Genomic Analysis of Heterosis in an Angus × Hereford Cattle Population
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data: Animals, Genotypes, and Phenotypes
2.2. Statistical Analysis
2.3. Genome-Wide Association Analyses
3. Results and Discussion
3.1. Genome-Wide Association
3.2. Additive Effects
3.3. Dominance Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gregory, K.; Cundiff, L. Crossbreeding in beef cattle: Evaluation of systems. J. Anim. Sci. 1980, 51, 1224–1242. [Google Scholar] [CrossRef]
- Bennett, G.L. Periodic rotational crosses. I. Breed and heterosis utilization. J. Anim. Sci. 1987, 65, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Fredeen, H.; Weiss, G.; Newman, J.; Lawson, J.; Rahnefeld, G. Lifetime reproductive efficiency of first-cross beef cows under contrasting environments. Can. J. Anim. Sci. 1981, 61, 539–554. [Google Scholar] [CrossRef]
- Fredeen, H.; Weiss, G.; Newman, J.; Rahnefeld, G.; Lawson, J. Environmental and genetic effects on preweaning performance of calves from first-cross cows. I. Calving ease and preweaning mortality. Can. J. Anim. Sci. 1982, 62, 35–49. [Google Scholar] [CrossRef]
- Gregory, K.; Cundiff, L.; Koch, R. Breed effects and heterosis in advanced generations of composite populations for reproduction and maternal traits of beef cattle. J. Anim. Sci. 1992, 70, 656–672. [Google Scholar] [CrossRef] [PubMed]
- Frisch, J.; O’Neill, C.; D’Occhio, M.; Gazzola, C. Maximizing Heterotic Advantage Using Systematic Crossbreeding; NAP Occasional Publication: Washington, DC, USA, 1998; pp. 96–102. [Google Scholar]
- Koger, M. Efective crossbreeding systems utilizing zebu cattle. J. Anim. Sci. 1980, 50, 1215–1220. [Google Scholar] [CrossRef]
- Gregory, K.; Cundiff, L.; Koch, R.; Dikeman, M.; Koohmaraie, M. Breed effects and retained heterosis for growth, carcass, and meat traits in advanced generations of composite populations of beef cattle. J. Anim. Sci. 1994, 72, 833–850. [Google Scholar] [CrossRef]
- Basarab, J.A.; Crowley, J.; Abo-Ismail, M.K.; Manafiazar, G.; Akanno, E.C.; Baron, V.S.; Plastow, G. Genomic retained heterosis effects on fertility and lifetime productivity in beef heifers. Can. J. Anim. Sci. 2018, 98, 642–655. [Google Scholar] [CrossRef]
- Falconer, D.S. Introduction to Quantitative Genetics; Pearson Education India: Noida, India, 1996. [Google Scholar]
- Vitezica, Z.G.; Varona, L.; Legarra, A. On the additive and dominant variance and covariance of individuals within the genomic selection scope. Genetics 2013, 195, 1223–1230. [Google Scholar] [CrossRef]
