Hierarchy Establishment in Growing Finishing Pigs: Impacts on Behavior, Growth Performance, and Physiological Parameters
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
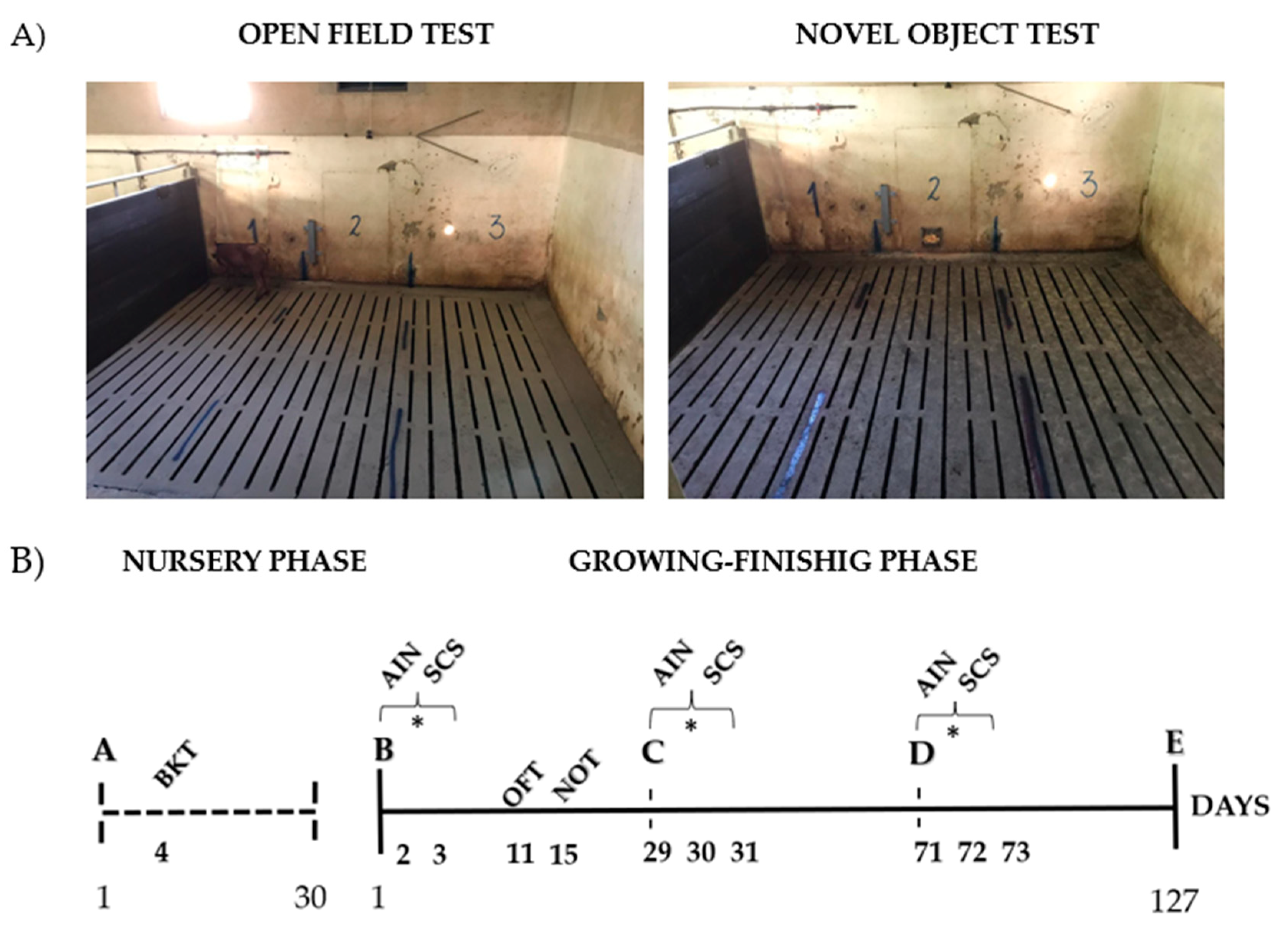
2.1. Nursery Phase
2.1.1. Blood Samples
2.1.2. Backtest
2.2. Growing–Finishing Phase
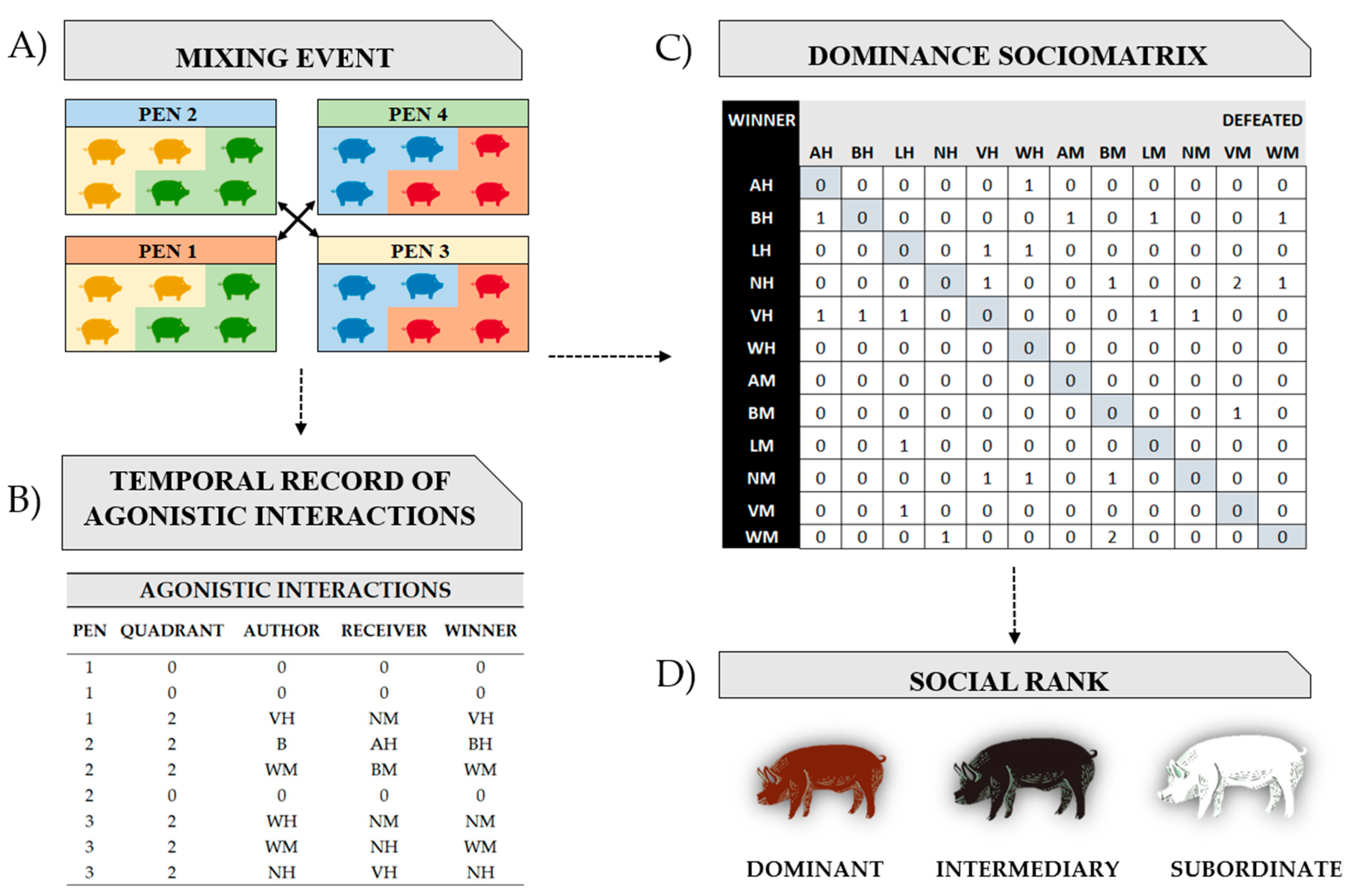
2.2.1. Social Stress
2.2.2. Sampling Methods after Mixing
2.2.3. Behavioral Tests
2.2.4. Growth Performance and Feeding Behavior
2.2.5. Hair Cortisol
2.2.6. Statistical Analysis and Dominance Sociomatrix
3. Results
3.1. Physiological Measures
3.2. Backtest
3.3. Performance and Feeding Behavior
3.4. Open Field and Novel Object Test
4. Discussion
4.1. Performance and Physiological Parameter
4.2. Backtest, OFT, and NOT
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meese, G.B.; Ewbank, R. The establishment and nature of the dominance hierarchy in the domesticated pig. Anim. Behav. 1973, 21, 326–334. [Google Scholar] [CrossRef]
- Drews, C. The concept and definition of dominance in animal behavior. Behaviour 1993, 125, 283–313. [Google Scholar] [CrossRef]
- Schmid, V.S.; de Vries, H. Finding a dominance order most consistent with a linear hierarchy: An improved algorithm for the I&SI method. Anim. Behav. 2013, 86, 1097–1105. [Google Scholar] [CrossRef]
- Tong, X.; Shen, C.; Chen, R.; Gao, S.; Liu, X.; Schinckel, A.P.; Zhou, B. Reestablishment of Social Hierarchies in Weaned Pigs after Mixing. Animals 2019, 10, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Holst, D. The concept of stress and its relevance for animal behavior. Adv. Study. Behav. 1998, 27, 1–131. [Google Scholar] [CrossRef]
- Fleshner, M.; Laudenslager, M.L.; Simons, L.; Maier, S.F. Reduced serum antibodies associated with social defeat in rats. Physiol. Behav. 1989, 45, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- de Groot, J.; Ruis, M.A.; Scholten, J.W.; Koolhaas, J.M.; and Boersma, W.J. Long-term effects of social stress on antiviral immunity in pigs. Physiol. Behav. 2001, 73, 145–158. [Google Scholar] [CrossRef]
- Dingemanse, N.J.; Wolf, M. Between-individual differences in behavioural plasticity within populations: Causes and consequences. Anim. Behav. 2013, 85, 1031–1039. [Google Scholar] [CrossRef]
- Gimsa, U.; Tuchscherer, M.; Kanitz, E. Psychosocial Stress and Immunity-What Can We Learn From Pig Studies? Front. Behav. Neurosci. 2018, 12, 64. [Google Scholar] [CrossRef] [Green Version]
