Monitoring of Body Condition in Dairy Cows to Assess Disease Risk at the Individual and Herd Level
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Study Population
2.2. Outcome and Predictors Variables
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meléndez, P.; Bartolomé, J. Advances on nutrition and fertility in dairy cattle: Review. Rev. Mex. Cienc. Pecu. 2017, 8, 407–417. [Google Scholar]
- Roche, J.R.; Friggens, N.C.; Kay, J.K.; Fisher, M.W.; Stafford, K.J.; Berry, D.P. Invited review: Body condition score and its association with dairy cow productivity, health, and welfare. J. Dairy Sci. 2009, 92, 5769–5801. [Google Scholar] [CrossRef]
- Carvalho, P.D.; Souza, A.H.; Amundson, M.C.; Hackbart, K.S.; Fuenzalida, M.J.; Herlihy, M.M.; Ayres, H.; Dresch, A.R.; Vieira, L.M.; Guenther, J.N.; et al. Relationships between fertility and postpartum changes in body condition and body weight in lactating dairy cows. J. Dairy Sci. 2014, 97, 3666–3683. [Google Scholar] [CrossRef] [PubMed]
- Dairy New Zealand. The InCalf Book for New Zealand Dairy Farmers, 2nd ed.; DairyNZ Ltd.: Hamilton, New Zealand, 2019. [Google Scholar]
- Dairy Australia. InCalf Book for Dairy Farmers, 2nd ed.; Dairy Australia Limited: Melbourne, Australia, 2017. [Google Scholar]
- Bedere, N.; Cutullic, E.; Delaby, L.; Garcia-Launay, F.; Disenhaus, C. Meta-analysis of the relationships between reproduction, milk yield and body condition score in dairy cows. Livest. Sci. 2018, 210, 73–84. [Google Scholar] [CrossRef]
- Dubuc, J.; Denis-Robichaud, J. A dairy herd-level study of postpartum diseases and their association with reproductive performance and culling. J. Dairy Sci. 2017, 100, 3068–3078. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.D.; Galligan, D.T.; Thomsen, N. Principal Descriptors of Body Condition Score in Holstein Cows. J. Dairy Sci. 1994, 77, 2695–2703. [Google Scholar] [CrossRef] [PubMed]
- Buonaiuto, G.; Lopez-Villalobos, N.; Niero, G.; Degano, L.; Dadati, E.; Formigoni, A.; Visentin, G. The application of Legendre Polynomials to model muscularity and body condition score in primiparous Italian Simmental cattle. Ital. J. Anim. Sci. 2022, 21, 350–360. [Google Scholar] [CrossRef]
