Etiopathogenesis of Canine Cruciate Ligament Disease: A Scoping Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
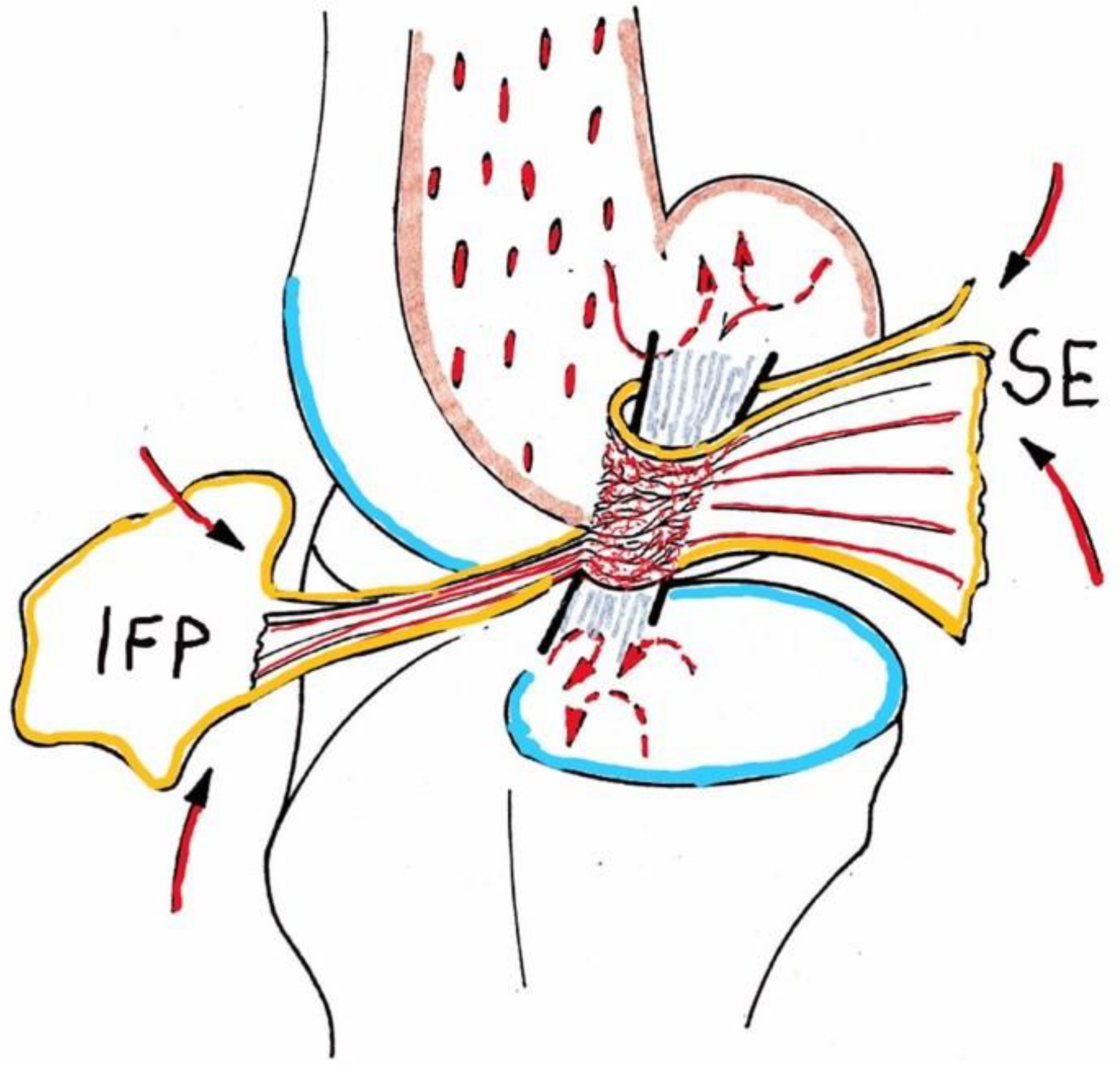
3.1. Cruciate Ligament Anatomy, Physiology, Biomechanical Features
3.2. Risk Factors: Breed, Sex, Neuter Status, Weight, Age, Activity
3.3. Genetics
3.4. Biomechanics/Joint Functional Anatomy/Orthopedic Conformation
3.5. Osteoarthritic Changes
3.5.1. Inflammation, Cytokines, Immune Mediation, Apoptosis
3.5.2. Synovial Membrane, Matrix Collagen, Ligaments, Menisci
3.5.3. Systemic Factors
3.5.4. Late-Stage Osteoarthritis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilke, V.L.; Robinson, D.A.; Evans, R.B.; Rothschild, M.F.; Conzemius, M.G. Estimate of the Annual Economic Impact of Treatment of Cranial Cruciate Ligament Injury in Dogs in the United States. J. Am. Vet. Med. Assoc. 2005, 227, 1604–1607. [Google Scholar] [CrossRef] [PubMed]
- Witsberger, T.H.; Villamil, J.A.; Schultz, L.G.; Hahn, A.W.; Cook, J.L. Prevalence of and Risk Factors for Hip Dysplasia and Cranial Cruciate Ligament Deficiency in Dogs. J. Am. Vet. Med. Assoc. 2008, 232, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Scavelli, T.D.; Schrader, S.C.; Matthiesen, D.T.; Skorup, D.E. Partial Rupture of the Cranial Cruciate Ligament of the Stifle in Dogs: 25 Cases (1982–1988). J. Am. Vet. Med. Assoc. 1990, 196, 1135–1138. [Google Scholar]
- Little, J.P.; Bleedorn, J.A.; Sutherland, B.J.; Sullivan, R.; Kalscheur, V.L.; Ramaker, M.A.; Schaefer, S.L.; Hao, Z.; Muir, P. Arthroscopic Assessment of Stifle Synovitis in Dogs with Cranial Cruciate Ligament Rupture. PLoS ONE 2014, 9, e97329. [Google Scholar] [CrossRef]
- Bleedorn, J.A.; Greuel, E.N.; Manley, P.A.; Schaefer, S.L.; Markel, M.D.; Holzman, G.; Muir, P. Synovitis in Dogs with Stable Stifle Joints and Incipient Cranial Cruciate Ligament Rupture: A Cross-Sectional Study. Vet. Surg. 2011, 40, 531–543. [Google Scholar] [CrossRef]
- Hayashi, K.; Frank, J.D.; Dubinsky, C.; Zhengling, H.; Markel, M.D.; Manley, P.A.; Muir, P. Histologic Changes in Ruptured Canine Cranial Cruciate Ligament. Vet. Surg. 2003, 32, 269–277. [Google Scholar] [CrossRef]
- Hayashi, K.; Manley, P.A.; Muir, P. Cranial Cruciate Ligament Pathophysiology in Dogs with Cruciate: A Review. J. Am. Anim. Hosp. Assoc. 2004, 40, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Rayward, R.M.; Thomson, D.G.; Davies, J.V.; Innes, J.F.; Whitelock, R.G. Progression of Osteoarthritis Following TPLO Surgery: A Radiographic Study of 40 Dogs. J. Small Anim. Pract. 2004, 45, 92–97. [Google Scholar] [CrossRef]
- Mölsä, S.H.; Hyytiäinen, H.K.; Hielm-Björkman, A.K.; Laitinen-Vapaavuori, O.M. Long-Term Functional Outcome after Surgical Repair of Cranial Cruciate Ligament Disease in Dogs. BMC Vet. Res. 2014, 10, 266. [Google Scholar] [CrossRef] [Green Version]
- Conzemius, M.G.; Evans, R.B.; Besancon, M.F.; Gordon, W.J.; Horstman, C.L.; Hoefle, W.D.; Nieves, M.A.; Wagner, S.D. Effect of Surgical Technique on Limb Function after Surgery for Rupture of the Cranial Cruciate Ligament in Dogs. J. Am. Vet. Med. Assoc. 2005, 226, 232–236. [Google Scholar] [CrossRef]
- Voss, K.; Damur, D.M.; Guerrero, T.; Haessig, M.; Montavon, P.M. Force Plate Gait Analysis to Assess Limb Function after Tibial Tuberosity Advancement in Dogs with Cranial Cruciate Ligament Disease. Vet. Comp. Orthop. Traumatol. 2008, 21, 243–249. [Google Scholar] [PubMed]
- Morgan, J.P.; Voss, K.; Damur, D.M.; Guerrero, T.; Haessig, M.; Montavon, P.M. Correlation of Radiographic Changes after Tibial Tuberosity Advancement in Dogs with Cranial Cruciate-Deficient Stifles with Functional Outcome. Vet. Surg. 2010, 39, 425–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paatsama, S. Ligament Injuries in the Canine Stifle Joint—A Clinical and Experimental Study; Kauppakirsapaino: Helsinki, Finland, 1952. [Google Scholar]
- Cook, J.L. Cranial Cruciate Ligament Disease in Dogs: Biology Versus. Vet. Surg. 2010, 39, 270–277. [Google Scholar] [CrossRef]
- Muir, P.; Schwartz, Z.; Malek, S.; Kreines, A.; Cabrera, S.Y.; Buote, N.J.; Bleedorn, J.A.; Schaefer, S.L.; Holzman, G.; Hao, Z. Contralateral Cruciate Survival in Dogs with Unilateral-Contact Cranial Cruciate Ligament Rupture. PLoS ONE 2011, 6, e25331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, C.; Ramaker, M.A.; Kaur, S.; Csomos, R.A.; Kroner, K.T.; Bleedorn, J.A.; Schaefer, S.L.; Muir, P. Radiographic Risk Factors for Contralateral Rupture in Dogs with unilateral Cranial Cruciate Ligament Rupture. PLoS ONE 2014, 9, e106389. [Google Scholar] [CrossRef]
- Fuller, M.C.; Hayashi, K.; Bruecker, K.A.; Holsworth, I.G.; Sutton, J.S.; Kass, P.H.; Kantrowitz, B.J.; Kapatkin, A.S. Evaluation of the Radiographic Infrapatellar Fat Pad Sign of the contralateral Stifle Joint as a Risk Factor for Subsequent Cranial Cruciate Ligament Rupture in Dogs with unilateral Rupture: 96 Cases (2006–2007). J. Am. Vet. Med. Assoc. 2014, 244, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Boden, B.P.; Sheehan, F.T.; Torg, J.S.; Hewett, T.E. Noncontact Anterior Cruciate Ligament Injuries: Mechanisms and Risk Factors. Am. Acad. Orthop. Surg. 2010, 18, 520–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binversie, E.E.; Walczak, B.E.; Cone, S.G.; Baker, L.A.; Scerpella, T.A.; Muir, P. Canine ACL Rupture: A Spontaneous Large Animal Model of Human ACL Rupture. BMC Musculoskelet Disord. 2022, 23, 116. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.M.; Johnson, A.L. Cranial Cruciate Ligament Rupture. Pathogenesis, Diagnosis, and postoperative Rehabilitation. Vet. Clin. N. Am. Small. Anim. Pract. 1993, 23, 717–733. [Google Scholar] [CrossRef]
