Evolutions in Commercial Meat Poultry Breeding
Abstract
:Simple Summary
Abstract
1. History of Commercial Poultry Breeding
1.1. Domestication
1.2. Development of Science and Commercial Breeding Structures
1.2.1. Development of Quantitative Genetics
1.2.2. Testing Stations
1.2.3. Crossbreeding
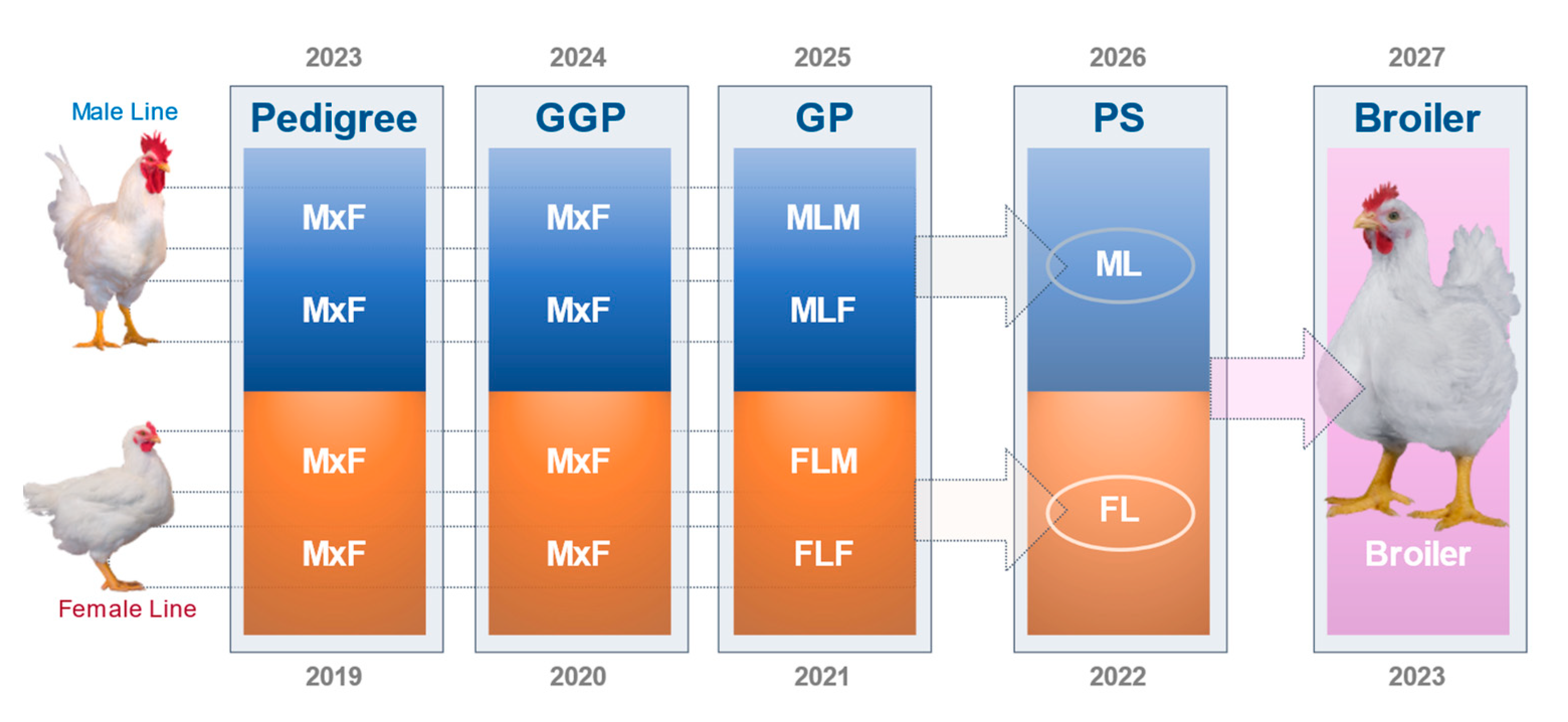
1.2.4. Pedigree Breeding Structure
1.2.5. Development of Genomic Selection
1.2.6. From Specialist Lines Breeders to Broad Spectrum Breeders
2. Evolution in Breeding Goals
2.1. Breeding Goal
2.2. What Determines a Breeding Goal
2.3. Expansion of Breeding Goals
3. Genetics Basis of Key Traits and the Management of Antagonisms
3.1. Traits
3.2. Development of Key Traits
3.2.1. Traits Covering Health and Welfare
3.2.2. Environmental Impact Traits
3.2.3. Robustness Traits
3.2.4. Meat Quality Traits
3.3. New Methods
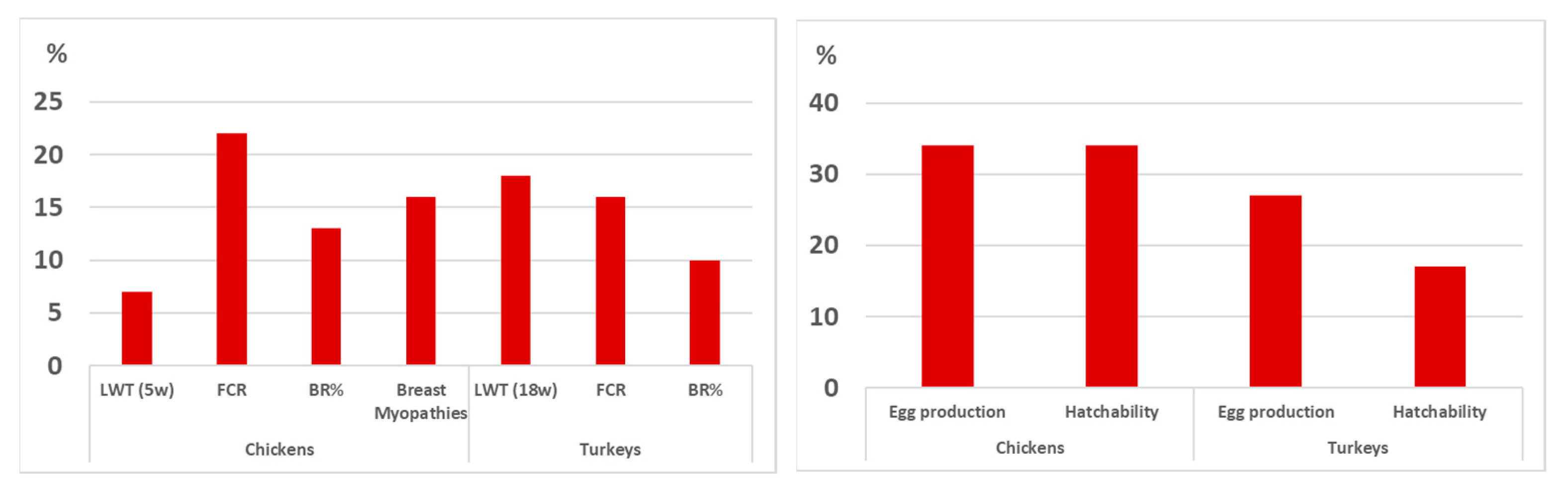
3.3.1. The Impact of Genomics Selection
3.3.2. Gut Health and Gut Microbiome
3.4. The Management of Trait Antagonisms
3.4.1. The Levels of Trait Antagonism
3.4.2. The Management of Antagonisms between Two Traits
4. Breeding for Sustainability: The Balance between Welfare and Environmental Impact
4.1. Crossbreds for Different Market Segments
4.2. Environmental Impact of Different Crossbreds
4.3. Outcomes at the Farm Level
5. A Future Outlook
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McKay, J.C. The Genetics of Modern Commercial Poultry. In Biology of Breeding Poultry; Poultry Science Symposium Series; Hocking, P., Ed.; CABI International: Wallingford, UK, 2009; Volume 29, pp. 3–9. [Google Scholar]
- Tixier-Boichard, M.; Leenstra, F.; Flock, D.K.; Hocking, P.M.; Weigend, S. A Century of Poultry Genetics. World’s Poult. Sci. J. 2012, 68, 307–321. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2023–2032; OECD Publishing: Paris, France, 2023; pp. 20–76+184–201. [Google Scholar]
- Zeuner, F.E. A History of Domesticated Animals; Hutchinson & Co: London, UK, 1963; p. 560. [Google Scholar]
- West, B.; Zhou, B.X. Did chickens go north? New evidence for domestication. J. Archaeol. Sci. 1988, 15, 515–533. [Google Scholar] [CrossRef]
- Tixier-Boichard, M.; Bedd’hom, B.; Rognon, X. Chicken domestication: From archaeology to genomics. C. R. Biol. 2011, 334, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Lawal, R.A.; Martin, S.H.; Vanmechelen, K.; Vereijken, A.; Silva, P.; Al-Atiyat, R.M.; Aljumaah, R.S.; Mwacharo, J.M.; Wu, D.-D.; Zhang, Y.-P.; et al. The wild species genome ancestry of domestic chickens. BMC Biol. 2020, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-S.; Thakur, M.; Peng, M.-S.; Jiang, Y.; Frantz, L.A.F.F.; Li, M.; Zhang, J.-J.; Wang, S.; Peters, J.; Otecko, N.O.; et al. 863 genomes reveal the origin and domestication of chicken. Cell Res. 2020, 30, 693–701. [Google Scholar] [CrossRef]