- Schouten, W.G.P.; Wiepkema, P.R. Coping styles of tethered sows. Behav. Process. 1991, 25, 125–132. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Korte, S.M.; De Boer, S.F.; Van Der Vegt, B.J.; Van Reenen, C.G.; Hopster, H.; De Jong, I.C.; Ruis, M.A.; Blokhuis, H.J. Coping styles in animals: Current status in behavior and stress physiology. Neurosci. Biobehav. Rev. 1999, 23, 925–935. [Google Scholar] [CrossRef]
- Sih, A.; Bell, A.M.; Johnson, J.C.; Ziemba, R.E. Behavioral syndromes: An integrative overview. Q. Rev. Biol. 2004, 79, 241–277. [Google Scholar] [CrossRef]
- Koolhaas, J.M. Coping style and immunity in animals: Making sense of individual variation. Brain Behav. Immun. 2008, 22, 662–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosling, S. From mice to men: What can we learn about personality from animal research. Psychol. Bull. 2001, 127, 45–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watters, J.V.; Powell, D.M. Measuring animal personality for use in population management in zoos: Suggested methods and rationale. Zoo Biol. 2011, 29, 1–12. [Google Scholar] [CrossRef]
- Forkman, B.; Boissy, A.; Meunier-Salaün, M.C.; Canali, E.; Jones, R.B. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 2007, 92, 340–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berlyne, D.E. Novelty and curiosity as determinants of exploratory behavior. Br. J. Psychol. 1950, 41, 68–80. [Google Scholar] [CrossRef]
- Archer, J. Tests for emotionality in rat and mice: A review. Anim. Behav. 1973, 21, 205–235. [Google Scholar] [CrossRef]
- Huang, P.; Kimball, R.T.; Mary, C.M.S. Does the use of a multi-trait, multi-test approach to measure animal personality yield different behavioural syndrome results? Behaviour 2018, 155, 115–150. [Google Scholar] [CrossRef]
- O’Malley, C.I.; Steibel, J.P.; Bates, R.O.; Ernst, C.W.; Siegford, J.M. The Social Life of Pigs: Changes in affiliative and agonistic behaviors following mixing. Animals 2022, 12, 206. [Google Scholar] [CrossRef]
- Zebunke, M.; Repsilber, D.; Nürnberg, G.; Wittenburg, D.; Puppe, B. The backtest in pigs revisited—An analysis of intra-situational behaviour. Appl. Anim. Behav. Sci. 2015, 169, 17–25. [Google Scholar] [CrossRef]
- Hessing, M.J.C.; Hagelso, A.M.; van Beek, J.A.M.; Wiepkema, P.R.; Schouten, W.G.P.; Krukow, R. Individual behavioural characteristics in pigs. Appl. Anim. Behav. Sci. 1993, 37, 285–295. [Google Scholar] [CrossRef]