- Griffon, D.J. A Review of the Pathogenesis of Canine Cranial Cruciate Ligament as a Basis for Future Preventive Strategies. Vet. Surg. 2010, 39, 399–409. [Google Scholar] [CrossRef]
- Todorović, A.Z.; Macanović, M.V.L.; Mitrović, M.B.; Krstić, N.E.; van Bree, H.J.J.; Gielen, I.M.L. The Role of Tibial Plateau Angle in Canine Cruciate Ligament Rupture—A Review of the Literature. Vet. Comp. Orthop. Traumatol. 2022, 35, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Spinella, G.; Arcamone, G.; Valentini, S. Cranial Cruciate Ligament Rupture in Dogs: Review on Biomechanics, Etiopathogenetic Factors and Rehabilitation. Vet. Sci. 2021, 8, 186. [Google Scholar] [CrossRef]
- Brioschi, V.; Arthurs, G.I. Cranial Cruciate Ligament Rupture in Small Dogs (<15 kg): A Narrative Literature Review. J. Small Anim. Pract. 2021, 62, 1037–1050. [Google Scholar] [CrossRef]
- Comerford, E.J.; Smith, K.; Hayashi, K. Update on the Aetiopathogenesis of Canine Cranial Cruciate Ligament Disease. Vet. Comp. Orthop. Traumatol. 2011, 24, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Arnoczky, S.P. Anatomy of the Anterior Cruciate Ligament. Clin. Orthop. Relat. Res. 1983, 14, 19–25. [Google Scholar] [CrossRef]
- Vasseur, P.B.; Arnoczky, S.P. Collateral Ligaments of the Canine Stifle Joint: Anatomic and Functional Analysis. Am. J. Vet. Res. 1981, 42, 1133–1137. [Google Scholar]
- Arnoczky, S.P.; Marshall, J.L. The Cruciate Ligaments of the Canine Stifle: An Anatomical and Functional Analysis. Am. J. Vet. Res. 1977, 38, 1807–1814. [Google Scholar] [PubMed]
- Dodds, J.A.; Arnoczky, S.P. Anatomy of the Anterior Cruciate Ligament: A Blueprint for Repair and Reconstruction. Arthroscopy 1994, 10, 132–139. [Google Scholar] [CrossRef]
- Vasseur, P.B.; Pool, R.R.; Arnoczky, S.P.; Lau, R.E. Correlative Biomechanical and Histologic Study of the Cranial Cruciate Ligament in Dogs. Am. J. Vet. Res. 1985, 46, 1842–1854. [Google Scholar] [PubMed]
- de Rooster, H.; de Bruin, T.; van Bree, H. Morphologic and Functional Features of the Canine Cruciate Ligaments. Vet. Surg. 2006, 35, 769–780. [Google Scholar] [CrossRef]
- Arnoczky, S.P. Blood Supply to the Anterior Cruciate Ligament and Supporting Structures. Orthop. Clin. N. Am. 1985, 16, 15–28. [Google Scholar] [CrossRef]
- Kobayashi, S.; Baba, H.; Uchida, K.; Negoro, K.; Sato, M.; Miyazaki, T.; Nomura, E.; Murakami, K.; Shimizubata, M.; Meir, A. Microvascular System of Anterior Cruciate Ligament in Dogs. J. Orthop. Res. 2006, 24, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.L.; Arnoczky, S.P.; Rubin, R.M.; Wickiewicz, T.L. Microvasculature of the Cruciate Ligaments. Phys. Sportsmed. 1979, 7, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Zahm, H. Die Ligament Decussata Im Gesunden Und Arterotischen Kniegelenk Des Hundes. Kleintierpraxis 1965, 10, 38–47. [Google Scholar]
- Kuroki, K.; Williams, N.; Ikeda, H.; Bozynski, C.C.; Leary, E.; Cook, J.L. Histologic Assessment of Ligament Vascularity and Synovitis in Dogs with Cranial Cruciate Ligament Disease. Am. J. Vet. Res. 2019, 80, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Bhandal, J.; Rodriguez, C.O.J.; Kim, S.Y.; Entwistle, R.; Naydan, D.; Kapatkin, A.; Stover, S.M. Vascular Distribution in Ruptured Canine Cranial Cruciate Ligament. Vet. Surg. 2011, 40, 198–203. [Google Scholar] [CrossRef]
- Slocum, B.; Devine, T. Cranial Tibial Thrust: A Primary Force in the Canine Stifle. J. Am. Vet. Med. Assoc. 1983, 183, 456–459. [Google Scholar] [PubMed]
- Slocum, B.; Slocum, T.D. Tibial Plateau Leveling Osteotomy for Repair of Cranial Cruciate Ligament Rupture in the Canine. Vet. Clin. N. Am. Small Anim. Pract. 1993, 23, 777–795. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Hans, E.C. Tibial Plateau Leveling Osteotomy for Cranial Cruciate Ligament Rupture in Canines: Patient Selection and Reported Outcomes. Vet. Med. Res. Rep. 2019, 10, 249–255. [Google Scholar] [CrossRef]
- Mazdarani, P.; Nielsen, M.B.M.; Gundersen, R.S.; von Wenck, A.; Miles, J.E. Geometric Modelling of CORA-Based Levelling Osteotomy in the Dog. Res. Vet. Sci. 2021, 135, 127–133. [Google Scholar] [CrossRef]
- Guénégo, L.; Vezzoni, A.; Vezzoni, L. Comparison of Tibial Anatomical-Mechanical Axis Angles and Patellar Positions between Tibial Plateau Levelling Osteotomy (TPLO) and Modified Cranial Closing Wedge Osteotomy (AMA-Based CCWO) for the Treatment of Cranial Cruciate Ligament Disease in Large Dogs with Tibial Plateau Slopes Greater than 30° and Clinically Normal Labradors Retrievers. BMC Vet. Res. 2021, 17, 368. [Google Scholar] [CrossRef]
- Montavon, P.M. Advancement of the Tibial Tuberosity for the Treatment of Cranial Cruciate DeficientCanine Stifle. In Proceedings of the 1st World Orthopaedic Veterinary Congress, Munich, Germany, 5–8 September 2002; p. 152. [Google Scholar]
- Aragosa, F.; Caterino, C.; della Valle, G.; Fatone, G. Tibial Tuberosity Advancement Techniques (TTAT): A Systematic Review. Animals 2022, 12, 2114. [Google Scholar] [CrossRef]
- Duerr, F.M.; Duncan, C.G.; Savicky, R.S.; Park, R.D.; Egger, E.L.; Palmer, R.H. Risk Factors for Excessive Tibial Plateau Angle in Large-Breed with Cranial Cruciate Ligament Disease. J. Am. Vet. Med. Assoc. 2007, 231, 1688–1691. [Google Scholar] [CrossRef]
- Inauen, R.; Koch, D.; Bass, M.; Haessig, M. Tibial Tuberosity Conformation as a Risk Factor for Cranial Cruciate Ligament Rupture in the Dog. Vet. Comp. Orthop. Traumatol. 2009, 22, 16–20. [Google Scholar] [PubMed]
- Duval, J.M.; Budsberg, S.C.; Flo, G.L.; Sammarco, J.L. Breed, Sex, and Body Weight as Risk Factors for Rupture of the Cranial Cruciate Ligament in Young Dogs. J. Am. Vet. Med. Assoc. 1999, 215, 811–814. [Google Scholar] [PubMed]
