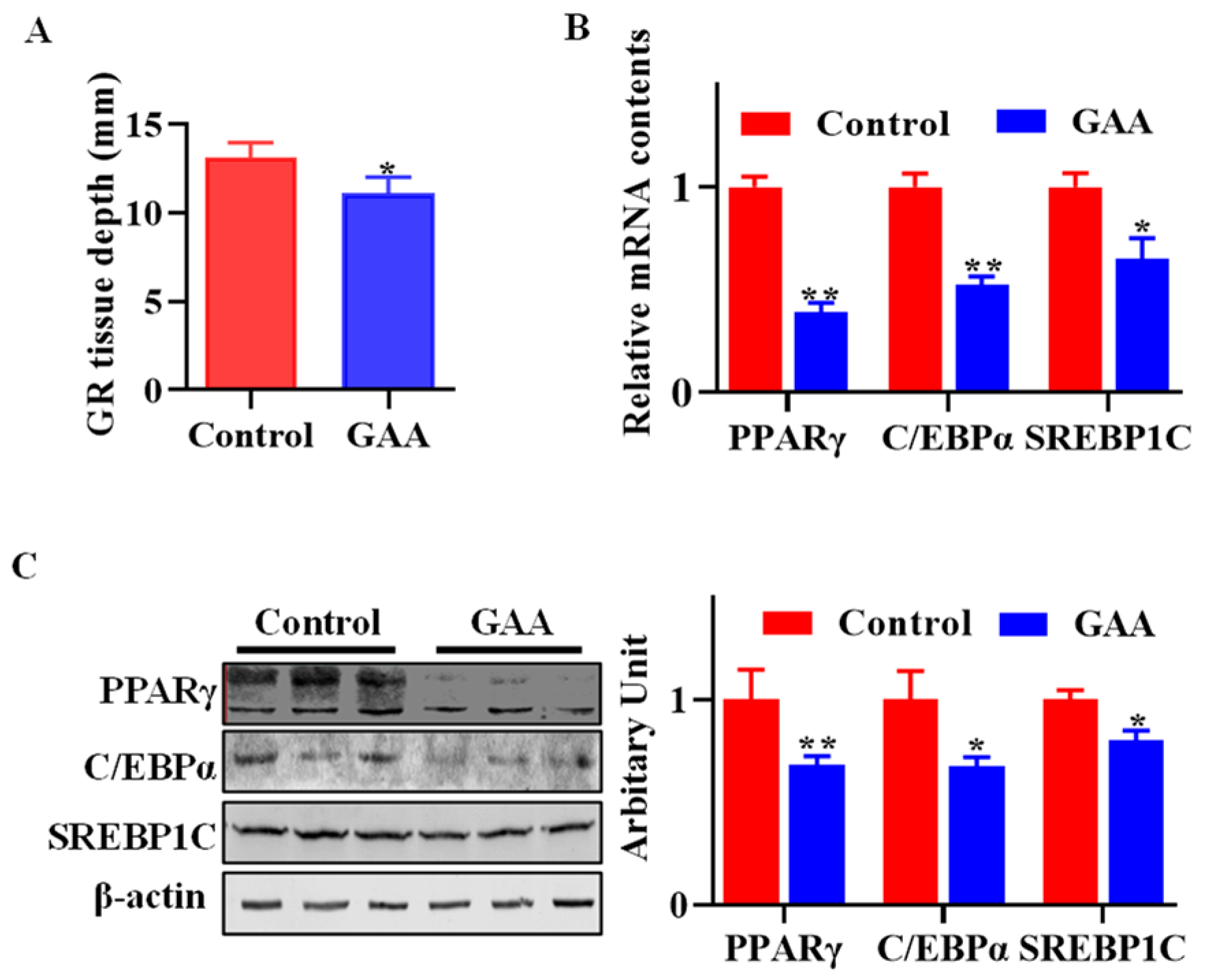
3.1. Dietary Effects of GAA on Body Fatness
3.2. GAA Inhibited SVF Cell Proliferation
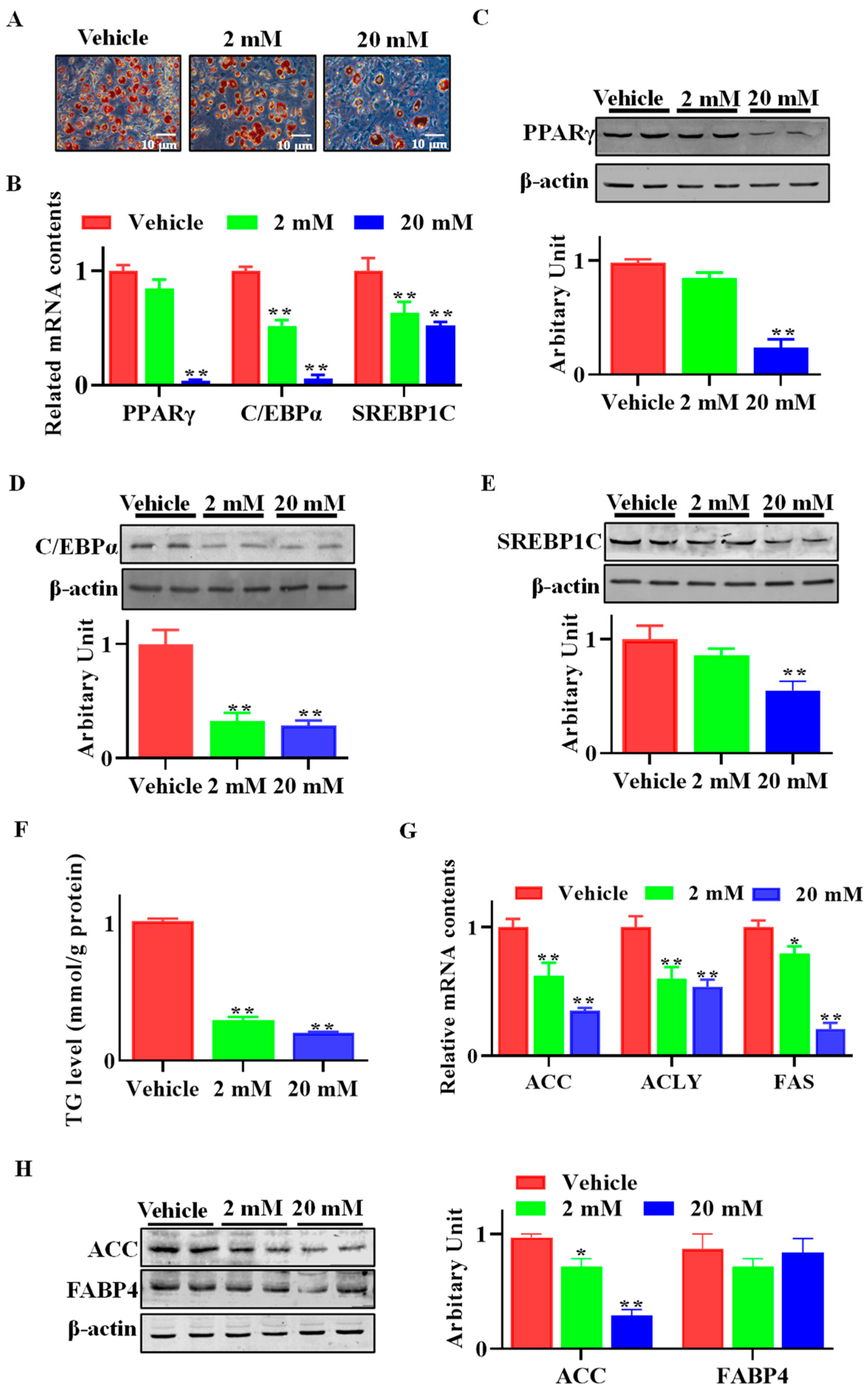
3.3. GAA Attenuated SVF Cell Adipogenic Differentiation
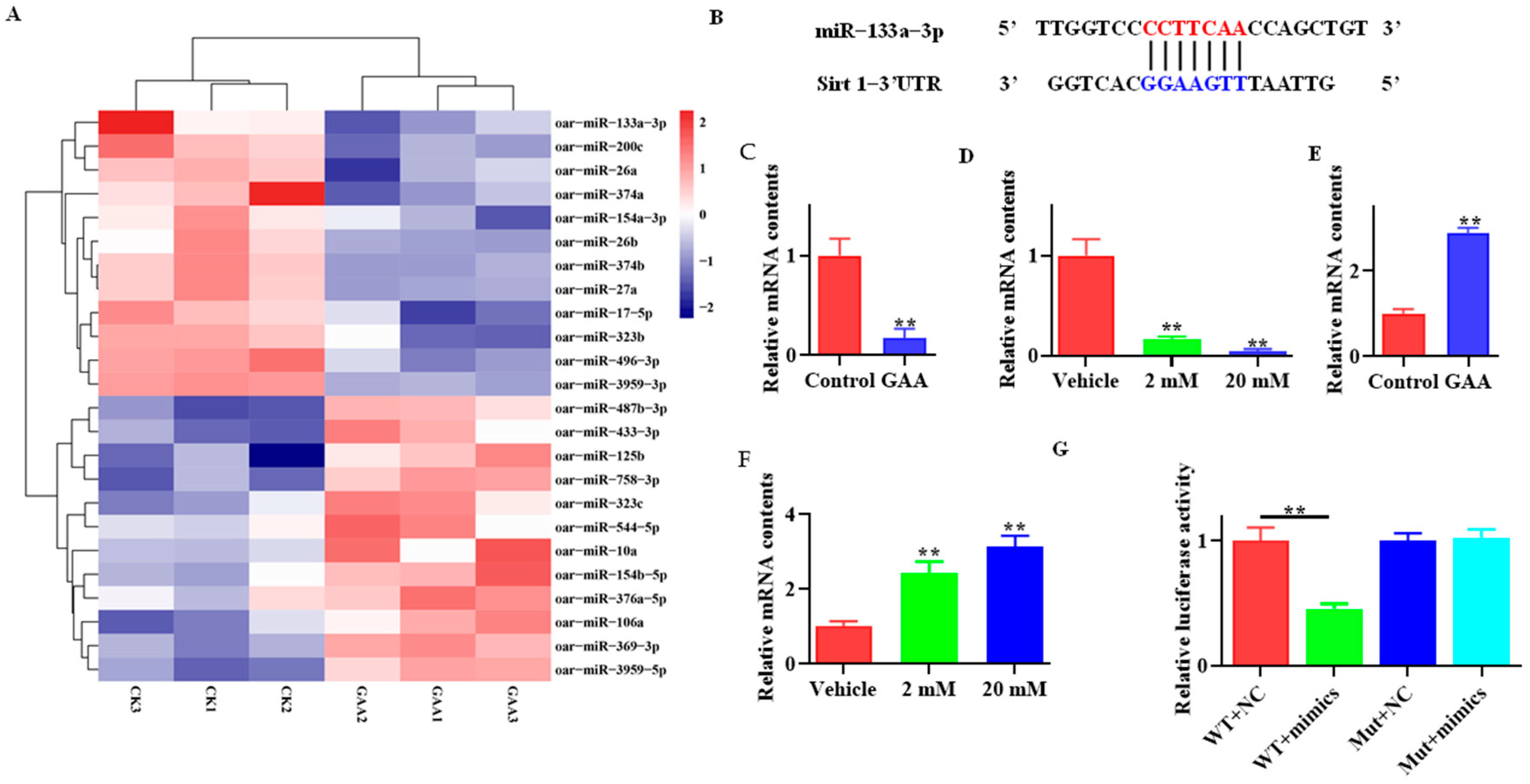
3.4. Effects of GAA on miR-133a and Sirt1 Expression
3.5. Effect of miR-133a on Adipogenic Differentiation of Sheep SVF Cells
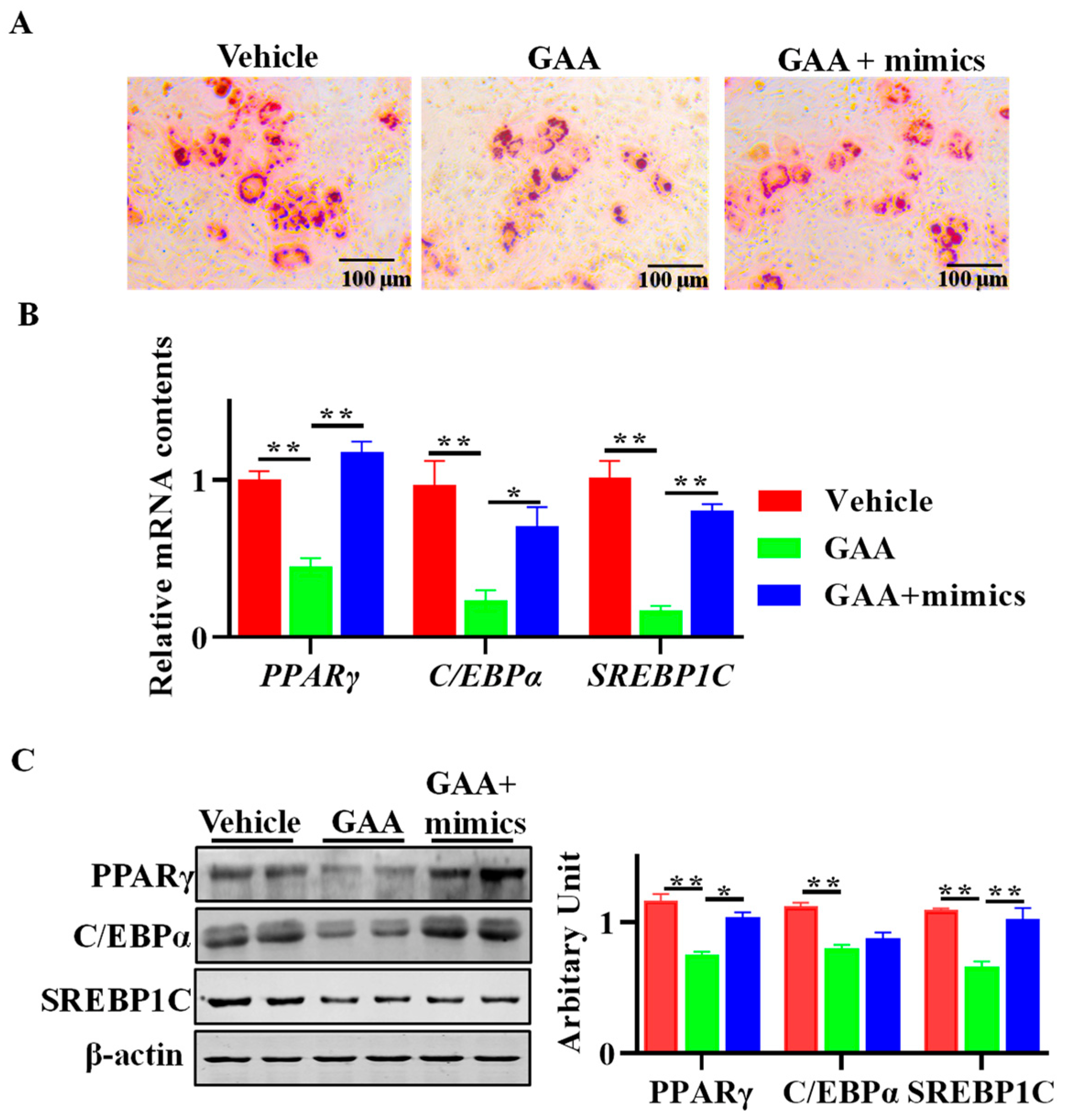
3.6. GAA Attenuated Adipogenesis via Regulation of miR-133a
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostojic, S.M.; Ratgeber, L.; Olah, A.; Betlehem, J.; Acs, P. Guanidinoacetic acid deficiency: A new entity in clinical medicine? Int. J. Med. Sci. 2020, 17, 2544. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; El Sayed, R.; Abdelfattah-Hassan, A.; Morshedy, A.M. Creatine or guanidinoacetic acid? Which is more effective at enhancing growth, tissue creatine stores, quality of meat, and genes controlling growth/myogenesis in Mulard ducks. J. Appl. Anim. Res. 2019, 47, 159–166. [Google Scholar] [CrossRef]
- Zhu, Z.; Gu, C.; Hu, S.; Li, B.; Zeng, X.; Yin, J. Dietary guanidinoacetic acid supplementation improved carcass characteristics, meat quality and muscle fibre traits in growing-finishing gilts. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Valini, G.A.d.C.; Duarte, M.d.S.; Rodrigues, G.d.A.; Veroneze, R.; Saraiva, A.; Hausman, G.; Rocha, G.C. Guanidinoacetic acid supplementation on growth performance and molecular mechanisms of lean mass gain in nursery pigs. Cienc. Rural 2020, 50, e20190948. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, C.; Wu, Z.Z.