- Hessing, M.J.C.; Hagelso, A.M.; Schouten, W.G.P.; Wiepkema, P.R.; Van Beek, J.A.M. Individual behavioral and physiological strategies in pigs. Physiol. Behav. 1994, 55, 39–46. [Google Scholar] [CrossRef]
- Ruis, M.A.W.; Te Brake, J.H.A.; Van de Burgwal, J.A.; De Jong, I.C.; Blokhuis, H.J.; Koolhaas, J.M. Personalities in female domesticated pigs: Behavioral and physiological indications. Appl. Anim. Behav. Sci. 2000, 66, 31–47. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–267. Available online: http://www.jstor.org/stable/4533591 (accessed on 4 July 2022). [CrossRef] [PubMed] [Green Version]
- Welfare Quality®. Welfare Quality® Assessment Protocol for Pigs (Sows and Piglets, Growing and Finishing Pigs); Welfare Quality® Consortium: Lelystad, The Netherlands, 2009. [Google Scholar]
- Fels, M.; Hartung, J.; Hoy, S. Social hierarchy formation in piglets mixed in different group compositions after weaning. Appl. Anim. Behav. Sci. 2014, 152, 17–22. [Google Scholar] [CrossRef]
- Scheffler, K.; Stamer, E.; Traulsen, I.; Krieter, J. Relationship between behavioural tests and agonistic interactions at different age levels in pigs. Appl. Anim. Behav. Sci. 2016, 177, 19–24. [Google Scholar] [CrossRef]
- Langbein, J.; Puppe, B. Analysing dominance relationships by sociometric methods—A plea for a more standardised and precise approach in farm animals. Appl. Anim. Behav. Sci. 2004, 87, 293–315. [Google Scholar] [CrossRef]
- Samarakone, T.S.; Gonyou, H.W. Domestic pigs alter their social strategy in response to social group size. Appl. Anim. Behav. Sci. 2009, 121, 8–15. [Google Scholar] [CrossRef]
- McGlone, J.J. A Quantitative Ethogram of Aggressive and Submissive Behaviors in Recently Regrouped Pigs. J Anim Sci. 1985, 61(3), 556–566. [Google Scholar] [CrossRef]
- Dalmau, A.; Fabrega, E.; Velarde, A. Fear assessment in pigs exposed to a novel object test. Appl. Anim. Behav. Sci. 2009, 117, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Colpoys, J.D.; Abell, C.E.; Gabler, N.K.; Keating, A.F.; Millman, S.T.; Siegford, J.M.; Young, J.M.; Johnson, A.K. Feed efficiency effects on barrow and gilt behavioral reactivity to novel stimuli tests. J. Anim. Sci. 2015, 93, 1267–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leliveld, L.M.C.; Düpjan, S.; Tuchscherer, A.; Puppe, B. Vocal correlates of emotional reactivity within and across contexts in domestic pigs (Sus scrofa). Physiol. Behav. 2017, 181, 117–126. [Google Scholar] [CrossRef]