- de la Riva, G.; Hart, B.L.; Farver, T.B.; Oberbauer, A.M.; Messam, L.L.M.; Willits, N.; Hart, L.A. Neutering Dogs: Effects on Joint Disorders and Cancers in Golden. PLoS ONE 2013, 8, e55937. [Google Scholar] [CrossRef] [PubMed]
- Grierson, J.; Asher, L.; Grainger, K. An Investigation into Risk Factors for Bilateral Canine Cruciate Ligament Rupture. Vet. Comp. Orthop. Traumatol. 2011, 24, 192–196. [Google Scholar] [CrossRef]
- Ragetly, C.A.; Evans, R.; Mostafa, A.A.; Griffon, D.J. Multivariate Analysis of Morphometric Characteristics to Evaluate Factors for Cranial Cruciate Ligament Deficiency in Labrador. Vet. Surg. 2011, 40, 327–333. [Google Scholar] [CrossRef]
- Sellon, D.C.; Marcellin-Little, D.J. Risk Factors for Cranial Cruciate Ligament Rupture in Dogs in Canine Agility. BMC Vet. Res. 2022, 18, 39. [Google Scholar] [CrossRef]
- Alm, A.; Strömberg, B. Vascular Anatomy of the Patellar and Cruciate Ligaments. A Microangiographic and Histologic Investigation in the Dog. Acta Chir. Scand. Suppl. 1974, 445, 25–35. [Google Scholar]
- Adams, P.; Bolus, R.; Middleton, S.; Moores, A.P.; Grierson, J. Influence of Signalment on Developing Cranial Cruciate Rupture in Dogs in the UK. J. Small Anim. Pract. 2011, 52, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Whitehair, J.G.; Vasseur, P.B.; Willits, N.H. Epidemiology of Cranial Cruciate Ligament Rupture in Dogs. J. Am. Vet. Med. Assoc. 1993, 203, 1016–1019. [Google Scholar]
- Rudd Garces, G.; Arizmendi, A.; Barrientos, L.S.; Crespi, J.A.; Morales, H.; Peral García, P.; Padula, G.; Giovambattista, G. Epidemiology of Cranial Cruciate Ligament Rupture and Patellar in Dogs from the Province of Buenos Aires, Argentina. Vet. Comp. Orthop. Traumatol. 2021, 34, 24–31. [Google Scholar] [CrossRef]
- Hans, E.C.; Barnhart, M.D.; Kennedy, S.C.; Naber, S.J. Comparison of Complications Following Tibial Tuberosity Advancement and Tibial Plateau Levelling Osteotomy in Very Large and Giant Dogs 50 Kg or More in Body Weight. Vet. Comp. Orthop. Traumatol. 2017, 30, 299–305. [Google Scholar] [CrossRef]
- Buote, N.; Fusco, J.; Radasch, R. Age, Tibial Plateau Angle, Sex, and Weight as Risk Factors for Contralateral Rupture of the Cranial Cruciate Ligament in Labradors. Vet. Surg. 2009, 38, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Engdahl, K.; Emanuelson, U.; Höglund, O.; Bergström, A.; Hanson, J. The Epidemiology of Cruciate Ligament Rupture in an Insured Dog Population. Sci. Rep. 2021, 11, 9546. [Google Scholar] [CrossRef] [PubMed]
- Boge, G.S.; Moldal, E.R.; Dimopoulou, M.; Skjerve, E.; Bergström, A. Breed Susceptibility for Common Surgically Treated Orthopaedic in 12 Dog Breeds. Acta Vet. Scand. 2019, 61, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Li, Z.; Hayward, J.J.; Hayashi, K.; Krotscheck, U.; Todhunter, R.J.; Tang You and Huang, M. Genomic Prediction of Two Complex Orthopedic Traits Across Multiple Pure and Mixed Breed Dogs. Front. Genet. 2021, 12, 666740. [Google Scholar] [CrossRef]
- Bellumori, T.P.; Famula, T.R.; Bannasch Danika, L.; Belanger, J.M.; Oberbauer, A.M. Prevalence of Inherited Disorders among Mixed-Breed and Purebred: 27,254 Cases (1995–2010). J. Am. Vet. Med. Assoc. 2013, 242, 1549–1555. [Google Scholar] [CrossRef] [Green Version]
- Döring, A.-K.; Junginger, J.; Hewicker-Trautwein, M. Cruciate Ligament Degeneration and Stifle Joint Synovitis in 56 with Intact Cranial Cruciate Ligaments: Correlation of histological Findings and Numbers and Phenotypes of Inflammatory with Age, Body Weight and Breed. Vet. Immunol. Immunopathol. 2017, 196, 5–13. [Google Scholar] [CrossRef]
- Wilke, V.L.; Conzemius, M.G.; Kinghorn, B.P.; Macrossan, P.E.; Cai, W.; Rothschild, M.F. Inheritance of Rupture of the Cranial Cruciate Ligament In. J. Am. Vet. Med. Assoc. 2006, 228, 61–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baird, A.E.G.; Carter, S.D.; Innes, J.F.; Ollier, W.E.; Short, A.D. Genetic Basis of Cranial Cruciate Ligament Rupture (CCLR) In. Connect. Tissue Res. 2014, 55, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.R.; Conzemius, M.G.; McCue, M.E.; Ekenstedt, K.J. SNP-Based Heritability and Genetic Architecture of Cranial Ligament Rupture in Labrador Retrievers. Anim. Genet. 2020, 51, 824–828. [Google Scholar] [CrossRef]
- Temwichitr, J.; Hazewinkel, H.A.W.; van Hagen, M.A.; Leegwater, P.A.J. Polymorphic Microsatellite Markers for Genetic Analysis of collagen Genes in Suspected Collagenopathies in Dogs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2007, 54, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Allaith, S.; Tew, S.R.; Hughes, C.E.; Clegg, P.D.; Canty-Laird, E.G.; Comerford, E.J. Characterisation of Key Proteoglycans in the Cranial Cruciate (CCLs) from Two Dog Breeds with Different to CCL Disease and Rupture. Vet. J. 2021, 272, 105657. [Google Scholar] [CrossRef]
- Comerford, E.J.; Tarlton, J.F.; Wales, A.; Bailey, A.J.; Innes, J.F. Ultrastructural Differences in Cranial Cruciate Ligaments from Dogs of Two Breeds with a Differing Predisposition to Ligament Degeneration and Rupture. J. Comp. Pathol. 2006, 134, 8–16. [Google Scholar] [CrossRef]
- Macias, C.; Mckee, W.M.; May, C. Caudal Proximal Tibial Deformity and Cranial Cruciate Ligament in Small-Breed Dogs. J. Small Anim. Pract. 2002, 43, 433–438. [Google Scholar] [CrossRef]
- Clements, D.N.; Carter, S.D.; Innes, J.F.; Ollier, W.E.R.; Day, P.J.R. Gene Expression Profiling of Normal and Ruptured Canine Anterior Cruciate Ligaments. Osteoarthr. Cartil. 2008, 16, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Healey, E.; Murphy, R.J.; Hayward, J.J.; Castelhano, M.; Boyko, A.R.; Hayashi, K.; Krotscheck, U.; Todhunter, R.J. Genetic Mapping of Distal Femoral, Stifle, and Tibial Morphology in Dogs with Cranial Cruciate Ligament. PLoS ONE 2019, 14, e0223094. [Google Scholar] [CrossRef]