- Misztal, I.; Varona, L.; Culbertson, M.; Bertrand, J.K.; Mabry, J.; Lawlor, T.J.; Van Tassel, C.P.; Gengler, N. Studies on the value of incorporating the effect of dominance in genetic evaluations of dairy cattle, beef cattle and swine. BASE 1998. [Google Scholar]
- Bolormaa, S.; Pryce, J.E.; Zhang, Y.; Reverter, A.; Barendse, W.; Hayes, B.J.; Goddard, M.E. Non-additive genetic variation in growth, carcass and fertility traits of beef cattle. Genet. Sel. Evol. 2015, 47, 26. [Google Scholar] [CrossRef] [PubMed]
- Akanno, E.C.; Abo-Ismail, M.K.; Chen, L.; Crowley, J.J.; Wang, Z.; Li, C.; Basarab, J.A.; MacNeil, M.D.; Plastow, G.S. Modeling heterotic effects in beef cattle using genome-wide SNP-marker genotypes. J. Anim. Sci. 2018, 96, 830–845. [Google Scholar] [CrossRef] [PubMed]
- Meuwissen, T.; Hayes, B.; Goddard, M. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Knapp, B.; Church, R.C.; Flower, A.E. Genetic history of the line 1 Hereford cattle at the United States range livestock experiment station. Mont. Agric. Exp. Sta. Bull. 1951, 479, 3–27. [Google Scholar]
- MacNeil, M.D. Invited Review: Research contributions from seventy-five years of breeding Line 1 Hereford cattle at Miles City, Montana1,2. J. Anim. Sci. 2009, 87, 2489–2501. [Google Scholar] [CrossRef]
- Leesburg, V.L.R.; MacNeil, M.D.; Neser, F.W.C. Influence of Miles City Line 1 on the United States Hereford population1,2,3. J. Anim. Sci. 2014, 92, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, M.; Vermeire, L. Effect of weather patterns on preweaning growth of beef calves in the Northern Great Plains. Agric. Sci. 2012, 3, 929. [Google Scholar] [CrossRef][Green Version]
- MacNeil, M.; Cardoso, F.; Hay, E. Genotype by environment interaction effects in genetic evaluation of preweaning gain for Line 1 Hereford cattle from Miles City, Montana. J. Anim. Sci. 2017, 95, 3833–3838. [Google Scholar] [CrossRef]
- MacNeil, M.D.; Urick, J.J.; Newman, S.; Knapp, B.W. Selection for postweaning growth in inbred Hereford cattle: The Fort Keogh, Montana line 1 example. J. Anim. Sci. 1992, 70, 723–733. [Google Scholar] [CrossRef]
- Sargolzaei, M.; Chesnais, J.P.; Schenkel, F.S. A new approach for efficient genotype imputation using information from relatives. BMC Genom. 2014, 15, 478. [Google Scholar] [CrossRef]
- Pitchford, W.S.; Pitchford, J.M.; Alexopoulos, J.G.; Hebart, M.L. Genomic Analysis of Purebred and Crossbred Angus Cows Quantifies Heterozygosity, Breed, and Additive Effects on Components of Reproduction. Animals 2021, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.M.; Laster, D.; Gregory, K.E. Characterization of biological types of cattle I. Dystocia and preweaning growth. J. Anim. Sci. 1976, 43, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Gregory, K.E.; Cundiff, L.V.; Smith, G.M.; Laster, D.; Fitzhugh, H., Jr. Characterization of biological types of cattle-Cycle II: I. Birth and weaning traits. J. Anim. Sci. 1978, 47, 1022–1030. [Google Scholar] [CrossRef]