- Appleby, M.C. The probability of linearity in hierarchies. Anim. Behav. 1983, 31, 600–608. [Google Scholar] [CrossRef]
- Heimburge, S.; Kanitz, E.; Otten, W. The use of hair cortisol for the assessment of stress in animals. Gen. Comp. Endocrinol. 2019, 270, 10–17. [Google Scholar] [CrossRef]
- Davenport, M.D.; Tiefenbacher, S.; Lutz, C.K.; Novaka, M.A.; Meyer, J.S. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen. Comp. Endocrinol. 2006, 147, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Abeni, F.; Petrera, F.; Prà, A.D.; Rapetti, L.; Crovetto, G.M.; Galassi, G. Blood parameters in fattening pigs from two genetic types fed diet with three different protein concentrations. Transl. Anim. Sci. 2018, 2, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.E.; Bowman, A.S.; Eckersall, D. Hematology of Pigs; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 1019–1025. ISBN 9781119500506. [Google Scholar] [CrossRef]
- Martínez-Miró, S.; Tecles, F.; Ramón, M.; Escribano, D.; Hernández, F.; Madrid, J.; Orengo, J.; Martínez-Subiela, S.; Manteca, X.; Cerón, J.J. Causes, consequences and biomarkers of stress in swine: An update. BMC Vet. Res. 2016, 12, 171. [Google Scholar] [CrossRef] [Green Version]
- Klem, T.B.; Bleken, E.; Morberg, H.; Thoresen, S.I.; Framstad, T. Hematologic and biochemical reference intervals for Norwegian crossbreed grower pigs. Vet. Clin. Pathol. 2010, 39, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Montoro, J.C.; Pessoa, J.; Solà-Oriol, D.; Muns, R.; Gasa, J.; Manzanilla, E.G. Effect of phase feeding, space allowance and mixing on productive performance of grower-finisher pigs. Animals 2022, 12, 390. [Google Scholar] [CrossRef]
- Foister, S.; Doeschl-Wilson, A.; Roehe, R.; Arnott, G.; Boyle, L.; Turner, S. Social network properties predict chronic aggression in commercial pig systems. PLoS ONE 2018, 13, 0205122. [Google Scholar] [CrossRef] [Green Version]
- Coutellier, L.; Arnould, C.; Boissy, A.; Orgeur, P. Pig’s responses to repeated social regrouping and relocation during the growing-finishing period. Appl. Anim. Behav. Sci. 2007, 105, 102–114. [Google Scholar] [CrossRef]
- Désautés, C.; Bidanel, J.P.; Mormède, P. Genetic study of behavioural and pituitary-adrenocortical reactivity in response to an environmental challenge in pigs. Physiol. Behav. 1997, 62, 337–345. [Google Scholar] [CrossRef]