- Alm, A.; Ekström, H.; Strömberg, B. Tensile Strength of the Anterior Cruciate Ligament in the Dog. Acta Chir. Scand. Suppl. 1974, 445, 15–23. [Google Scholar]
- Ueda, H.; Matsukawa, T.; Watanabe, T.; Hosaka, Y.; Takehana, K. Morphological, Biochemical and Mechanical Features of the Cranial Cruciate and Lateral Collateral Ligaments in Dogs. Okajimas. Folia. Anat. Jpn. 2006, 83, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Arnoczky, S.P.; Rubin, R.M.; Marshall, J.L. Microvasculature of the Cruciate Ligaments and Its Response to Injury. An Experimental Study in Dogs. J. Bone Joint. Surg. Am. 1979, 61, 1221–1229. [Google Scholar] [CrossRef]
- Hayashi, K.; Frank, J.; Hao, Z.; Schamberger, G.M.; Markel, M.D.; Manley, P.A.; Muir, P. Evaluation of Ligament Fibroblast Viability in Ruptured Cranial Ligament of Dogs. Am. J. Vet. Res. 2003, 64, 1010–1016. [Google Scholar] [CrossRef]
- Menzel, E.J.; Niebauer, G.; Smolen, J.S. Demonstration of C 1 Q-Binding Immune Complexes in Dogs with arthritis of the Femoro-Tibial Joints Accompanied by Rupture of the Anterior Cruciate Ligaments. Zentralbl. Veterinarmed B 1980, 27, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Tirgari, M. Changes in the Canine Stifle Joint Following Rupture of the Anterior Cruciate Ligament. J. Small Anim. Pract. 1978, 19, 17–26. [Google Scholar] [CrossRef]
- Comerford, E.J.; Tarlton, J.F.; Innes, J.F.; Johnson, K.A.; Amis, A.A.; Bailey, A.J. Metabolism and Composition of the Canine Anterior Cruciate Ligament Relate to Differences in Knee Joint Mechanics and Predisposition to Ligament Rupture. J. Orthop. Res. 2005, 23, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Comerford, E.J.; Tarlton, J.F.; Avery, N.C.; Bailey, A.J.; Innes, J.F. Distal Femoral Intercondylar Notch Dimensions and Their Relationship to Composition and Metabolism of the Canine Anterior Cruciate Ligament. Osteoarthr. Cartil. 2006, 14, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Krier, E.M.; Johnson, T.A.; Breiteneicher, A.H.; Peycke, L.E.; Hulse, D.A. Articular Cartilage Lesions Associated with Complete Lateral Tears in the Dog. Vet. Surg. 2018, 47, 958–962. [Google Scholar] [CrossRef]
- Kaufman, K.; Beale, B.S.; Thames, H.D.; Saunders, W.B. Articular Cartilage Scores in Cranial Cruciate Ligament-Deficient with or without Bucket Handle Tears of the Medial Meniscus. Vet. Surg. 2016, 46, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Spreng, D.; Sigrist, N.; Jungi, T.; Busato, A.; Lang, J.; Pfister, H.; Schawalder, P. Nitric Oxide Metabolite Production in the Cranial Cruciate Ligament, Synovial Membrane, and Articular Cartilage of Dogs with Cranial Cruciate Ligament Rupture. Am. J. Vet. Res. 2000, 61, 530–536. [Google Scholar] [CrossRef]
- Klocke, N.W.; Snyder, P.W.; Widmer, W.R.; Zhong, W.; McCabe, G.P.; Breur, G.J. Detection of Synovial Macrophages in the Joint Capsule of dogs with Naturally Occurring Rupture of the Cranial Cruciate Ligament. Am. J. Vet. Res. 2005, 66, 493–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galloway, R.H.; Lester, S.J. Histopathological Evaluation of Canine Stifle Joint Synovial Collected at the Time of Repair of Cranial Cruciate Rupture. J. Am. Anim. Hosp. Assoc. 1995, 31, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Heffron, L.E.; Campbell, J.R. Morphology, Histology and Functional Anatomy of the Canine Cranial Cruciate Ligament. Vet. Rec. 1978, 102, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, C.; Amis, A.A.; Stead, A.C.; Law, H.T. Comparison of the Biomechanical Properties of Rottweiler and Racing Greyhound Cranial Cruciate Ligaments. J. Small Anim. Pract. 2000, 41, 303–307. [Google Scholar] [CrossRef]
- Jackson, J.; Vasseur, P.B.; Griffey, S.; Walls, C.M.; Kass, P.H. Pathologic Changes in Grossly Normal Menisci in Dogs with Rupture of the Cranial Cruciate Ligament. J. Am. Vet. Med. Assoc. 2001, 218, 1281–1284. [Google Scholar] [CrossRef]
- Franklin, S.P.; Gilley, R.S.; Palmer, R.H. Meniscal Injury in Dogs with Cranial Cruciate Ligament Rupture. Compend. Contin. Educ. Vet. 2010, 32, E1–E10. quiz E11. [Google Scholar]
- Hayes, G.M.; Langley-Hobbs, S.J.; Jeffery, N.D. Risk Factors for Medial Meniscal Injury in Association with Cranial Cruciate Ligament Rupture. J. Small Anim. Pract. 2010, 51, 630–634. [Google Scholar] [CrossRef]
- Dillon, D.E.; Gordon-Evans, W.J.; Griffon, D.J.; Knap, K.M.; Bubb, C.L.; Evans, R.B. Risk Factors and Diagnostic Accuracy of Clinical Findings for Meniscal Disease in Dogs with Cranial Cruciate Ligament Disease. Vet. Surg. 2014, 43, 446–450. [Google Scholar] [CrossRef]
- Guastella, D.B.; Fox, D.B.; Cook, J.L. Tibial Plateau Angle in Four Common Canine Breeds with Cranial Ligament Rupture, and Its Relationship to Meniscal Tears. Vet. Comp. Orthop. Traumatol. 2008, 21, 125–128. [Google Scholar]
- Plesman, R.; Gilbert, P.; Campbell, J. Detection of Meniscal Tears by Arthroscopy and Arthrotomy in Dogs with Cranial Cruciate Ligament Rupture: A Retrospective, Cohort Study. Vet. Comp. Orthop. Traumatol. 2013, 26, 42–46. [Google Scholar] [CrossRef]
- Laube, R.L.; Kerstetter, K.K. Prevalence and Risk Factors for Bilateral Meniscal Tears during Treatment for Cranial Cruciate Ligament disease via Tibial Plateau Levelling Osteotomy in Dogs. Vet. Comp. Orthop. Traumatol. 2021, 34, 37–42. [Google Scholar] [CrossRef]