- Gregory, K.E.; Smith, G.M.; Cundiff, L.; Koch, R.; Laster, D. Characterization of biological types of cattle-cycle III: I. Birth and weaning traits. J. Anim. Sci. 1979, 48, 271–279. [Google Scholar] [CrossRef]
- Cundiff, L.V.; Gregory, K.; Koch, R. Germplasm evaluation in beef cattle-cycle IV: Birth and weaning traits. J. Anim. Sci. 1998, 76, 2528–2535. [Google Scholar] [CrossRef]
- Gregory, K.; Cundiff, L.; Koch, R. Breed effects and heterosis in advanced generations of composite populations for birth weight, birth date, dystocia, and survival as traits of dam in beef cattle. J. Anim. Sci. 1991, 69, 3574–3589. [Google Scholar] [CrossRef]
- Casas, E.; Thallman, R.; Cundiff, L. Birth and weaning traits in crossbred cattle from Hereford, Angus, Norwegian Red, Swedish Red and White, Wagyu, and Friesian sires. J. Anim. Sci. 2012, 90, 2916–2920. [Google Scholar] [CrossRef]
- Williams, J.; Aguilar, I.; Rekaya, R.; Bertrand, J. Estimation of breed and heterosis effects for growth and carcass traits in cattle using published crossbreeding studies. J. Anim. Sci. 2010, 88, 460–466. [Google Scholar] [CrossRef]
- Schiermiester, L.N.; Thallman, R.; Kuehn, L.; Kachman, S.D.; Spangler, M.L. Estimation of breed-specific heterosis effects for birth, weaning, and yearling weight in cattle. J. Anim. Sci. 2015, 93, 46–52. [Google Scholar] [CrossRef]
- Akanno, E.C.; Chen, L.; Abo-Ismail, M.K.; Crowley, J.; Wang, Z.; Li, C.; Basarab, J.; MacNeil, M.; Plastow, G. Genomic prediction of breed composition and heterosis effects in Angus, Charolais, and Hereford crosses using 50K genotypes. Can. J. Anim. Sci. 2017, 97, 431–438. [Google Scholar] [CrossRef]
- Snelling, W.; Allan, M.; Keele, J.; Kuehn, L.; Mcdaneld, T.; Smith, T.; Sonstegard, T.; Thallman, R.; Bennett, G. Genome-wide association study of growth in crossbred beef cattle. J. Anim. Sci. 2010, 88, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Marín-Garzón, N.; Magalhães, A.; Mota, L.; Fonseca, L.; Chardulo, L.; Albuquerque, L. Genome-wide association study identified genomic regions and putative candidate genes affecting meat color traits in Nellore cattle. Meat Sci. 2021, 171, 108288. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; Wiggans, G.R.; Ma, L.; Sonstegard, T.S.; Lawlor, T.J.; Crooker, B.A.; Van Tassell, C.P.; Yang, J.; Wang, S.; Matukumalli, L.K. Genome-wide association analysis of thirty one production, health, reproduction and body conformation traits in contemporary US Holstein cows. BMC Genom. 