- Mormède, P.; Andanson, S.; Aupérin, B.; Beerda, B.; Guémené, D.; Malmkvist, J.; Manteca, X.; Manteuffel, G.; Prunet, P.; van Reenen, C.G.; et al. Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiol. Behav. 2007, 92, 317–339. [Google Scholar] [CrossRef] [PubMed]
- Ekkel, E.D.; Dieleman, S.J.; Schouten, W.G.P.; Portela, A.; Corni-Lissen, G.; Tielen, M.J.M.; Halberg, F. The circadian rhythm of cortisol in the saliva of young pigs. Physiol. Behav. 1996, 60, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Ruis, M.A.W.; Brake, J.H.A.; Engel, B.; Ekkel, E.D.; Buist, W.G.; Blokhuis, H.J.; Koolhaas, J.M. The circadian rhythm of salivary cortisol in growing pigs: Effects of age, gender, and stress. Physiol Behav. 1997, 62, 623–630. [Google Scholar] [CrossRef]
- Leiber-Schotte, C. Influence of the Ranking in Young Sires in the Self-Performance Test on Feed Intake and Feed Intake Behavior, Taking into Account Endocrinological and Immunological Parameters. Ph.D. thesis, University of Veterinary Medicine, Hannover, Germany, 2009. [Google Scholar]
- Parois, S.; Larzul, C.; Prunier, A. Associations between the dominance status and sexual development, skin lesions or feeding behaviour of intact male pigs. Appl. Anim. Behav. Sci. 2017, 187, 15–22. [Google Scholar] [CrossRef]
- Bus, J.D.; Boumans, I.J.M.M.; Webb, L.E.; Bokkers, E.A.M. The potential of feeding patterns to assess generic welfare in growing-finishing pigs. Appl. Anim. Behav. Sci. 2021, 241, 1053–1083. [Google Scholar] [CrossRef]
- Morrow, A.T.S.; Walker, N. A note on changes to feeding behaviour of growing pigs by fitting stalls to single-space feeders. Anim. Prod. 1994, 59, 151–153. [Google Scholar] [CrossRef]
- Botermans, J.A.M.; Svendsen, J. Effect of feeding environment on performance, injuries and behaviour in growing-finishing pigs: Group-based studies. Acta Agric. Scand. Sect. A—Anim. Sci. 2000, 50, 237–249. [Google Scholar] [CrossRef]
- Andersen, I.L.; Andenæs, H.; Bøe, K.E.; Jensen, P.; Bakken, M. The effects of weight asymmetry and resource distribution on aggression in groups of unacquainted pigs. Appl. Anim. Behav. Sci. 2000, 68, 107–120. [Google Scholar] [CrossRef]
- McBride, G.; James, J.; Hodgens, N. Social behaviour of domestic animals. IV. Growing pigs. Anim. Prod. 1964, 6, 129–139. [Google Scholar] [CrossRef]