- Olive, J.; d’Anjou, M.-A.; Cabassu, J.; Chailleux, N.; Blond, L. Fast Presurgical Magnetic Resonance Imaging of Meniscal Tears and Concurrent Subchondral Bone Marrow Lesions. Study of Dogs with Naturally Occurring Cranial Cruciate Ligament Rupture. Vet. Comp. Orthop. Traumatol. 2014, 27, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, C.A.; Horstman, C.L.; Mason, D.R.; Evans, R.B. Severity of Patellar Luxation and Frequency of Concomitant Cranial Cruciate Ligament Rupture in Dogs: 162 Cases (2004–2007). J. Am. Vet. Med. Assoc. 2010, 236, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, T.G.; Geyer, H.; Hässig, M.; Montavon, P.M. Effect of Conformation of the Distal Portion of the Femur and proximal Portion of the Tibia on the Pathogenesis of Cranial Ligament Disease in Dogs. Am. J. Vet. Res. 2007, 68, 1332–1337. [Google Scholar] [CrossRef]
- Johnson, K.A.; Hay, C.W.; Chu, Q.; Roe, S.C.; Caterson, B. Cartilage-Derived Biomarkers of Osteoarthritis in Synovial Fluid of Dogs with Naturally Acquired Rupture of the Cranial Cruciate Ligament. Am. J. Vet. Res. 2002, 63, 775–781. [Google Scholar] [CrossRef]
- Han, S.; Cheon, H.; Cho, H.; Kim, J.; Kang, J.H.; Yang, M.P.; Lee, Y.; Lee, H.; Chang, D. Evaluation of Partial Cranial Cruciate Ligament Rupture with Positive Contrast Computed Tomographic Arthrography in Dogs. J. Vet. Sci. 2008, 9, 395–400. [Google Scholar] [CrossRef] [Green Version]
- de Rooster, H.; van Bree, H. Use of Compression Stress Radiography for the Detection of Partial Tears of the Canine Cranial Cruciate Ligament. J. Small Anim. Pract. 1999, 40, 573–576. [Google Scholar] [CrossRef]
- Schwandt, C.S.; Bohorquez-Vanelli, A.; Tepic, S.; Hassig, M.; Dennler, R.; Vezzoni, A.; Montavon, P.M. Angle between the Patellar Ligament and Tibial Plateau in Dogs with Partial Rupture of the Cranial Cruciate Ligament. Am. J. Vet. Res. 2006, 67, 1855–1860. [Google Scholar] [CrossRef]
- Krayer, M.; Rytz, U.; Oevermann, A.; Doherr, M.G.; Forterre, F.; Zurbriggen, A.; Spreng, D.E. Apoptosis of Ligamentous Cells of the Cranial Cruciate Ligament from Stable Stifle Joints of Dogs with Partial Cranial Cruciate Ligament Rupture. Am. J. Vet. Res. 2008, 69, 625–630. [Google Scholar] [CrossRef]
- Skytte, D.; Schmökel, H.; Miles, J. Partial Rupture of the Cranial Cruciate Ligament Treated with Tibial Tuberosity Advancement without Debridement of the Remaining Ligament: A Clinical Study of 18 Cases. Schweiz. Arch. Tierheilkd. 2014, 156, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, P.; Brühschwein, A.; Winkels, P.; Werner, H.; Ludewig, E.; Grevel, V.; Oechtering, G. Value of Low-Field Magnetic Resonance Imaging in Diagnosing Meniscal Tears in the Canine Stifle: A Prospective Study Evaluating Sensitivity and Specificity in Naturally Occurring Cranial Cruciate Ligament Deficiency with Arthroscopy as the Gold Standard. Vet. Surg. 2010, 39, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Agnello, K.A.; Brown, D.C.; Zyla, S.G.; Hayashi, K. Arthroscopic Caudal Cruciate Ligament Damage in Canine Stifles with Cranial Cruciate Ligament Disease. Vet. Comp. Orthop. Traumatol. 2022, 35, 263–269. [Google Scholar] [CrossRef]
- Ashour, A.E.; Hoffman, C.L.; Muir, P. Correlation between Orthopaedic and Radiographic Examination and Arthroscopic Ligament Fibre Damage in Dogs with cruciate Ligament Rupture. Aust. Vet. J. 2019, 97, 490–498. [Google Scholar] [CrossRef]
- van den Berg, W.B. Lessons from Animal Models of Osteoarthritis. Curr. Opin. Rheumatol. 2001, 13, 452–456. [Google Scholar] [CrossRef]
- Pond, M.J.; Campbell, J.R. The Canine Stifle Joint. I. Rupture of the Anterior Cruciate Ligament. An Assessment of Conservative and Surgical Treatment. J. Small Anim. Pract. 1972, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meeson, R.L.; Todhunter, R.J.; Blunn, G.; Nuki, G.; Pitsillides, A.A. Spontaneous Dog Osteoarthritis–A One Medicine Vision. Nat. Rev. Rheumatol. 2019, 15, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Mizokami, N.; Ichinohe, T.; Kanno, N.; Suzuki, S.; Yogo, T.; Harada, Y.; Hara, Y. Long-Term Outcome and Progression of Osteoarthritis in Uncomplicated Cases of Cranial Cruciate Ligament Rupture Treated by Tibial Plateau Leveling Osteotomy in Dogs. J. Vet. Med. Sci. 2020, 82, 908–916. [Google Scholar] [CrossRef]
- Bureau, S. Owner Assessment of the Outcome of Tibial Plateau Levelling Osteotomy without Meniscal Evaluation for Treatment of Naturally Occurring Cranial Cruciate Ligament Rupture: 130 Cases (2009 to 2013). J. Small Anim. Pract. 2017, 58, 468–475. [Google Scholar] [CrossRef]
- Priddy, N.H., 2nd; Tomlinson, J.L.; Dodam, J.R.; Hornbostel, J.E. Complications with and Owner Assessment of the Outcome of Tibial Plateau Leveling Osteotomy for Treatment of Cranial Cruciate Ligament Rupture in Dogs: 193 Cases (1997–2001). J. Am. Vet. Med. Assoc. 2003, 222, 1726–1732. [Google Scholar] [CrossRef]
- Berger, B.; Knebel, J.; Steigmeier-Raith, S.; Reese, S.; Meyer-Lindenberg, A. Long-Term Outcome after Surgical Treatment of Cranial Cruciate Ligament Rupture in Small Breed Dogs. Comparison of Tibial Plateau Leveling Osteotomy and Extra-Articular Stifle Stabilization. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2015, 43, 373–380. [Google Scholar] [CrossRef]
- Girling, S.L.; Bell, S.C.; Whitelock, R.G.; Rayward, R.M.; Thomson, D.G.; Carter, S.C.; Vaughan-Thomas, A.; Innes, J.F. Use of Biochemical Markers of Osteoarthritis to Investigate the Potential Disease-Modifying Effect of Tibial Plateau Levelling Osteotomy. J. Small Anim. Pract. 2006, 47, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Hurley, C.R.; Hammer, D.L.; Shott, S. Progression of Radiographic Evidence of Osteoarthritis Following Plateau Leveling Osteotomy in Dogs with Cranial Cruciate Rupture: 295 Cases (2001–2005). J. Am. Vet. Med. Assoc. 2007, 230, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Heffron, L.E.; Campbell, J.R. Osteophyte Formation in the Canine Stifle Joint Following for Rupture of the Cranial Cruciate Ligament. J. Small Anim. Pract. 1979, 20, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.F.; Costello, M.; Barr, F.J.; Rudorf, H.; Barr, A.R.S. Radiographic Progression of Osteoarthritis of the Canine Stifle Joint: A Prospective Study. Vet. Radiol. Ultrasound 2004, 45, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Ritzo, M.E.; Ritzo, B.A.; Siddens, A.D.; Summerlott, S.; Cook, J.L. Incidence and Type of Meniscal Injury and Associated Long-Term Clinical Outcomes in Dogs Treated Surgically for Cranial Cruciate Ligament Disease. Vet. Surg. 2014, 43, 952–958. [Google Scholar] [CrossRef]