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, J.; Chen, L.; Zhang, Y.L.; Pei, C.X.; et al. Effects of guanidinoacetic acid supplementation on growth performance, nutrient digestion, rumen fermentation and blood metabolites in Angus bulls. Animal 2020, 14, 2535–2542. [Google Scholar] [CrossRef]
- Li, Z.; Liang, H.; Xin, J.; Xu, L.; Li, M.; Yu, H.; Zhang, W.; Ge, Y.; Li, Y.; Qu, M. Effects of Dietary Guanidinoacetic Acid on the Feed Efficiency, Blood Measures, and Meat Quality of Jinjiang Bulls. Front. Vet. Sci. 2021, 8, 684295. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Song, P.; Zhao, J.; Zhang, J.; Zhao, J. Skeletal muscle mass, meat quality and antioxidant status in growing lambs supplemented with guanidinoacetic acid. Meat Sci. 2022, 192, 108906. [Google Scholar] [CrossRef]
- Li, W.J.; Jiang, Y.W.; Cui, Z.Y.; Wu, Q.C.; Zhang, F.; Chen, H.W.; Wang, Y.L.; Wang, W.K.; Lv, L.K.; Xiong, F.L.; et al. Dietary Guanidine Acetic Acid Addition Improved Carcass Quality with Less Back-Fat Thickness and Remarkably Increased Meat Protein Deposition in Rapid-Growing Lambs Fed Different Forage Types. Foods 2023, 12, 641. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Fu, Y.; Li, Y.; Jiang, Y.; Zhou, G.; Gao, F. Creatine Monohydrate and Guanidinoacetic Acid Supplementation Affects the Growth Performance, Meat Quality, and Creatine Metabolism of Finishing Pigs. J. Agric. Food Chem. 2018, 66, 9952–9959. [Google Scholar] [CrossRef]
- Portocarero, N.; Braun, U. The physiological role of guanidinoacetic acid and its relationship with arginine in broiler chickens. Poult. Sci. 2021, 100, 101203. [Google Scholar] [CrossRef]