- Hafez, E.S.E.; Signoret, J.P. The behaviour of swine. In The Behaviour of Domestic Animals; Hafez, E.S.E., Ed.; Tindall et Cassell, Baillière: London, UK, 1969; pp. 349–390. [Google Scholar]
- Meese, G.B.; Ewbank, R. A note on instability of the dominance hierarchy and variations in level of aggression within groups of fattening pigs. Anim. Prod. 1972, 14, 359–362. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Van Reenen, C.G. Interaction between coping style/personality, stress, and welfare: Relevance for domestic farm animals. Am. Soc. Anim. Sci. 2016, 94, 2284–2296. [Google Scholar] [CrossRef]
- Finkemeier, M.A.; Langbein, J.; Puppe, B. Personality research in mammalian farm animals: Concepts, measures, and relationship to welfare. Front. Vet. Sci. 2018, 5, 131. [Google Scholar] [CrossRef]
- O’Malley, C.I.; Turner, S.P.; D’Eath, R.B.; Steibel, J.P.; Bates, R.O.; Ernst, C.W.; Siegford, J.M. Animal personality in the management and welfare of pigs. Appl. Anim. Behav. Sci. 2019, 218, 104821. [Google Scholar] [CrossRef]
- Zebunke, M.; Nürnberg, G.; Melzer, N.; Puppe, B. The backtest in pigs revisited Inter-situational behaviour and animal classification. Appl. Anim. Behav. Sci. 2017, 194, 7–13. [Google Scholar] [CrossRef]
- Camerlink, I.; Ursinus, W.W.; Bartels, A.C.; Bijma, P.; Bolhuis, J.E. Indirect genetic effects for growth in pigs affect behaviour and weight around weaning. Behav. Genet. 2018, 48, 413–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, D. The vocalizations and other behaviour of growing pigs in an ‘open field’ test. Appl. Anim. Ethol. 1974, 1, 3–16. [Google Scholar] [CrossRef]


| Term | * Definition |
|---|---|
| Agonistic interaction | Physical contact between individuals with or without reaction on the part of the receiver, leading to an attack (unilateral action—the receiver does not bite back) or a fight (bilateral action—the receiver counter-attacks) |
| Submissive behavior | Body-turning (receiver pig turns whole body 180 degrees to protect head and ear) and is usually accompanied by an ear bite and flight response of the attacked pig |
| Author | Attacking pig |
| Receiver | Pig being attacked |
| Winner | A pig is considered a winner when the other individual involved in the agonistic interaction shows a flight and/or submissive behavior. The winner may be the individual who started the agonistic interaction or not |