- Guo, Y.; Ou, J.; Zan, Y.; Wang, Y.; Li, H.; Zhu, C.; Chen, K.; Zhou, X.; Hu, X.; Carlborg, Ö. Researching on the fine structure and admixture of the worldwide chicken population reveal connections between populations and important events in breeding history. Evol. Appl. 2021, 15, 553–564. [Google Scholar] [CrossRef]
- Madge, S.; McGowan, P. Pheasants, Partridges & Grouse: A Guide to the Pheasants, Partridges, Quails, Grouse, Guineafowl, Buttonquails and Sandgrouse of the World; Christopher Hem: London, UK, 2002; pp. 1–23. [Google Scholar]
- Speller, C.F.; Kemp, B.M.; Wyatt, S.D.; Monroe, C.; Lipe, W.D.; Arndt, U.M.; Yang, D.Y. Ancient mitochondrial DNA analysis reveals complexity of indigenous North American turkey domestication. Proc. Natl. Acad. Sci. USA 2010, 107, 2807–2812. [Google Scholar] [CrossRef]
- Katajamaa, R.; Jensen, P. Tameness correlates with domestication related traits in a Red Junglefowl intercross. Genes Brain Behav. 2020, 20, e12704. [Google Scholar] [CrossRef]
- Rutkauskaite, A.; Jensen, P. Domestication effects on social information transfer in chickens. Anim. Cogn. 2022, 25, 1473–1478. [Google Scholar] [CrossRef]
- Jull, M.A. Poultry Breeding; John Wiley & Sons, Inc.: New York, NY, USA; Chapman & Hall, Ltd.: London, UK, 1932; p. 376. [Google Scholar]
- Laughlin, K.F. The Evolution of Genetics, Breeding and Production; Temperton Fellowship; Harper Adams University: Newport, UK, 2007; Volume 15, pp. 1–55. [Google Scholar]
- Muir, W.M.; Wong, G.K.-S.; Zhang, Y.; Wang, J.; Groenen, M.A.M.; Crooijmans, R.P.M.A.; Megens, H.-J.; Zhang, H.; Okimoto, R.; Vereijken, A.; et al. Genome-wide assessment of worldwide chicken SNP genetic diversity indicates significant absence of rare alleles in commercial breeds. Proc. Natl. Acad. Sci. USA 2008, 105, 17312–17317. [Google Scholar] [CrossRef]
- Nieuwenhoven, A.-M.N.-V.; Knap, P.; Avendaño, S. The Role of Commercial Pig and Poultry Breeding for Food Security. Anim. Front. 2013, 3, 52–57. [Google Scholar] [CrossRef]
- Department for Environment, Food and Rural Affairs (DEFRA). Poultry in the United Kingdom. The Genetic Resources of the National Flocks; Department for Environment, Food and Rural Affairs (DEFRA): London, UK, 2010; pp. 1–24.