- Yan, Z.; Yan, Z.; Liu, S.; Yin, Y.; Yang, T.; Chen, Q. Regulative Mechanism of Guanidinoacetic Acid on Skeletal Muscle Development and Its Application Prospects in Animal Husbandry: A Review. Front. Nutr. 2021, 8, 714567. [Google Scholar] [CrossRef] [PubMed]
- Vishvanath, L.; Gupta, R.K. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J. Clin. Investig. 2019, 129, 4022–4031. [Google Scholar] [CrossRef]
- Hilton, C.; Neville, M.; Karpe, F. MicroRNAs in adipose tissue: Their role in adipogenesis and obesity. Int. J. Obes. 2013, 37, 325–332. [Google Scholar] [CrossRef]
- Lee, N.; Kim, I.; Park, S.; Han, D.; Ha, S.; Kwon, M.; Kim, J.; Byun, S.-H.; Oh, W.; Jeon, H.B. Creatine inhibits adipogenesis by downregulating insulin-induced activation of the phosphatidylinositol 3-kinase signaling pathway. Stem Cells Dev. 2015, 24, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Kazak, L.; Chouchani, E.T.; Lu, G.Z.; Jedrychowski, M.P.; Bare, C.J.; Mina, A.I.; Kumari, M.; Zhang, S.; Vuckovic, I.; Laznik-Bogoslavski, D. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metab. 2017, 26, 660–671.e3. [Google Scholar] [CrossRef] [PubMed]
- Khajali, F.; Lemme, A.; Rademacher-Heilshorn, M. Guanidinoacetic acid as a feed supplement for poultry. World’s Poult. Sci. J. 2020, 76, 270–291. [Google Scholar] [CrossRef]
- Hearnden, R.; Sandhar, B.; Vyas, V.; Longhi, M.P. Isolation of stromal vascular fraction cell suspensions from mouse and human adipose tissues for downstream applications. STAR Protoc. 2021, 2, 100422. [Google Scholar] [CrossRef]
- Cao, Y.; Deng, B.; Zhang, S.; Gao, H.; Song, P.; Zhang, J.; Zhao, J. Astragalus polysaccharide regulates brown adipogenic differentiation through miR-1258-5p-modulated cut-like homeobox 1 expression. Acta Biochim. Biophys. Sin. 2021, 53, 1713–1722. [Google Scholar] [CrossRef]