- Flo, G.L. Meniscal Injuries. Vet. Clin. N. Am. Small. Anim. Pract. 1993, 23, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Case, J.B.; Hulse, D.; Kerwin, S.C.; Peycke, L.E. Meniscal Injury Following Initial Cranial Cruciate Ligament Stabilization Surgery in 26 Dogs (29 Stifles). Vet. Comp. Orthop. Traumatol. 2008, 21, 365–367. [Google Scholar] [CrossRef]
- Henrotin, Y.; Martel-Pelletier, J.; Msika, P.; Guillou, G.B.; Deberg, M. Usefulness of Specific OA Biomarkers, Coll2-1 and Coll2-1NO2, in the Anterior Cruciate Ligament OA Canine Model. Osteoarthr. Cartil. 2012, 20, 787–790. [Google Scholar] [CrossRef]
- Chockalingam, P.S.; Glasson, S.S.; Lohmander, L.S. Tenascin-C Levels in Synovial Fluid Are Elevated after Injury to the Human and Canine Joint and Correlate with Markers of Inflammation and Matrix Degradation. Osteoarthr. Cartil. 2013, 21, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Toth, S.A.; Siegel, M.I. Canine Cruciate Ligament Ruptures: Implications for Financial Costs and Human Health. Anat. Rec. 2021, 304, 222–230. [Google Scholar] [CrossRef]
- Boland, L.; Danger, R.; Cabon, Q.; Rabillard, M.; Brouard, S.; Bouvy, B.; Gauthier, O. MMP-2 as an Early Synovial Biomarker for Cranial Cruciate Ligament Disease in Dogs. Vet. Comp. Orthop. Traumatol. 2014, 27, 210–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabillard, M.; Danger, R.; Doran, I.P.; Niebauer, G.W.; Brouard, S.; Gauthier, O. Matrix Metalloproteinase Activity in Stifle Synovial Fluid of Cranial Cruciate Ligament Deficient Dogs and Effect of Postoperative Doxycycline Treatment. Vet. J. 2012, 193, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Hegemann, N.; Kohn, B.; Brunnberg, L.; Schmidt, M.F. Biomarkers of Joint Tissue Metabolism in Canine Osteoarthritic and Arthritic Joint Disorders. Osteoarthr. Cartil. 2002, 10, 714–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malek, S.; Weng, H.Y.; Martinson, S.A.; Rochat, M.C.; Béraud, R.; Riley, C.B. Evaluation of Serum MMP-2 and MMP-3, Synovial Fluid IL-8, MCP-1, and KC Concentrations as Biomarkers of Stifle Osteoarthritis Associated with Naturally Occurring Cranial Cruciate Ligament Rupture in Dogs. PLoS ONE 2020, 15, e0242614. [Google Scholar] [CrossRef]
- Niebauer, G.W.; Lubec, G. Collagenase activity in the ruptured cruciate ligament in dogs and its inhibition in vitro. Zent. Vet. A 1980, 27, 628–634. [Google Scholar] [CrossRef]
- Muir, P.; Danova, N.A.; Argyle, D.J.; Manley, P.A.; Hao, Z. Collagenolytic Protease Expression in Cranial Cruciate Ligament and Stifle Synovial Fluid in Dogs with Cranial Cruciate Ligament Rupture. Vet. Surg. 2005, 34, 482–490. [Google Scholar] [CrossRef]
- Niebauer, G.W.; Niedermüller, H.; Skalicky, M. Collagen cross-links in the cruciate ligament of dogs and their relation to pathological cruciate ligament rupture. Zent. Vet. A 1983, 30, 688–693. [Google Scholar] [CrossRef]
- Hayashi, K.; Kim, S.-Y.; Lansdowne, J.L.; Kapatkin, A.; Déjardin, L.M. Evaluation of a Collagenase Generated Osteoarthritis Biomarker in naturally Occurring Canine Cruciate Disease. Vet. Surg. 2009, 38, 117–121. [Google Scholar] [CrossRef]
- de Bruin, T.; de Rooster, H.; van Bree, H.; Cox, E. Interleukin-8 MRNA Expression in Synovial Fluid of Canine Joints with Osteoarthritis. Vet. Immunol. Immunopathol. 2005, 108, 387–397. [Google Scholar] [CrossRef]
- de Bruin, T.; de Rooster, H.; van Bree, H.; Duchateau, L.; Cox, E. Cytokine MRNA Expression in Synovial Fluid of Affected and contralateral Stifle Joints and the Left Shoulder Joint in dogs with Unilateral Disease of the Stifle Joint. Am. J. Vet. Res. 2007, 68, 953–961. [Google Scholar] [CrossRef]
- Fujita, Y.; Hara, Y.; Nezu, Y.; Orima, H.; Tagawa, M. Biomarkers in Dogs Surgically Treated for Ruptured Cranial Cruciate Ligaments. Vet. Rec. 2012, 171, 426. [Google Scholar] [CrossRef]
- Steffey, M.A.; Miura, N.; Todhunter, R.J.; Nykamp, S.G.; Freeman, K.P.; Scarpino, V.; Vernier-Singer, M.A.; Erb, H.N.; MacLeod, J.N.; Lust, G.; et al. The Potential and Limitations of Cartilage-Specific (V+C)(-) Fibronectin and Cartilage Oligomeric Matrix Protein as Osteoarthritis Biomarkers in Canine Synovial Fluid. Osteoarthr. Cartil. 2004, 12, 818–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, C.W.; Chu, Q.; Budsberg, S.C.; Clayton, M.K.; Johnson, K.A. Synovial Fluid Interleukin 6, Tumor Necrosis Factor, and Nitric Values in Dogs with Osteoarthritis Secondary to Cranial Ligament Rupture. Am. J. Vet. Res. 1997, 58, 1027–1032. [Google Scholar] [PubMed]
- Forterre, S.; Zurbriggen, A.; Spreng, D. Nitric Oxide Induces Cell Death in Canine Cruciate Ligament Cells by Activation of Tyrosine Kinase and Reactive Oxygen Species. BMC Vet. Res. 2012, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Louis, E.; Remer, K.A.; Doherr, M.G.; Neumann, U.; Jungi, T.; Schawalder, P.; Spreng, D. Nitric Oxide and Metalloproteinases in Canine Articular: A Comparison between the Cranial Cruciate, the Medial Collateral and the Femoral Head Ligament. Vet. J. 2005, 172, 466–472. [Google Scholar] [CrossRef]