- De Vries, H.; Correns, C.; Von Tschermak, E. The discovery of Mendel’s papers. In Plant Hybridization before Mendel; Roberts, H.F., Ed.; Princeton University Press: Princeton, NJ, USA, 1929; pp. 320–358. [Google Scholar]
- Bateson, W.; Saunders, E.R. Experimental studies on the physiology of heredity. Experiments with poultry. In Reports on the Evolution Committee of the Royal Society; The Royal Society: London, UK, 1902; Volume 1, pp. 87–124. [Google Scholar]
- Fisher, R.A. The correlation between relatives on the supposition of mendelian inheritance. Trans. R. Soc. 1918, 52, 399–433. [Google Scholar] [CrossRef]
- Hardy, G.H. Mendelian proportions in a mixed population. Science 1908, 28, 49–50. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, W. Über den Nachweis der Vererbung beim Menschen. Jahresh. Ver. Vaterl. Naturkd. Wurttemb. 1908, 64, 369–382. [Google Scholar]
- Punnett, R.C.; Bailey, P.G. On inheritance of weight in poultry. J. Genet. 1914, 4, 23. [Google Scholar] [CrossRef]
- Sustainable Farm Animal Breeding and Reproduction Technology Platform (FABRE-TP). A Vision for 2025; European Forum of Farm Animal Breeders (EFFAB): Wageningen, The Netherlands, 2006; 30p. [Google Scholar]
- Sustainable Farm Animal Breeding and Reproduction Technology Platform (FABRE-TP). Strategic Research Agenda; European Forum of Farm Animal Breeders (EFFAB): Wageningen, The Netherlands, 2008; p. 26. [Google Scholar]
- Hazel, L.N.; Lush, J.L. The efficiency of three methods of selection. J. Hered. 1942, 33, 393–399. [Google Scholar] [CrossRef]
- Duggan, B.; Ralph, J.; Avendaño, S.; Neeteson, A.-M.; Burnside, T.A.; Koerhuis, A. Decades of Welfare and Sustainability Selection at Aviagen; Aviagen Group: Newbridge, UK, 2023; 17p. [Google Scholar]
- Osborne, R. The use of sire and dam family averages in increasing the efficiency of selective breeding under a hierarchical mating system. Heredity 1957, 11, 93–116. [Google Scholar] [CrossRef]
- Henderson, C.R. Best linear unbiased estimation and prediction under a selection model. Biometrics 1975, 31, 423–447. [Google Scholar] [CrossRef]
- Fiers, W.; Contreras, R.; Duerinck, F.; Haegeman, G.; Iserentant, D.; Merregaert, J.; Jou, W.M.; Molemans, F.; Raeymaekers, A.; Berghe, A.V.D.; et al. Complete nucleotide sequence of bacteriophage MS2 RNA: Primary and secondary structure of the replicase gene. Nature 1976, 260, 500–507. [Google Scholar] [CrossRef]
- Venter, J.C.; Adams, M.D.; Myers, E.W.; Li, P.W.; Mural, R.J.; Sutton, G.G.; Smith, H.O.; Yandell, M.; Evans, C.A.; Holt, R.A.; et al. The sequence of the human genome. Science 2001, 291, 1304–1351. [Google Scholar] [CrossRef]
- Wallis, J.W.; Aerts, J.; Groenen, M.A.M.; Crooijmans, R.P.M.A.; Layman, D.; Graves, T.A.; Scheer, D.E.; Kremitzki, C.; Fedele, M.J.; Mudd, N.K.; et al. A physical map of the chicken genome. Nature 2004, 432, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.L.; Bastiaansen, J.W.M.; Elferink, M.G.; Megens, H.-J.; Richard, P.M.A.; Crooijmans, R.P.M.A.; Blomberg, L.A.; Fleischer, R.C.; Van Tassell, C.P.; Sonstegard, T.S.; et al. Whole genome SNP discovery and analysis of genetic diversity in Turkey (Meleagris gallopavo). BMC Genom. 2012, 13, 391. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Thompson, R. Efficiency of marker-assisted selection in the improvement of quantitative traits. Genetics 1990, 124, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Meuwissen, T.H.E.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, S.J.; Napel, J.T. Study of the Impact of Genetic Selection on the Welfare of Chickens Bred and Kept for Meat Production; DG SANCO 2011/12254; IBF International Consulting: Brussels, Belgium, 2013; p. 118. [Google Scholar]
- Neeteson, A.-M.; McAdam, J.; Swalander, M.; Koerhuis, A. Decades of Welfare and Sustainability Selection in Aviagen; Aviagen Group: Newbridge, UK, 2016; p. 11. [Google Scholar]
- Goddard, M.E. Consensus and debate in the definition of breeding objectives. J. Dairy Sci. 1998, 81, 6–18. [Google Scholar] [CrossRef]
- Farm Animal Welfare Committee (FAWC). Opinion on the Welfare Implications of Breeding and Breeding Technologies in Commercial Livestock Agriculture; Department for Environment, Food and Rural Affairs (DEFRA): London, UK, 2012; pp. 1–29.