- Buonaiuto, G.; Lopez-Villalobos, N.; Costa, A.; Niero, G.; Degano, L.; Mammi, L.M.E.; Cavallini, D.; Palmonari, A.; Formigoni, A.; Visentin, G. Stayability in Simmental cattle as affected by muscularity and body condition score between calvings. Front. Vet. Sci. 2023, 10, 1141286. [Google Scholar] [CrossRef]
- Shmueli, G. To Explain or to Predict? Stat. Sci. 2010, 25, 289–310. [Google Scholar] [CrossRef]
- Wisnieski, L.; Norby, B.; Pierce, S.J.; Becker, T.; Sordillo, L.M. Prospects for predictive modeling of transition cow diseases. Anim. Health Res. Rev. 2019, 20, 19–30. [Google Scholar] [CrossRef]
- Chapinal, N.; LeBlanc, S.J.; Carson, M.E.; Leslie, K.E.; Godden, S.; Capel, M.; Santos, J.E.P. Herd-level association of serum metabolites in the transition period with disease, milk production, and early lactation reproductive performance. J. Dairy Sci. 2012, 95, 5676–5682. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Association between the proportion of sampled transition cows with increased nonesterified fatty acids and β-hydroxybutyrate and disease incidence, pregnancy rate, and milk production at the herd level. J. Dairy Sci. 2010, 93, 3595–3601. [Google Scholar] [CrossRef] [PubMed]
- Jordan, D.; McEwen, S.A. Herd-level test performance based on uncertain estimates of individual test performance, individual true prevalence and herd true prevalence. Prev. Vet. Med. 1998, 36, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.A.; McArt, J.A.; Overton TR Stokol, T.; Nydam, D.V. Using Nonesterified Fatty Acids and b-Hydroxybutyrate Concentrations During the Transition Period for Herd-Level Monitoring of Increased Risk of Disease and Decreased Reproductive and Milking Performance. Vet. Clin. Food Anim. 2013, 29, 387–412. [Google Scholar] [CrossRef] [PubMed]
- Giuliodori, M.J.; Magnasco, R.P.; Becu-Villalobos, D.; Lacau-Mengido, I.M.; Risco, C.A.; de la Sota, R.L. Metritis in dairy cows: Risk factors and reproductive performance. J. Dairy. Sci. 2013, 96, 3621–3631. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining postpartum uterine disease in cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef]