2011, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Leal-Gutiérrez, J.D.; Rezende, F.M.; Reecy, J.M.; Kramer, L.M.; Peñagaricano, F.; Mateescu, R.G. Whole genome sequence data provides novel insights into the genetic architecture of meat quality traits in beef. Front. Genet. 2020, 1046. [Google Scholar] [CrossRef]
- Saatchi, M.; Schnabel, R.D.; Taylor, J.F.; Garrick, D.J. Large-effect pleiotropic or closely linked QTL segregate within and across ten US cattle breeds. BMC Genom. 2014, 15, 442. [Google Scholar] [CrossRef]
- Höglund, J.K.; Guldbrandtsen, B.; Lund, M.S.; Sahana, G. Analyzes of genome-wide association follow-up study for calving traits in dairy cattle. BMC Genet. 2012, 13, 1–9. [Google Scholar] [CrossRef]
- Akanno, E.C.; Chen, L.; Abo-Ismail, M.K.; Crowley, J.J.; Wang, Z.; Li, C.; Basarab, J.A.; MacNeil, M.D.; Plastow, G.S. Genome-wide association scan for heterotic quantitative trait loci in multi-breed and crossbred beef cattle. Genet. Sel. Evol. 2018, 50, 48. [Google Scholar] [CrossRef]



| Breed | Trait | n | Mean (SD) |
|---|---|---|---|
| Line 1 | Birth Weight (kg) | 722 | 36.92 (4.75) |
| Line 1 | Weaning Weight (kg) | 718 | 194.41 (37.29) |
| Line 1 | Yearling Weight (kg) | 718 | 298.42 (93.19) |
| Angus | Birth Weight (kg) | 608 | 35.20 (5.29) |
| Angus | Weaning Weight (kg) | 608 | 231.91 (42.24) |
| Angus | Yearling Weight (kg) | 589 | 347.96 (56.2) |
| Angus * × Line1 | Birth Weight (kg) | 215 | 37.82 (2.31) |
| Angus * × Line 1 | Weaning Weight (kg) | 199 | 209.19 (24.10) |
| Angus * × Line 1 | Yearling Weight (kg) | 169 | 335.69 (16.65) |
| Angus * × Line 1 F2 | Birth Weight (kg) | 121 | 35.58 (2.62) |
| Angus * × Line 1 F2 | Weaning Weight (kg) | 119 | 224.56 (18.37) |
| Angus * × Line 1 F2 | Yearling Weight (kg) | 90 | 306.67 (11.28) |
| Line 1 * × Angus | Birth Weight (kg) | 345 | 38.26 (2.41) |
| Line 1 * × Angus | Weaning Weight (kg) | 335 | 225.46 (23.98) |
| Line 1 * × Angus | Yearling Weight (kg) | 220 | 332.33 (26.96) |
| Line 1 * × Angus F2 | Birth Weight (kg) | 84 | 37.08 (1.26) |
| Line 1 * × Angus F2 | Weaning Weight (kg) | 119 | 229.41 (11.75) |
| Line 1 * × Angus F2 | Yearling Weight (kg) | 90 | 311.68 (5.23) |
| Trait | H * Effect | p-Value |
|---|---|---|
| Birth Weight (kg) | 0.03 (0.006) | 0.0001 |
| Weaning Weight (kg) | 5.13 (0.04) | 0.0001 |
| Yearling weight (kg) | 6.02 (0.08) | 0.0001 |
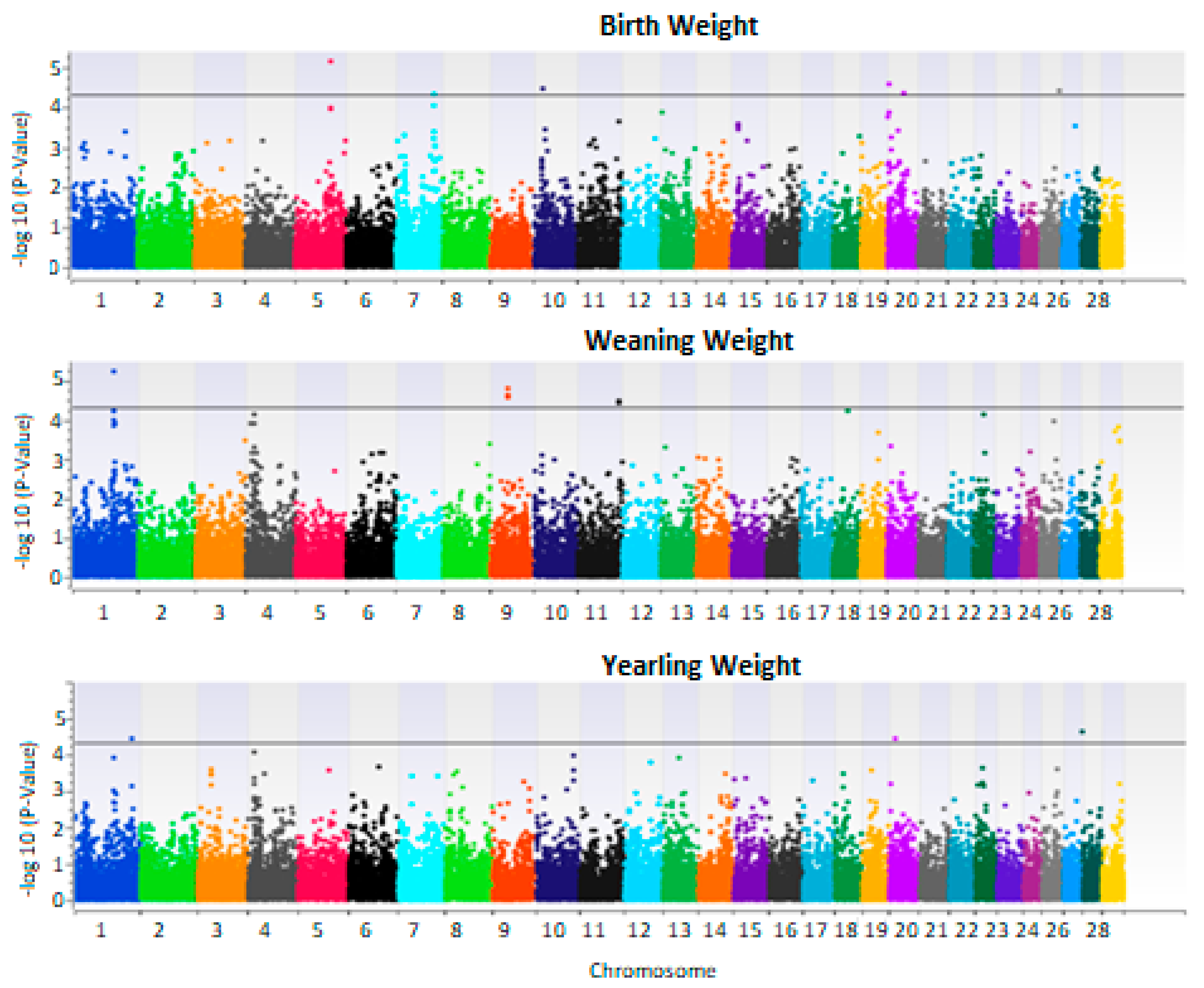
| Trait | SNP | Chromosome | Position | −log10 (p-Value) |
|---|---|---|---|---|
| BW | BovineHD0500023948 | 5 | 84635051 | 5.15 |