- Neeteson, A.-M.; Avendaño, S.; Koerhuis, A. Poultry Breeding for Sustainability and Welfare. In The Economics of Farm Animal Welfare. Theory, Evidence and Policy; Ahmadi, B.V., Moran, D., D’Eath, R., Eds.; CABI International: Wallingford, UK, 2020; pp. 117–146. [Google Scholar]
- Howie, J.A.; Avendano, S.; Tolkamp, B.J.; Kyriazakis, I. Genetic parameters of feeding behavior traits and their relationship with live performance traits in modern broiler lines. Poult. Sci. 2011, 90, 1197–1205. [Google Scholar] [CrossRef]
- Rusakovica, J.; Kremer, V.D.; Plötz, T.; Rohlf, P.; Kyriazakis, I. The genetic basis of novel water utilisation and drinking behaviour traits and their relationship with biological performance in turkeys. Genet. Sel. Evol. 2017, 49, 72. [Google Scholar] [CrossRef]
- Kapell, D.N.R.G.; Hill, W.G.; Neeteson, A.M.; Mc Adam, J.; Koerhuis, A.N.M.; Avendaño, S. Twenty-five years of selection for improved leg health in purebred broiler lines and underlying genetic parameters. Poult. Sci. 2012, 91, 3032–3043. [Google Scholar] [CrossRef]
- Kapell, D.N.R.G.; Hocking, P.M.; Glover, P.K.; Kremer, V.D.; Avendaño, S. Genetics of leg health in turkeys. Genetic basis of leg health and its relationship with body weight in purebred turkey lines. Poult. Sci. 2017, 96, 1553–1562. [Google Scholar] [CrossRef]
- Agriculture and Agri-Food Canada. Chicken and Turkey Condemnations. Poultry Condemnation Report by Species for Federally Inspected Plants. Government of Canada. Gouvernement du Canada. Available online: https://agriculture.canada.ca/en/canadas-agriculture-sectors/animal-industry/poultry-and-egg-market-information (accessed on 20 January 2023).
- Kapell, D.N.R.G.; Hill, W.G.; Neeteson, A.M.; McAdam, J.; Koerhuis, A.N.M.; Avendaño, S. Genetic parameters of foot-pad dermatitis and body weight in purebred broiler lines in 2 contrasting environments. Poult. Sci. 2012, 91, 565–574. [Google Scholar] [CrossRef]
- Mayne, R.K.; Else, R.W.; Hocking, P.M. High litter moisture alone is sufficient to cause footpad dermatitis in growing turkeys. Brit. Poult. Sci. 2007, 48, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Hocking, P.M. Turkeys are equally susceptible to foot pad dermatitis from 1 to 10 weeks of age and foot pad scores were minimized when litter moisture was less than 30%. Poult. Sci. 2011, 90, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Hocking, P.M.; Wu, K. Traditional and commercial turkeys show similar susceptibility to foot pad dermatitis and behavioural evidence of pain. Brit. Poult. Sci. 2013, 54, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Hocking, P.M. Role of litter management on foot pad dermatitis and productivity of traditional and commercial turkeys. In Proceedings of the 8th Turkey Science and Production Conference, Chester, UK, 27–28 March 2014; pp. 33–36. [Google Scholar]
- Royal Society for the Protection of Cruelty against Animals (RSPCA). RSPCA Broiler Breed Welfare Assessment Protocol. 2017. Available online: https://www.rspca.org.uk/documents/1494935/9042554/RSPCA+Broiler+Welfare+Assessment+Protocol+May17.pdf/a8dfa8ff-69ed-e1ab-c908-b1523e4ec642?t=1553171031284&download=true (accessed on 26 July 2023).
- Avendaño, S.A. Adaptability, Robustness and Biological Efficiency in Poultry. In Proceedings of the XIth European Symposium on Poultry Genetics. Session 6. New Breeding Goals for Poultry Species, Prague, Czech Republic, 23–25 October 2019; pp. 53–56. [Google Scholar]
- Howie, J.A.; Tolkamp, B.J.; Bley, T.; Kyriazakis, I. Short-term feeding behaviour has a similar structure in broilers, turkeys and ducks. Brit. Poult. Sci. 2010, 51, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Tolkamp, B.J.; Allcroft, D.J.; Barrio, J.P.; Bley, T.A.; Howie, J.A.; Jacobsen, T.B.; Morgan, C.A.; Schweitzer, D.P.; Wilkinson, S.; Yeates, M.P.; et al. The temporal structure of feeding behavior. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, 378–393. [Google Scholar] [CrossRef]
- Decuypere, E.; Hocking, P.M.; Tona, K.; Onagbesan, O.; Bruggeman, V.; Jones, E.K.M.; Cassy, S.; Rideau, N.; Metayer, S.; Jego, Y. Broiler breeder paradox: A project report. World’s Poult. Sci. J. 2006, 62, 443–453. [Google Scholar] [CrossRef]
- Hill, W.G.; Wolc, A.; O’Sullivan, N.P.; Avendaño, S. Chapter 11. Breeding for sustainability: Maintaining and enhancing multi-trait genetic improvement. In Sustainable Poultry Production in Europe (Poultry Science Symposium Series Book 31); Burton, E., Gatcliffe, J., O’Neill, H.M., Scholey, D., Eds.; CABI Publishers: Oxforshire, UK, 2016; pp. 193–213. [Google Scholar]
- Ricard, F.H. A trial of selecting chickens on their growth curve pattern experimental design and 1st general results. Ann. Genet. Sel. Anim. 1975, 7, 427–444. [Google Scholar]