- Fowler, S.M.; Morris, S.; Hopkins, D.L. Assessment of a probe to measure fat depth of lamb carcasses. Meat Sci. 2020, 159, 107937. [Google Scholar] [CrossRef]
- Oksbjerg, N.; Gondret, F.; Vestergaard, M. Basic principles of muscle development and growth in meat-producing mammals as affected by the insulin-like growth factor (IGF) system. Domest. Anim. Endocrinol. 2004, 27, 219–240. [Google Scholar] [CrossRef]
- Mohammadabadi, M.; Bordbar, F.; Jensen, J.; Du, M.; Guo, W. Key genes regulating skeletal muscle development and growth in farm animals. Animals 2021, 11, 835. [Google Scholar] [CrossRef] [PubMed]
- Buonaiuto, G.; Lopez-Villalobos, N.; Niero, G.; Degano, L.; Dadati, E.; Formigoni, A.; Visentin, G. The application of legendre polynomials to model muscularity and body condition score in primiparous Italian simmental cattle. Ital. J. Anim. Sci. 2022, 21, 350–360. [Google Scholar] [CrossRef]
- Fowler, S.M.; Hoban, J.M.; van de Ven, R.; Gardner, G.; Pethick, D.W.; Hopkins, D.L. The effect of lamb carcase weight and GR depth on the production of value-added cuts–A short communication. Meat Sci. 2017, 131, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Handbook of Australian Meat, 7th ed.; AUS-MEAT: Tingalpa, Australia, 2005.
- Fajas, L. Adipogenesis: A cross-talk between cell proliferation and cell differentiation. Ann. Med. 2003, 35, 79–85. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research techniques made simple: Analysis of collective cell migration using the wound healing assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Zeng, C.; Pan, F.; Jones, L.A.; Lim, M.M.; Griffin, E.A.; Sheline, Y.I.; Mintun, M.A.; Holtzman, D.M.; Mach, R.H. Evaluation of 5-ethynyl-2′-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system. Brain Res. 2010, 1319, 21–32. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Qiu, W.; Zhang, J.; Feng, S.; Zhou, X.; Wang, X.; Jin, L.; Long, K.; Liu, L.; et al. Guanidinoacetic Acid Regulates Myogenic Differentiation and Muscle Growth Through miR-133a-3p and miR-1a-3p Co-mediated Akt/mTOR/S6K Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2837. [Google Scholar] [CrossRef]
- Tang, Q.; Otto, T.C.; Lane, M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 44–49. [Google Scholar] [CrossRef]
- Elkhawaga, S.Y.; Ismail, A.; Elsakka, E.G.; Doghish, A.S.; Elkady, M.A.; El-Mahdy, H.A. miRNAs as cornerstones in adipogenesis and obesity. Life Sci. 2023, 315, 121382. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Zhang, W.; Hu, X.; Wei, H.; Peng, J.; Jiang, S. SIRT1 inhibits adipogenesis and promotes myogenic differentiation in C3H10T1/2 pluripotent cells by regulating Wnt signaling. Cell Biosci. 2015, 5, 61. [Google Scholar] [CrossRef]
- Pang, W.; Wang, Y.; Wei, N.; Xu, R.; Xiong, Y.; Wang, P.; Shen, Q.; Yang, G. Sirt1 inhibits akt2-mediated porcine adipogenesis potentially by direct protein-protein interaction. PLoS ONE 2013, 8, e71576. [Google Scholar] [CrossRef] [PubMed]