| Hapmap57038-rs29015626 | 20 | 4567765 | 4.58 | |
| BovineHD1000006398 | 10 | 19462017 | 4.45 | |
| BovineHD2600013437 | 26 | 46999583 | 4.40 | |
| BovineHD2000010948 | 20 | 38376008 | 4.35 | |
| BovineHD0700027153 | 7 | 92917772 | 4.33 | |
| WW | BovineHD0100028626 | 1 | 100258442 | 5.23 |
| BovineHD0900011585 | 9 | 41673609 | 4.79 | |
| ARS-BFGL-NGS-114241 | 11 | 100472048 | 4.48 | |
| YW | BovineHD2800000001 | 28 | 5302 | 4.62 |
| Hapmap20495-rs29024564 | 1 | 138692649 | 4.43 | |
| BovineHD2000004465 | 20 | 14291458 | 4.41 |
| Trait | SNP | Chromosome | Position | −log10 (p-Value) |
|---|---|---|---|---|
| BW | BovineHD1900011884 | 19 | 41700252 | 5.67 |
| BovineHD0200032395 | 2 | 112608207 | 5.51 | |
| BovineHD0100013823 | 1 | 49017600 | 5.41 | |
| BovineHD1100009030 | 11 | 30187133 | 4.82 | |
| BovineHD1400016579 | 14 | 59818285 | 4.81 | |
| BTA-99960-no-rs | 22 | 8765709 | 4.74 | |
| ARS-BFGL-NGS-32774 | 23 | 13371915 | 4.71 | |
| Hapmap60225-rs29010786 | 4 | 37385962 | 4.56 | |
| UA-IFASA-7751 | 3 | 62689947 | 4.42 | |
| BovineHD1900017738 | 19 | 61764496 | 4.40 | |
| BovineHD0800026507 | 8 | 89344276 | 4.33 | |
| WW | BovineHD0400004973 | 4 | 16939402 | 5.68 |
| BovineHD1200009887 | 12 | 33674092 | 5.17 | |
| ARS-BFGL-NGS-7266 | 13 | 59514859 | 4.90 | |
| BovineHD0700022399 | 7 | 76343644 | 4.80 | |
| BovineHD2800004761 | 28 | 16946164 | 4.63 | |
| BovineHD0900011239 | 9 | 40542770 | 4.57 | |
| Hapmap57378-rs29020063 | 29 | 42749808 | 4.57 | |
| YW | ARS-BFGL-NGS-119888 | 6 | 15252879 | 5.49 |
| BTB-00942520 | 26 | 37956801 | 5.33 | |
| BovineHD0400004973 | 4 | 16939402 | 5.02 | |
| BovineHD2100000540 | 21 | 3518772 | 4.73 | |
| Hapmap44304-BTA-87076 | 9 | 18372617 | 4.71 | |
| BovineHD0800029668 | 8 | 100388737 | 4.65 | |
| BovineHD0300002944 | 3 | 8918173 | 4.62 | |
| BovineHD1200024816 | 12 | 85647505 | 4.36 | |
| Hapmap53672-rs29025245 | 2 | 94314937 | 4.36 | |
| BovineHD0600009773 | 6 | 55115455 | 4.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hay, E.H.; Roberts, A. Genomic Analysis of Heterosis in an Angus × Hereford Cattle Population. Animals 2023, 13, 191. https://doi.org/10.3390/ani13020191
Hay EH, Roberts A. Genomic Analysis of Heterosis in an Angus × Hereford Cattle Population. Animals. 2023; 13(2):191. https://doi.org/10.3390/ani13020191
Chicago/Turabian StyleHay, El Hamidi, and Andy Roberts. 2023. "Genomic Analysis of Heterosis in an Angus × Hereford Cattle Population" Animals 13, no. 2: 191. https://doi.org/10.3390/ani13020191
APA StyleHay, E. H., & Roberts, A. (2023). Genomic Analysis of Heterosis in an Angus × Hereford Cattle Population. Animals, 13(2), 191. https://doi.org/10.3390/ani13020191