- Dawkins, M.S.; Layton, R. Breeding for better welfare: Genetic goals for broiler chickens and their parents. Anim. Welf. 2012, 21, 147–155. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Panel on Animal Health and Welfare (AHAW). Scientific Opinion on welfare aspects of the management and housing of the grand-parent and parent stocks raised and kept for breeding purposes. EFSA J. 2009, 8, 1667. [Google Scholar]
- Dunn, I.C.; Meddle, S.L.; Wilson, P.W.; Wardle, C.A.; Law, A.S.; Bishop, V.R.; Hindar, C.; Robertson, G.W.; Burt, D.W.; Ellison, S.J.H.; et al. Decreased expression of the satiety signal receptor CCKAR is responsible for increased growth and body weight during the domestication of chickens. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E909–E921. [Google Scholar] [CrossRef]
- Dixon, L.M.; Brocklehurst, S.; Sandilands, V.; Bateson, M.; Tolkamp, B.J.; D’Eath, R.B. Measuring Motivation for Appetitive Behaviour: Food-Restricted Broiler Breeder Chickens Cross a Water Barrier to Forage in an Area of Wood Shavings without Food. PLoS ONE 2014, 9, e102322. [Google Scholar] [CrossRef] [PubMed]
- Van Emous, R.A.; Kwakkel, R.; Van Krimpen, M.; Hendriks, W. Effects of growth pattern and dietary protein level during rearing on feed intake, eating time, eating rate, behavior, plasma corticosterone concentration, and feather cover in broiler breeder females during the rearing and laying period. Appl. Anim. Behav. Sci. 2014, 150, 44–54. [Google Scholar] [CrossRef]
- Van Emous, R.A. Body Composition and Reproduction in Broiler Breeders: Impact of Feeding Strategies. Ph.D. Thesis, Wageningen University—Graduate School of Wageningen Institute of Animal Sciences (WIAS), Wageningen, The Netherlands, 2015; 173p. [Google Scholar]
- Chang, A.; Halley, J.; Silva, M. Can feeding the broiler breeder improve chick quality and offspring performance? Anim. Prod. Sci. 2016, 56, 1254–1262. [Google Scholar] [CrossRef]
- Vanderhoydonck, C. Transgenerationele Beïnvloeding van de Performantie van Vleeskuikenmoederdieren en Vleeskuikens als Gevolg van een Aangepaste Voederstrategie bij de Vleeskuiken(Groot)Moederdieren (Transgenerational Effect on the Performance of Breeders and Broilers as a Result of an Adapted Feeding Strategy of Their (Grand)Parents). Ph.D. Thesis, KU Leuven Bio-Ingenieurswetenschappen, Leuven, Belgium, 2016; 134p. [Google Scholar]
- Lesuisse, J.; Li, C.; Schallier, S.; Leblois, J.; Everaert, N.; Buyse, J. Feeding broiler breeders a reduced balanced protein diet during the rearing and laying period impairs reproductive performance but enhances broiler offspring performance. Poult. Sci. 2017, 96, 3949–3959. [Google Scholar] [CrossRef] [PubMed]
- Li, C. Transgenerational Effects of Controlled Feed Allocation and Reduced Balanced Protein Diet on the Welfare, Cognition and Behaviour of Broiler Breeders. Ph.D. Thesis, KU Leuven Bio-Ingenieurswetenschappen, Leuven, Belgium, 2018; 155p. [Google Scholar]
- Cherrie, G.; Avendaño, S.; Dunn, I.C.; D’Eath, R.B.; Dixon, L. The effect of different feeding strategies on female broiler breeder satiety. In Proceedings of the 52nd Congress of the International Society for Applied Ethology, Bristol, UK, 30 July–3 August 2018. 1p. [Google Scholar]
- Van Emous, R.A. Effects of feeding strategies during lay on broiler breeder production performance, eggshell quality, incubation traits, and behavior. Poult. Sci. 2023, 102, 102630. [Google Scholar] [CrossRef]
- Jones, H.E. A Study of the Scope for the Application of Research in Animal Genomics and Breeding to Reduce Nitrogen and Methane Emissions from Livestock Based Food Chains; Defra Project Report AC204; Department of Food, Agriculture and Rural Affairs (Defra), United Kingdom Government: London, UK, 2008; 22p.
- Burnside, T.A.; Ralph, J.H. Updates on Welfare and Sustainability for the European Turkey Industry. In Proceedings of the 15th Turkey Science and Production Conference, Chester, UK, 22–24 March 2023; pp. 44–51. [Google Scholar]
- British United Turkeys (B.U.T.). Big6 Commercial Performance Objectives; British United Turkeys: Broughton, UK, 1993. [Google Scholar]
- British United Turkeys (B.U.T.). Big6 Commercial Performance Objectives; British United Turkeys: Broughton, UK, 2002. [Google Scholar]
- Aviagen Turkeys. B.U.T. 6 Commercial Performance Objectives; Aviagen Turkeys: Tattenhall, UK, 2012. [Google Scholar]
- Aviagen Turkeys. B.U.T. 6 Commercial Performance Objectives; Aviagen Turkeys: Tattenhall, UK, 2020; Available online: https://www.aviagenturkeys.com/uploads/2022/03/16/POCLLB6_V2_BUT%206-Commercial%20Goals_UK_2022.pdf (accessed on 14 July 2023).
- Aviagen. Ross 308 Broiler Performance Objectives; Aviagen: Newbridge, UK, 1972. [Google Scholar]
- Aviagen. Ross 308 Broiler Performance Objectives; Aviagen: Newbridge, UK, 1994. [Google Scholar]
- Aviagen. Ross 308 Broiler Performance Objectives; Aviagen: Newbridge, UK, 2003. [Google Scholar]
- Aviagen. Ross 308 Broiler Performance Objectives; Aviagen: Newbridge, UK, 2022; Available online: https://aviagen.com/assets/Tech_Center/Ross_Broiler/RossxRoss308-BroilerPerformanceObjectives2022-EN.pdf (accessed on 14 July 2023).