- Baskal, S.; Beckmann, B.; Stahmer, L.; Peter, C.; Bohnhorst, B.; Das, A.M.; Tsikas, D. Possible role of SIRT1 and SIRT3 in post-translational modifications in human breast milk during the neonatal period. Amino Acids 2022, 54, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-specific microRNAs in skeletal muscle development. Dev. Biol. 2016, 410, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Bezprozvannaya, S.; Williams, A.H.; Qi, X.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008, 22, 3242–3254. [Google Scholar] [CrossRef] [PubMed]
- Trajkovski, M.; Ahmed, K.; Esau, C.C.; Stoffel, M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat. Cell Biol. 2012, 14, 1330–1335. [Google Scholar] [CrossRef]
- Qiang, L.; Wang, L.; Kon, N.; Zhao, W.; Lee, S.; Zhang, Y.; Rosenbaum, M.; Zhao, Y.; Gu, W.; Farmer, S.R. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell 2012, 150, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Song, T.; Peng, J.; Zhou, Z.; Wei, H.; Zhou, R.; Jiang, S.; Peng, J. SIRT1 suppresses adipogenesis by activating Wnt/β-catenin signaling in vivo and in vitro. Oncotarget 2016, 7, 77707. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Wang, Y.; Li, Y.; Wang, Y.; Zhu, J.; Lin, Y. Screening of key miRNAs related with the differentiation of subcutaneous adipocytes and the validation of miR-133a-3p functional significance in goats. Anim. Biosci. 2023, 36, 144–155. [Google Scholar] [CrossRef]


| Gene | Sequence (5′-3′) | |
|---|---|---|
| PCNA | Forward | GAGGCGTCTCAGGCGTTC |
| Reverse | TTAAGCGCCTCCAGCACTTT | |
| Cdk4 | Forward | GCCCCGAGATGTCTCTCTAC |
| Reverse | AGAGATTCGCTTGTGTGGGT | |
| CyclinD1 | Forward | GTCCTGGTGTTTTCCTTTGCTC |
| Reverse | CTCTTCCCTCTCTTCAGCTCAG | |
| PPARγ | Forward | CCGGCGCAGTTGTTCAG |
| Reverse | CGGCATCTCTGTGTCAACCA | |
| C/EBPα | Forward | AGACGTCCATCGACATCAGC |
| Reverse | CCCGGGTAGTCAAAGTCGTT | |