- Gyger, O.; Botteron, C.; Doherr, M.; Zurbriggen, A.; Schawalder, P.; Spreng, D. Detection and Distribution of Apoptotic Cell Death in Normal and Diseased Canine Cranial Cruciate Ligaments. Vet. J. 2007, 174, 371–377. [Google Scholar] [CrossRef]
- Hofer, D.; Forterre, S.; Schweighauser, A.; Krayer, M.; Doherr, M.; Schawalder, P.; Zurbriggen, A.; Spreng, D. Selective INOS-Inhibition Does Not Influence Apoptosis in ruptured Canine Cranial Cruciate Ligaments. Vet. Comp. Orthop. Traumatol. 2009, 22, 198–203. [Google Scholar] [CrossRef]
- Forterre, S.; Zurbriggen, A.; Spreng, D. In Vitro Effect of Different Mediators of Apoptosis on Canine Cranial and Caudal Cruciate Ligament Fibroblasts and Its Reversibility by Pancaspase Inhibitor ZVAD.Fmk. Vet. Immunol. Immunopathol. 2011, 139, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Pardy, C.K.; Matyas, J.R.; Zernicke, R.F. Doxycycline Effects on Mechanical and Morphometrical properties of Early- and Late-Stage Osteoarthritic Bone Following Anterior Ligament Injury. J. Appl. Physiol. (1985) 2004, 97, 1254–1260. [Google Scholar] [CrossRef] [Green Version]
- Jauernig, S.; Schweighauser, A.; Reist, M.; von Rechenberg, B.; Schawalder, P.; Spreng, D. The Effects of Doxycycline on Nitric Oxide and Stromelysin in Dogs with Cranial Cruciate Ligament Rupture. Vet. Surg. 2001, 30, 132–139. [Google Scholar] [CrossRef]
- Kraeutler, M.J.; Aliberti, G.M.; Scillia, A.J.; McCarty, E.C.; Mulcahey, M.K. A Systematic Review of Basic Science and Animal Studies on the Use of Doxycycline to Reduce the Risk of Posttraumatic Osteoarthritis After Anterior Cruciate Ligament Rupture/Transection. Am. J. Sports Med. 2021, 49, 2255–2261. [Google Scholar] [CrossRef] [PubMed]
- Hewicker-Trautwein, M.; Carter, S.D.; Bennett, D.; Kelly, D.F. Immunocytochemical Demonstration of Lymphocyte Subsets and MHC II Antigen Expression in Synovial Membranes from dogs with Rheumatoid Arthritis and Degenerative Joint Disease. Vet. Immunol. Immunopathol. 1999, 67, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Faldyna, M.; Zatloukal, J.; Leva, L.; Kohout, P.; Nečas, A.; Toman, M. Lymphocyte Subsets in Stifle Joint Synovial Fluid of Dogs with Spontaneous Rupture of the Cranial Cruciate Ligament. Acta Vet. Brno 2004, 73, 79–84. [Google Scholar] [CrossRef]
- Muir, P.; Schamberger, G.M.; Manley, P.A.; Hao, Z. Localization of Cathepsin K and Tartrate-Resistant Acid Phosphatase in Synovium and Cranial Cruciate Ligament in Dogs with Cruciate Disease. Vet. Surg. 2005, 34, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Muir, P.; Hayashi, K.; Manley, P.A.; Colopy, S.A.; Hao, Z. Evaluation of Tartrate-Resistant Acid Phosphatase and Cathepsin in Ruptured Cranial Cruciate Ligaments in Dogs. Am. J. Vet. Res. 2002, 63, 1279–1284. [Google Scholar] [CrossRef]
- Bhandal, J.; Hayashi, K.; Kim, S.-Y.; Klein, M.; Wong, A.; Toupadakis, C.A.; Muir, P.; Yellowley, C.E. Detection of Bacterial DNA by PCR in Dogs with Stifle Pathology. Vet. Surg. 2013, 42, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Niebauer, G.W.; Menzel, E.J. Immunological Changes in Canine Cruciate Ligament Rupture. Res. Vet. Sci. 1982, 32, 235–241. [Google Scholar] [CrossRef]
- Niebauer, G.W.; Wolf, B.; Bashey, R.I.; Newton, C.D. Antibodies to Canine Collagen Types I and II in Dogs with Spontaneous Cruciate Ligament Rupture and Osteoarthritis. Arthritis Rheum 1987, 30, 319–327. [Google Scholar] [CrossRef]
- Carter, S.D.; Bell, S.C.; Bari, A.S.; Bennett, D. Immune Complexes and Rheumatoid Factors in Canine Arthritides. Ann. Rheum Dis. 1989, 48, 986–991. [Google Scholar] [CrossRef]
- Muir, P.; Schaefer, S.L.; Manley, P.A.; Svaren, J.P.; Oldenhoff, W.E.; Hao, Z. Expression of Immune Response Genes in the Stifle Joint of dogs with Oligoarthritis and Degenerative Cranial Cruciate Ligament. Vet. Immunol. Immunopathol. 2007, 119, 214–221. [Google Scholar] [CrossRef]
- de Bruin, T.; de Rooster, H.; van Bree, H.; Cox, E. Evaluation of Anticollagen Type I Antibody Titers in Synovial of Both Stifle Joints and the Left Shoulder Joint of dogs with Unilateral Cranial Cruciate Disease. Am. J. Vet. Res. 2007, 68, 283–289. [Google Scholar] [CrossRef] [PubMed]
- de Bruin, T.; de Rooster, H.; Bosmans, T.; Duchateau, L.; van Bree, H.; Gielen, I. Radiographic Assessment of the Progression of Osteoarthrosis in the Contralateral Stifle Joint of Dogs with a Ruptured Cranial Cruciate Ligament. Vet. Rec. 2007, 161, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Hara, Y.; Ochi, H.; Nezu, Y.; Harada, Y.; Yogo, T.; Orima, H.; Tagawa, M. The Possible Role of the Tibial Plateau Angle for the Severity of Osteoarthritis in Dogs with Cranial Cruciate Ligament Rupture. J. Vet. Med. Sci. 2006, 68, 675–679. [Google Scholar] [CrossRef] [Green Version]
- Tirgari, M. The Surgical Significance of the Blood Supply of the Canine Stifle Joint. J. Small Anim. Pract. 1978, 19, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Kyllar, M.; Čížek, P. Cranial Cruciate Ligament Structure in Relation to the Tibial Slope and Intercondylar Notch Width in Dogs. J. Vet. Sci. 2018, 19, 699–707. [Google Scholar] [CrossRef]
- Lewis, B.A.; Allen, D.A.; Henrikson, T.D.; Lehenbauer, T.W. Computed Tomographic Evaluation of the Canine Intercondylar notch in Normal and Cruciate Deficient Stifles. Vet. Comp. Orthop. Traumatol. 2008, 21, 119–124. [Google Scholar]
- Fujita, Y.; Hara, Y.; Nezu, Y.; Schulz, K.S.; Tagawa, M. Proinflammatory Cytokine Activities, Matrix Metalloproteinase-3 Activity, and Sulfated Glycosaminoglycan Content in Synovial Fluid of Dogs with Naturally Acquired Cranial Cruciate Ligament Rupture. Vet. Surg. 2006, 35, 369–376. [Google Scholar] [CrossRef]