- Kelton, D.F.; Lissemore, K.D.; Martin, R.E. Recommendations for recording and calculating the incidence of selected clinical diseases of dairy cattle. J. Dairy Sci. 1998, 81, 2502–2509. [Google Scholar] [CrossRef] [PubMed]
- Greiner, M.; Pfeiffer, D.; Smith, R.D. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev. Vet. Med. 2000, 45, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Dohoo, I.; Martin, W.; Stryhn, H. (Eds.) Measures of Association, Chapter 6; Introduction to Clustered Data, Chapter 20. In Veterinary Epidemiologic Research; AVC Inc.: Charlottetown, PE, Canada, 2009; pp. 139–152, 563–576. [Google Scholar]
- Beam, S.W.; Butler, W.R. Energy balance and ovarian follicle development prior to the first ovulation postpartum in dairy cows receiving three levels of dietary fat. Biol. Reprod. 1997, 56, 133–142. [Google Scholar] [CrossRef]
- Beam, S.W.; Butler, W.R. Energy balance, metabolic hormones, and early postpartum follicular development in dairy cows fed prilled lipid. J. Dairy Sci. 1998, 81, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Beam, S.W.; Butler, W.R. Effects of energy balance on follicular development and first ovulation in postpartum dairy cows. J. Reprod. Fertil. 1999, 54, 411–424. [Google Scholar] [CrossRef]
- Giuliodori, M.J.; Magnasco, R.P.; Becu-Villalobos, D.; Lacau-Mengido, I.M.; Risco, C.A.; de la Sota, R.L. Clinical endometritis in an Argentinean herd of dairy cows: Risk factors and reproductive efficiency. J. Dairy Sci. 2013, 96, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Giuliodori, M.J.; Magnasco, M.; Magnasco, R.P.; Lacau-Mengido, I.M.; de la Sota, R.L. Purulent vaginal discharge in grazing dairy cows: Risk factors, reproductive performance and prostaglandin F2α treatment. J. Dairy Sci. 2017, 100, 3805–3815. [Google Scholar] [CrossRef] [PubMed]