- Bailey, R.A.; Watson, K.A.; Bilgili, S.F.; Avendaño, S. The genetic basis of pectoralis major myopathies in modern broiler chicken lines. Poult. Sci. 2015, 94, 2870–2879. [Google Scholar] [CrossRef] [PubMed]
- Siller, W.G.; Wight, P.A.L. The pathology of deep pectoral myopathy of turkeys. Avian Pathol. 1978, 7, 583–617. [Google Scholar] [CrossRef]
- Richardson, J.A.; Burgener, J.; Winterfield, R.W.; Dhillon, A.S. Deep pectoral myopathy in seven-week-old broiler chickens. Avian Dis. 1980, 24, 1054–1059. [Google Scholar] [CrossRef]
- Siller, W.G.; Wight, P.A.L.; Martindale, L. Exercise-induced deep pectoral myopathy in broiler fowls and turkeys. Vet. Sci. Commun. 1978, 2, 331–336. [Google Scholar] [CrossRef]
- Barbut, S. Recent myopathies in broiler’s breast meat fillets. World’s Poult. Sci. J. 2019, 75, 559–582. [Google Scholar] [CrossRef]
- Bailey, R.A.; Souza, E.; Avendaño, S. Characterising the Influence of Genetics on Breast Muscle Myopathies in Broiler Chickens. Front. Physiol. 2020, 11, 1041. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.A. Strategies and opportunities to control breast myopathies: An opinion paper. Front. Physiol. 2023, 14, 1173564. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, S.; Watson, K.A.; Kranis, A. Genomics in poultry breeding: From Utopia to deliverables. In Proceedings of the 9th World Congress on Genetics Applied to Livestock Production, Leipzig, Germany, 1–6 August 2010. Session 07-01. [Google Scholar]
- Avendaño, S.; Watson, K.A.; Kranis, A. Genomics in poultry breeding—Into consolidation phases. In Proceedings of the 24th World’s Poultry Congress, Salvador, Bahia, Brazil, 5–9 August 2012. [Google Scholar]
- Kranis, A.; Glover, P.; Ralph, J.; Avendano, S.; Watson, K.A. Genomics selection in Turkeys, current position and future potential. In Proceedings of the 10th Turkey Science and Production Conference, Chester, UK, 9–11 March 2016; pp. 23–25. [Google Scholar]
- Wolc, A.; Kranis, A.; Arango, J.; Settar, P.; Fulton, J.E.; O’Sullivan, N.; Avendaño, S.; Watson, K.A.; Hickey, J.M.; de los Campos, G.; et al. Implementation of Genomic Selection in the Poultry Industry. Anim. Front. 2016, 6, 23–31. [Google Scholar] [CrossRef]
- Avendaño, S.; Ralph, J. Economic challenges facing a global breeding company. In Proceedings of the 11th World Congress of Genetics in Livestock Production, Auckland, New Zealand, 11–16 February 2017. [Google Scholar]
- Ros-Freixedes, R.; Johnsson, M.; Whalen, A.; Chen, C.-Y.; Valente, B.D.; Herring, W.O.; Gorjanc, G.; Hickey, J.M. Genomic prediction with whole-genome sequence data in intensely selected pig lines. Gen. Sel. Evol. 2022, 54, 65. [Google Scholar] [CrossRef] [PubMed]
- Functional Annotation of Animal Genomes (FAANG). Available online: www.faang.org (accessed on 10 July 2023).
- Clark, E.L.; Archibald, A.L.; Daetwyler, H.D.; Groenen, M.A.M.; Harrison, P.W.; Houston, R.D.; Kühn, C.; Lien, S.; Macqueen, D.J.; Reecy, J.M.; et al. From FAANG to fork: Application of highly annotated genomes to improve farmed animal production. Genome Biol. 2020, 21, 285. [Google Scholar] [CrossRef]
- Genotype-Tissue Expression (GTEx). Available online: Gtexportal.org/home/ (accessed on 10 July 2023).
- The GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020, 369, 318–1330. [Google Scholar]
- Cisek, A.A.; Binek, M. Chicken intestinal microbiota function with a special emphasis on the role of probiotic bacteria. Pol. J. Vet. Sci. 2014, 17, 385–394. [Google Scholar] [CrossRef]
- Hooper, L.V.; Gordon, J.I. Commensal host-bacterial relationships in the gut. Science 2001, 292, 1115–1118. [Google Scholar] [CrossRef]
- Rodrigues, D.R.; Winson, E.; Wilson, K.M.; Briggs, W.N.; Duff, A.F.; Chasser, K.M.; Bielke, L.R. Intestinal Pioneer Colonizers as Drivers of Ileal Microbial Composition and Diversity of Broiler Chickens. Front. Microbiol. 2020, 10, 482842. [Google Scholar] [CrossRef]
- Guevarra, R.B.; Lee, J.H.; Lee, S.H.; Seok, M.-J.; Kim, D.W.; Na Kang, B.; Johnson, T.J.; Isaacson, R.E.; Kim, H.B. Piglet gut microbial shifts early in life: Causes and effects. J. Anim. Sci. Biotechnol. 2019, 10, 1. [Google Scholar] [CrossRef]
- Bindari, Y.R.; Gerber, P.F. Centennial Review: Factors affecting the chicken gastrointestinal microbial composition and their association with gut health and productive performance. Poult. Sci. 2022, 101, 101612. [Google Scholar] [CrossRef]
- Mulder, I.E.; Schmidt, B.; Stokes, C.R.; Lewis, M.; Bailey, M.; Aminov, R.I.; Prosser, J.I.; Gill, B.P.; Pluske, J.R.; Mayer, C.-D.; et al. Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces. BMC Biol. 2009, 7, 79. [Google Scholar] [CrossRef]
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.J.; Hermes, G.D.A.; Stegeman, J.A.; Smidt, H. Host and Environmental Factors Affecting the Intestinal Microbiota in Chickens. Front. Microbiol. 2018, 9, 235. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Bai, L.; Qu, Q.; Zhou, S.; Yang, M.; Guo, S.; Li, Q.; Liu, C. Impact of gut microbiota structure in heat-stressed broilers. Poult. Sci. 2019, 98, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.K.; Kelly, S.A.; Legge, R.; Ma, F.; Low, S.J.; Kim, J.; Zhang, M.; Oh, P.L.; Nehrenberg, D.; Hua, K.; et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc. Natl. Acad. Sci. USA 2010, 107, 18933–18938. [Google Scholar] [CrossRef]
- Singh, K.M.; Shah, T.M.; Reddy, B.; Deshpande, S.; Rank, D.N.; Joshi, C.G. Taxonomic and gene-centric metagenomics of the fecal microbiome of low and high feed conversion ratio (FCR) broilers. J. Appl. Genet. 2014, 55, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Dittoe, D.K.; Olson, E.G.; Ricke, S.C. Impact of the gastrointestinal microbiome and fermentation metabolites on broiler performance. Poult. Sci. 2022, 101, 101786. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Stewart, S.N.; Robinson, K.; Yang, Q.; Lyu, W.; Whitmore, M.A.; Zhang, G. Linkage between the intestinal microbiota and residual feed intake in broiler chickens. J. Anim. Sci. Biotechnol. 2021, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Difford, G.F.; Plichta, D.R.; Løvendahl, P.; Lassen, J.; Noel, S.J.; Højberg, O.; Wright, A.-D.G.; Zhu, Z.; Kristensen, L.; Nielsen, H.B.; et al. Host genetics and the rumen microbiome jointly associate with methane emissions in dairy cows. PLoS Genet. 2018, 14, 10. [Google Scholar] [CrossRef]