- Muir, P.; Kelly, J.L.; Marvel, S.J.; Heinrich, D.A.; Schaefer, S.L.; Manley, P.A.; Tewari, K.; Singh, A.; Suresh, M.; Hao, Z.; et al. Lymphocyte Populations in Joint Tissues from Dogs with Inflammatory Stifle Arthritis and Associated Degenerative Cranial Cruciate Ligament Rupture. Vet. Surg. 2011, 40, 753–761. [Google Scholar] [CrossRef]
- Barrett, J.G.; Hao, Z.; Graf, B.K.; Kaplan, L.D.; Heiner, J.P.; Muir, P. Inflammatory Changes in Ruptured Canine Cranial and Human Anterior Cruciate Ligaments. Am. J. Vet. Res. 2005, 66, 2073–2080. [Google Scholar] [CrossRef]
- Muir, P.; Manley, P.A.; Hao, Z. Collagen Fragmentation in Ruptured Canine Cranial Cruciate Ligament Explants. Vet. J. 2006, 172, 121–128. [Google Scholar] [CrossRef]
- Doom, M.; de Bruin, T.; de Rooster, H.; van Bree, H.; Cox, E. Immunopathological Mechanisms in Dogs with Rupture of the Cranial Ligament. Vet. Immunol. Immunopathol. 2008, 125, 143–161. [Google Scholar] [CrossRef] [Green Version]
- Newton, C.D. Rheumatoid Arthritis in Dogs. J. Am. Vet. Med. Assoc. 1976, 168, 113–121. [Google Scholar]
- Cox, T.; Comerford, E.J.; Wegg, M.; Mills, A.; Barrett, S.D.; Smith, K.D.; Sherratt, M.J.; Akhtar, R. Investigation of Fibrillin Microfibrils in the Canine Cruciate in Dogs with Different Predispositions to Ligament. Res. Vet. Sci. 2020, 133, 53–58. [Google Scholar] [CrossRef]
- Innes, J. Do Hormones Play a Role in Canine Cruciate Disease? J. Small Anim. Pract. 2003, 44, 520. [Google Scholar]
- Slauterbeck, J.R.; Pankratz, K.; Xu, K.T.; Bozeman, S.C.; Hardy, D.M. Canine Ovariohysterectomy and Orchiectomy Increases the prevalence of ACL Injury. Clin. Orthop. Relat. Res. 2004, 429, 301–305. [Google Scholar] [CrossRef]
- Powell, B.S.; Dhaher, Y.Y.; Szleifer, I.G. Review of the Multiscale Effects of Female Sex Hormones on Matrix Metalloproteinase-Mediated Collagen Degradation. Crit. Rev. Biomed. Eng. 2015, 43, 401–428. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.; van Bell, C.; Rogers, V.; Cooney, T. The Relationship between Serum Relaxin and Knee Joint Laxity in Female Athletes. Orthopedics 2002, 25, 669–673. [Google Scholar] [CrossRef]
- Toth, A.P.; Cordasco, F.A. Anterior Cruciate Ligament Injuries in the Female Athlete. J. Gend. Specif. Med. 2001, 4, 25–34. [Google Scholar] [PubMed]
- Dragoo, J.L.; Padrez, K.; Workman, R.; Lindsey, D.P. The Effect of Relaxin on the Female Anterior Cruciate Ligament: Analysis of Mechanical Properties in an Animal Model. Knee 2009, 16, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Dragoo, J.L.; Lee, R.S.; Benhaim, P.; Finerman, G.A.M.; Hame, S.L. Relaxin Receptors in the Human Female Anterior Cruciate Ligament. Am. J. Sports Med. 2003, 31, 577–584. [Google Scholar] [CrossRef]
- Wood, M.L.; Luthin, W.N.; Lester, G.E.; Dahners, L.E. Tendon Creep Is Potentiated by NKISK and Relaxin Which Produce Collagen Fiber Sliding. Iowa Orthop. J. 2003, 23, 75–79. [Google Scholar]
- Konopka, J.A.; DeBaun, M.R.; Chang, W.; Dragoo, J.L. The Intracellular Effect of Relaxin on Female Anterior Cruciate Ligament Cells. Am. J. Sports Med. 2016, 44, 2384–2392. [Google Scholar] [CrossRef] [PubMed]
- Restucci, B.; Sgadari, M.; Fatone, G.; della Valle, G.; Aragosa, F.; Caterino, C.; Ferrara, G.; Niebauer, G.W. Immunoexpression of Relaxin and Its Receptors in Stifle Joints of Dogs with Cranial Cruciate Ligament Disease. Animals 2022, 12, 819. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J.P.; Markel, M.D.; Muir, P. Caudal Cruciate Ligament Damage in Dogs with Cranial Cruciate Ligament Rupture. Vet. Surg. 2010, 39, 936–941. [Google Scholar] [CrossRef]
- Comerford, E.J.; Innes, J.F.; Tarlton, J.F.; Bailey, A.J. Investigation of the Composition, Turnover, and Thermal Properties of Ruptured Cranial Cruciate Ligaments of Dogs. Am. J. Vet. Res. 2004, 65, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, D.H.; Rockwood, C.A., Jr.; Frank, G.R.; Jack, S.C.; Kenyon, R. Repair of the Anterior Cruciate Ligament in Dogs. J. Bone Joint Surg. 1966, 48A, 503–519. [Google Scholar] [CrossRef]
- Pozzi, A.; Kim, S.E.; Conrad, B.P.; Horodyski, M.; Banks, S.A. Ex Vivo Pathomechanics of the Canine Pond-Nuki Model. PLoS ONE 2013, 8, e81383. [Google Scholar] [CrossRef]
- Vasseur, P. Clinical Results Following Nonoperative Management for Rupture of the Cranial Cruciate Ligament in Dogs. Vet. Surg. 1984, 13, 243–246. [Google Scholar] [CrossRef]
- de Rooster, H.; Cox, E.; van Bree, H. Prevalence and Relevance of Antibodies to Type-I and -II Collagen in Synovial Fluid of Dogs with Cranial Cruciate Ligament Damage. Am. J. Vet. Res. 2000, 61, 1456–1461. [Google Scholar] [CrossRef]
- Imholt, K.M.; Möller, S.; Fehr, M.; Meyer-Lindenberg, A. Lameness and osteoarthritis development following Tibial Plateau Leveling Osteotomy (TPLO) and potential prognostic predictors. A long-term retrospective study. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2011, 39, 323–335. [Google Scholar] [CrossRef]
- Ng, H.H.; Shen, M.; Samuel, C.S.; Schlossmann, J.; Bennett, R.G. Relaxin and Extracellular Matrix Remodeling: Mechanisms and Signaling Pathways. Mol. Cell Endocrinol. 2019, 487, 59–65. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niebauer, G.W.; Restucci, B. Etiopathogenesis of Canine Cruciate Ligament Disease: A Scoping Review. Animals 2023, 13, 187. https://doi.org/10.3390/ani13020187
Niebauer GW, Restucci B. Etiopathogenesis of Canine Cruciate Ligament Disease: A Scoping Review. Animals. 2023; 13(2):187. https://doi.org/10.3390/ani13020187
Chicago/Turabian StyleNiebauer, Gert W., and Brunella Restucci. 2023. "Etiopathogenesis of Canine Cruciate Ligament Disease: A Scoping Review" Animals 13, no. 2: 187. https://doi.org/10.3390/ani13020187
APA StyleNiebauer, G. W., & Restucci, B. (2023). Etiopathogenesis of Canine Cruciate Ligament Disease: A Scoping Review. Animals, 13(2), 187. https://doi.org/10.3390/ani13020187