- Chagas, L.M.; Rhodes, F.M.; Blache, D.; Gore, P.J.S.; Macdonald, K.A.; Verkerk, G.A. Precalving Effects on Metabolic Responses and Postpartum Anestrus in Grazing Primiparous Dairy Cows. J. Dairy Sci. 2006, 89, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Friggens, N.C. Body lipid reserves and the reproductive cycle: Towards a better understanding. Livest. Prod. Sci. 2003, 83, 219–236. [Google Scholar] [CrossRef]
- Rothman, K.J.; Greenland, S.; Lash, T.L.; Rothman, K.J.; Greenland, S.; Poole, C.; Lash, T.L. Causation and causal inference. In Modern Epidemiology, 3rd ed.; Philadelphia Lippincott Williams-Wilkins: Philadelphia, PA, USA, 2008; pp. 5–31. [Google Scholar]
- Wisnieski, L.; Norby, B.; Pierce, S.J.; Becker, T.; Gandy, J.C.; Sordillo, S. Predictive models for early lactation diseases in transition dairy cattle at dry-off. Prev. Vet. Med. 2019, 163, 68–78. [Google Scholar] [CrossRef]
- Wisnieski, L.; Norby, B.; Pierce, S.J.; Becker, T.; Gandy, J.C.; Sordillo, S. Cohort-level disease prediction by extrapolation of individual-level predictions in transition dairy cattle. Prev. Vet. Med. 2019, 169, 104692. [Google Scholar] [CrossRef]
- Wisnieski, L.; Norby, B.; Pierce, S.J.; Becker, T.; Gandy, J.C.; Sordillo, S. Cohort-level disease prediction using aggregate biomarker data measured at dry-off in transition dairy cattle: A proof-of-concept study. Prev. Vet. Med. 2019, 169, 104701. [Google Scholar] [CrossRef]
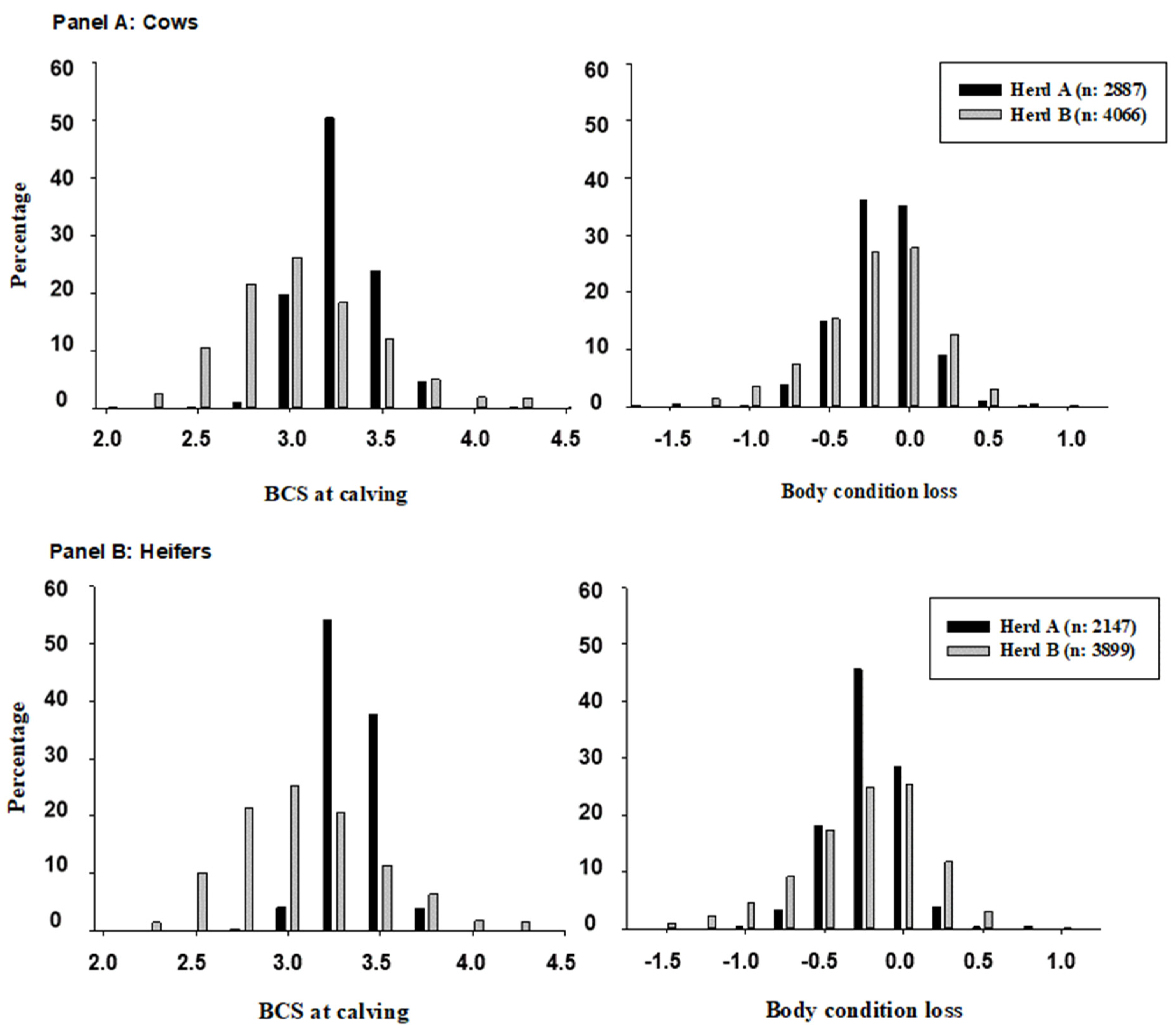
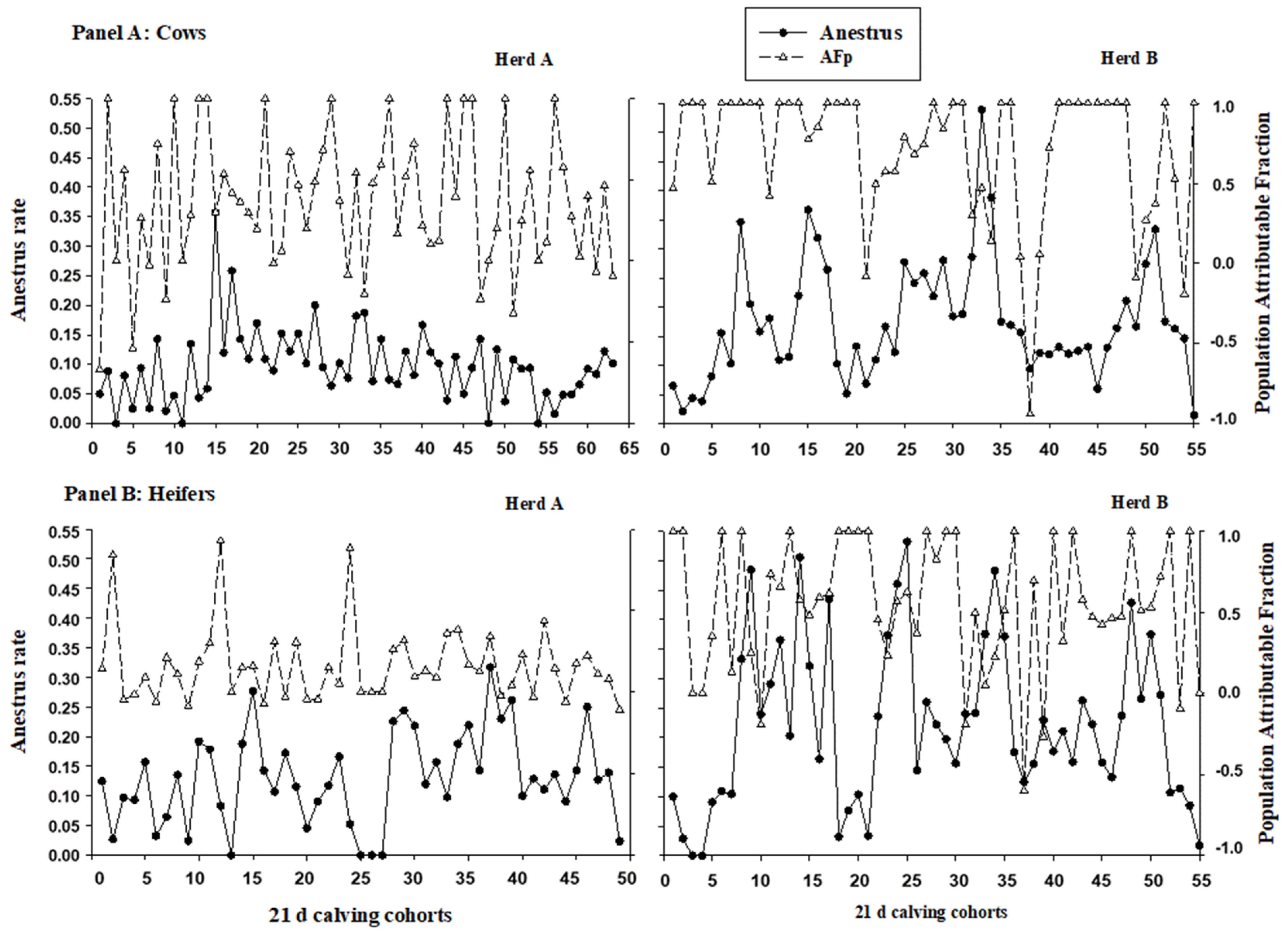
| Herd | Parity (n) | Anestrus 1 (%) | Metritis 2 (%) | Mastitis 3 (%) | PRE100 4 (%) | AI80 5 (%) | Milk 6 (Mean) |
|---|---|---|---|---|---|---|---|
| A | Heifers (2.147) | 14.0 | 22.8 | 7.1 | 31.9 | 64.9 | 24.8 |
| Cows (2.887) | 10.4 | 26.1 | 14.0 | 29.0 | 70.0 | 29.9 | |
| B | Heifers (3.899) | 23.5 | 27.0 | 30.0 | 31.7 | 61.2 | 24.7 |
| Cows (4.066) | 16.5 | 12.3 | 28.1 | 20.5 | 58.9 | 33.6 |
| OR (95%CI) 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Herd | Parity | Predictor | Anestrus 2 | Metritis 3 | Mastitis 4 | PRE100 5 | AI80 6 | Milk 7 |
| A | Heifers (n = 2.147) | BCS 8 | 0.07 (0.04–0.12) | 0.31 (0.21–0.48) | 1.12 (0.60–2.07) | 2.35 (1.63–3.41) | 4.48 (3.11–6.44) | 1.33 (0.82) |
| ΔBCS 9 | 0.36 (0.30–0.42) | 0.60 (0.52–0.70) | 0.75 (0.60–0.93) | 1.49 (1.31–1.70) | 1.89 (1.66–2.16) | 0.18 (0.58) | ||