| SREBP1C | Forward | GCCTTCTATTACATCCACAACCTTG |
| Reverse | AGATTATCCAGCATCCGCATGAG | |
| ACC | Forward | CTCGCCCTCAACGTAGGAAG |
| Reverse | GAACACATCGAGGGGAAGCA | |
| ACLY | Forward | TAACACCATCATCTGTGCTCGG |
| Reverse | GTCGAAGGCCTTGCTGAACA | |
| FAS | Forward | CTGGTGACGGCCAACTGTAT |
| Reverse | CACTGGCCCTGGGTTATGTT | |
| Sirt 1 | Forward | ACACCTGTCACTGTGGTAGAG |
| Reverse | GAACCGTAACCGGGGTCTG | |
| miRNA-133a | Forward | CGTTTGGTCCCCTTCAACC |
| Reverse | AGTGCAGGGTCCGAGGTATT | |
| U6 | Forward | CTCGCTTCGGCAGCACA |
| Forward | AACGCTTCACGAATTTGCGT | |
| 18S rRNA | Forward | CGGCTACCACATCCAAGGAA |
| Reverse | GCTGGAATTACCGCGGCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.-M.; Li, F.-Q.-Y.; Li, X.-Y.; Jiao, D.-R.; Liu, X.-D.; Lv, X.-Y.; Zhao, J.-X. Guanidinoacetic Acid Attenuates Adipogenesis through Regulation of miR-133a in Sheep. Animals 2023, 13, 3108. https://doi.org/10.3390/ani13193108
Zhao J-M, Li F-Q-Y, Li X-Y, Jiao D-R, Liu X-D, Lv X-Y, Zhao J-X. Guanidinoacetic Acid Attenuates Adipogenesis through Regulation of miR-133a in Sheep. Animals. 2023; 13(19):3108. https://doi.org/10.3390/ani13193108
Chicago/Turabian StyleZhao, Jia-Min, Fan-Qin-Yu Li, Xv-Ying Li, Dan-Rong Jiao, Xiang-Dong Liu, Xiao-Yang Lv, and Jun-Xing Zhao. 2023. "Guanidinoacetic Acid Attenuates Adipogenesis through Regulation of miR-133a in Sheep" Animals 13, no. 19: 3108. https://doi.org/10.3390/ani13193108
APA StyleZhao, J.-M., Li, F.-Q.-Y., Li, X.-Y., Jiao, D.-R., Liu, X.-D., Lv, X.-Y., & Zhao, J.-X. (2023). Guanidinoacetic Acid Attenuates Adipogenesis through Regulation of miR-133a in Sheep. Animals, 13(19), 3108. https://doi.org/10.3390/ani13193108