- Camarinha-Silva, A.; Maushammer, M.; Wellmann, R.; Vital, M.; Preuss, S.; Bennewitz, J. Host Genome Influence on Gut Microbial Composition and Microbial Prediction of Complex Traits in Pigs. Genetics 2017, 206, 1637–1644. [Google Scholar] [CrossRef]
- Zhou, Q.; Lan, F.; Gu, S.; Li, G.; Wu, G.; Yan, Y.; Li, X.; Jin, J.; Wen, C.; Sun, C.; et al. Genetic and microbiome analysis of feed efficiency in laying hens. Poult. Sci. 2023, 102, 102393. [Google Scholar] [CrossRef] [PubMed]
- Weishaar, R.; Wellmann, R.; Camarinha-Silva, A.; Rodehutscord, M.; Bennewitz, J. Selecting the hologenome to breed for an improved feed efficiency in pigs—A novel selection index. J. Anim. Breed. Genet. 2020, 137, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, S.; Neeteson, A.; Fancher, B. Broiler Breeding for Sustainability and Welfare—Are there Trade Offs? In Proceedings of the Poultry Beyond 2023, 6th International Broiler Nutritionists’ Conference, Queenstown, New Zealand, 16–20 October 2017. 17p. [Google Scholar]
- Chambers, J.R. Genetics of growth and meat production in chickens. In Poultry Breeding and Genetics; Crawford, R.D., Ed.; Elsevier Science Publishing Co.: New York, NY, USA, 1990; pp. 599–643. [Google Scholar]
- Nieuwenhoven, A.-M.N.V.; Avendaño, S.; Ralph, J.; Burnside, T. Improving welfare and sustainability of poultry meat production. In Proceedings of the International Poultry Meat Congress, Antalya, Turkey, 1–6 March 2023; Ceylan, N., Ed.; European Association for Animal Production: Rome, Italy 9p. [Google Scholar]
- Whitton, C.; Bogueva, D.; Marinova, D.; Phillips, C.J.C. Are We Approaching Peak Meat Consumption? Analysis of Meat Consumption from 2000 to 2019 in 35 Countries and Its Relationship to Gross Domestic Product. Animals 2021, 11, 3466. [Google Scholar] [CrossRef]
- Hockenhull Turkeys. Available online: http://www.hockenhullturkeys.co.uk/index.html (accessed on 4 June 2023).
- Better Chicken Commitment. Available online: www.betterchickencommitment.com (accessed on 4 July 2023).
- Autoriteit Consument en Markt (ACM). Analyse ACM van Duurzaamheidsafspraken ‘De Kip van Morgen’. 2015. Available online: https://www.acm.nl/sites/default/files/old_publication/publicaties/13758_analyse-acm-kip-van-duurzaamheidsafspraken-kip-van-morgen-2015-01-26.pdf (accessed on 4 June 2023).
- Dierenbescherming Beter Leven Keurmerk/Over de Dieren/Alle Dieren/Vleeskuikens. Available online: https://beterleven.dierenbescherming.nl/over-de-dieren/alle-dieren/vleeskuikens/ (accessed on 4 June 2023).
- Royal Society for the Prevention of Cruelty to Animals (RSPCA). RSPCA Assured/Farm Animal Welfare/Chickens. 2023. Available online: https://www.rspcaassured.org.uk/farm-animal-welfare/chickens/ (accessed on 4 June 2023).
- Red Tractor Our Poultry Standards. Available online: https://redtractor.org.uk/our-standards/poultry/ (accessed on 4 June 2023).
- Deutscher Tierschutzbund Tierschutzlabel “Für Mehr Tierschutz”. Available online: https://www.tierschutzlabel.info/ (accessed on 4 June 2023).
- Williams, A.G.; Audsley, E.; Sandars, D.L. Determining the Environmental Burdens and Resource Use in the Production of Agricultural and Horticultural Commodities; Main Report; Defra Research Project IS0205; Cranfield University: Bedford, UK, 2006; 22p, Available online: www.silsoe.cranfield.ac.uk (accessed on 11 June 2023).
- Leinonen, I.; Williams, A.G.; Wiseman, J.; Guy, J.; Kyriazakis, I. Predicting the Environmental Impacts of Chicken Systems in the United Kingdom Through a Life Cycle Assessment: Broiler Production Systems. Poult. Sci. 2012, 91, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Havlík, P.; Valin, H.; Notenbaert, A.; Rufino, M.C.; Thornton, P.K.; Blümmel, M.; Weiss, F.; Grace, D.; Obersteiner, M. Biomass Use, Production, Feed Efficiencies, and Greenhouse Gas Emissions from Global Livestock Systems. Proc. Natl. Acad. Sci. USA 2013, 110, 20888–20893. [Google Scholar] [CrossRef]
- Kremer, V.D.; Glover, P.K.; Kapell, D.N.R.G. Selection for Sustainable Resource Utilisation in Turkeys. In Proceedings of the 8th Turkey Science and Production Conference, Chester, UK, 27–28 March 2014; pp. 70–73. [Google Scholar]
- Ralph, J.H. Fit for Purpose, Balanced Breeding for Environmental Sustainability. In Proceedings of the 14th Turkey Science and Production Conference, Chester, UK, 4–6 March 2020; pp. 23–26. [Google Scholar]
- Leinonen, I.; Williams, A.G.; Kyriazakis, I. Comparing UK Turkey Production Systems Using Analytical Error Propagation in Uncertainty Analysis. In Proceedings of the 9th International Conference LCA of Food, San Francisco, CA, USA, 8–10 October 2014; Schenck, R., Huizenga, D., Eds.; ACLCA: Vashon, WA, USA, 2014. 9p. [Google Scholar]
- Leinonen, I.; Williams, A.G.; Nixey, C.; Kyriazakis, I. Assessment of the Environmental Impact of UK Turkey Production Systems. In Proceedings of the 8th Turkey Science and Production Conference, Chester, UK, 27–28 March 2014; pp. 74–77. [Google Scholar]
- Leinonen, I.; Williams, A.G.; Kyriazakis, I. Comparing the Environmental Impacts of UK Turkey Production Systems Using Analytical Error Propagation in Uncertainty Analysis. J. Clean. Prod. 2016, 112, 141–148. [Google Scholar] [CrossRef]
- De Jong, I. AF-17023 Greenwell (BO-63-001-018, BO-47-001-033). Project Description. Available online: https://research.wur.nl/en/projects/af-17023-greenwell-bo-63-001-018-bo-47-001-033 (accessed on 28 July 2023).