| Cows (n = 2.887) | BCS | 0.05 (0.04–0.08) | 0.40 (0.31–0.53) | 0.96 (0.70–1.30) | 1.67 (1.30–2.14) | 3.09 (2.37–4.02) | 2.59 (0.80) * | |
| ΔBCS | 0.33 (0.28–0.39) | 0.69 (0.62–0.76) | 0.94 (0.83–1.08) | 1.43 (1.28–1.59) | 1.72 (1.54–1.93) | −2.53 (0.68) * | ||
| B | Heifers (n = 3.899) | BCS | 0.13 (0.11–0.17) | 0.83 (0.70–0.99) | 0.90 (0.79–1.03) | 2.02 (1.76–2.31) | 3.08 (2.65–3.58) | −0.68 (0.41) |
| ΔBCS | 0.41 (0.38–0.46) | 0.83 (0.76–0.91) | 1.02 (0.95–1.08) | 1.32 (1.23–1.41) | 1.62 (1.51–1.74) | −2.24 (0.41) * | ||
| Cows (n = 4.066) | BCS | 0.12 (0.10–0.15) | 0.67 (0.56–0.79) | 0.97 (0.87–1.08) | 1.42 (1.25–1.62) | 2.20 (1.94–2.48) | −0.01 (0.46) | |
| ΔBCS | 0.37 (0.34–0.42) | 0.70 (0.45–1.10) | 1.00 (0.94–1.06) | 1.13 (1.06–1.21) | 1.42 (1.34–1.52) | −1.46 (0.49) * |
| Herd | Parity | Threshold 1 | OR (95%CI) 2 | Se 3 | Sp 4 | AUC 5 |
|---|---|---|---|---|---|---|
| A | Heifers 6 | Q50 (6%) | 0.22 (0.07–0.76) | 69.2% | 66.6% | 0.679 |
| A | Cows 7 | Q25 (20%) | 0.45 (0.14–1.47) | 81.5% | 33.3% | 0.574 |
| B | Heifers 8 | Q50 (74%) | 0.25 (0.06–0.73) | 66% | 68% | 0.673 |
| B | Cows 9 | Q75 (78%) | 0.29 (0.08–1.11) | 37% | 85% | 0.611 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rearte, R.; Lorenti, S.N.; Dominguez, G.; de la Sota, R.L.; Lacau-Mengido, I.M.; Giuliodori, M.J. Monitoring of Body Condition in Dairy Cows to Assess Disease Risk at the Individual and Herd Level. Animals 2023, 13, 3114. https://doi.org/10.3390/ani13193114
Rearte R, Lorenti SN, Dominguez G, de la Sota RL, Lacau-Mengido IM, Giuliodori MJ. Monitoring of Body Condition in Dairy Cows to Assess Disease Risk at the Individual and Herd Level. Animals. 2023; 13(19):3114. https://doi.org/10.3390/ani13193114
Chicago/Turabian StyleRearte, Ramiro, Santiago Nicolas Lorenti, German Dominguez, Rodolfo Luzbel de la Sota, Isabel María Lacau-Mengido, and Mauricio Javier Giuliodori. 2023. "Monitoring of Body Condition in Dairy Cows to Assess Disease Risk at the Individual and Herd Level" Animals 13, no. 19: 3114. https://doi.org/10.3390/ani13193114