- Bos, B.; van Kernebeek, H.; Mostert, P.; van Harn, J.; van Horne, P.; de Jong, I. Welfare, Environmental Impact and Economy of Broiler Chicken Production. An Overview of the Lessons Learned from the Greenwell Project. 2023. 12p. Available online: https://edepot.wur.nl/629412 (accessed on 28 July 2023).
- De Jong, I.C.; te Beest, D. Development of the Greenwell Welfare Assessment Model: 2. Data for all Production Stages and Calculation of Scores for the Broiler On-Farm Stage; Report 1260; Wageningen Livestock Research: Wageningen, The Netherlands, 2020; 84p, Available online: https://edepot.wur.nl/524330 (accessed on 28 July 2023).
- De Jong, I.C.; Bos, B.; Van Harn, J.; Mostert, P.; Te Beest, D. Differences and Variation in Welfare Performance of Broiler Flocks in Three Production Systems. Poult. Sci. 2022, 101, 101933. [Google Scholar] [CrossRef]
- International Poultry Welfare Alliance (IPWA). Key Welfare Indicators (KWIs) Reference Guide. Broiler Chickens. Available online: https://poultrywelfare.org/files/IPWA%20Broiler%20Chickens%20Guide%20V13.pdf (accessed on 4 June 2023).
- International Poultry Welfare Alliance (IPWA). Key Welfare Indicators (KWIs) Reference Guide. Turkeys. Available online: https://poultrywelfare.org/files/IPWA%20Turkey%20Guide%20V8.pdf (accessed on 4 June 2023).
- OECD/FAO. OECD-FAO Agricultural Outlook 2022–2031; OECD Publishing: Paris, France, 2022; pp. 189–206. [Google Scholar] [CrossRef]
- Stanton, A.V.; Leroy, F.; Elliott, C.; Mann, N.; Wall, P.; De Smet, S. 36-fold higher estimate of deaths attributable to red meat intake in GBD 2019: Is this reliable? Lancet 2022, 399, e23–e26. [Google Scholar] [CrossRef]
- GBD 2019 Diseases and Injuries Collaborators. Global Burden of Disease Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Clapp, J.; Anderson, M.; Rahmanian, M.; Suárez, S.M. An IPCC for Food? How the UN Food Systems Summit Is Being Used to Advance a Problematic New Science-Policy Agenda; iPES Food Briefing Note 1 on the governance of food systems; International Panel of Experts on Sustainable Food Systems: Brussels, Belgium, 2021; 19p, Available online: https://www.ipes-food.org/_img/upload/files/GovBrief.pdf (accessed on 20 July 2023).
- Leroy, F.; Smith, N.W.; Adesogan, A.T.; Beal, T.; Iannotti, L.; Moughan, P.J.; Mann, N. The role of meat in the human diet: Evoluationary aspects and nutritional value. Anim. Front. 2023, 13, 11–18. [Google Scholar] [CrossRef] [PubMed]























| Crossbred | ADG | Days to 2.5kg | FCR 2.5kg | % Evis | % Breast | % Liveability |
|---|---|---|---|---|---|---|
| Ross 308 | 66.3 | 37.7 | 1.53 | 73.4 | 25.3 | 96.5 |
| Ross 708 | 64.1 | 39.0 | 1.53 | 74.5 | 27.0 | 97.0 |
| Rustic Classic | 57.0 | 43.9 | 1.68 | 72.7 | 24.4 | 97.5 |
| Rustic Gold | 52.0 | 48.1 | 1.75 | 71.9 | 23.2 | 98.0 |
| Ranger Classic | 48.7 | 51.3 | 1.80 | 71.3 | 22.3 | 98.1 |
| Ranger Gold | 44.0 | 56.8 | 1.87 | 70.5 | 20.9 | 98.3 |
| Rambler Ranger | 33.0 | 76.0 | 2.15 | 69.3 | 18.1 | 98.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neeteson, A.-M.; Avendaño, S.; Koerhuis, A.; Duggan, B.; Souza, E.; Mason, J.; Ralph, J.; Rohlf, P.; Burnside, T.; Kranis, A.; et al. Evolutions in Commercial Meat Poultry Breeding. Animals 2023, 13, 3150. https://doi.org/10.3390/ani13193150
Neeteson A-M, Avendaño S, Koerhuis A, Duggan B, Souza E, Mason J, Ralph J, Rohlf P, Burnside T, Kranis A, et al. Evolutions in Commercial Meat Poultry Breeding. Animals. 2023; 13(19):3150. https://doi.org/10.3390/ani13193150
Chicago/Turabian StyleNeeteson, Anne-Marie, Santiago Avendaño, Alfons Koerhuis, Brendan Duggan, Eduardo Souza, James Mason, John Ralph, Paige Rohlf, Tim Burnside, Andreas Kranis, and et al. 2023. "Evolutions in Commercial Meat Poultry Breeding" Animals 13, no. 19: 3150. https://doi.org/10.3390/ani13193150
APA StyleNeeteson, A.-M., Avendaño, S., Koerhuis, A., Duggan, B., Souza, E., Mason, J., Ralph, J., Rohlf, P., Burnside, T., Kranis, A., & Bailey, R. (2023). Evolutions in Commercial Meat Poultry Breeding. Animals, 13(19), 3150. https://doi.org/10.3390/ani13193150