| Loser | The loser of an agonistic interaction is the one who showed an escape movement during the event. The loser may be the individual who initiated the agonistic interaction or not |
| Measure | Description | |
|---|---|---|
| Reactivity | Activity | -No movement of any portion of the pig’s body was visible for a period > 2 s. The duration includes the beginning of the inactivity until any movement of the body. |
| -No movement of any portion of the pig’s body was visible during the entire testing period (freezing). | ||
| Exploration | -Time it takes to move at least three feet (seconds/minute). | |
| -Time spent manipulating/exploring the floor or walls with its nose (seconds). | ||
| Escape attempts | -Number of times the animal raised its front legs against the wall (seconds). | |
| Quadrant occupation | -Number of times the animals crossed the line of a quadrant with both front legs. | |
| Vocalization | -Number of vocalizations (squeals and grunts). | |
| Eliminatory Conducts | -Urinate and defecate (yes/no). | |
| Novel object manipulation * | -Latency to touch the feeder with apples (seconds). | |
| -Time spent manipulating/touching the apples (seconds). | ||
| Initial Values | Final Values | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 Item | - | SUB | INT | DOM | p-Value | SUB | INT | DOM | p-Value |
| CBC—Complete Blood Count | |||||||||
| HTC | % | 37.66 ± 0.96 | 36.36 ± 0.69 | 36.88 ± 1.05 | 0.551 | 42.02 ± 1.32 | 42.11 ± 0.96 | 42.32 ± 1.32 | 0.987 |
| HMB | g/dL | 10.42 ± 0.29 | 10.35 ± 0.16 | 10.44 ± 0.24 | 0.932 | 12.85 ± 0.35 | 13.14 ± 0.25 | 12.77 ± 0.35 | 0.641 |
| HMT (106) | /µL | 6.31 ± 0.12 | 6.10 ± 0.09 | 6.38 ± 0.13 | 0.166 | 7.33 ± 0.23 | 7.19 ± 0.17 | 6.96 ± 0.23 | 0.521 |
| MCV | fL | 59.76 ± 1.33 | 59.67 ± 0.96 | 57.94 ± 1.46 | 0.574 | 57.22 ± 1.48 | 58.67 ± 1.07 | 61.57 ± 1.48 | 0.114 |
| MCH | pg | 16.53 ± 0.29 | 17.00 ± 0.21 | 16.38 ± 0.32 | 0.204 | 17.55 ± 0.41 | 18.31 ± 0.29 | 18.58 ± 0.41 | 0.180 |
| MCHC | g/dL | 27.76 ± 0.52 | 28.60 ± 0.38 | 28.31 ± 0.57 | 0.434 | 30.72 ± 0.31 AB | 31.22 ± 0.22 A | 30.24 ± 0.31 B | 0.040 |
| PLT (105) | /µL | 3.87 ± 0.55 | 3.78 ± 0.37 | 4.04 ± 0.58 | 0.932 | 3.00 ± 0.34 | 2.40 ± 0.24 | 2.72 ± 0.34 | 0.330 |
| WBC—White Blood Cells | |||||||||
| Lkc (103) | % | 15.61 ± 0.08 | 14.73 ± 0.06 | 17.42 ± 0.09 | 0.036 | 16.58 ± 0.11 AB | 18.21 ± 0.08 A | 14.53 ± 0.1 B | 0.044 |
| Eos (102) | /µL | 5.86 ± 1.17 | 3.61 ± 0.08 | 4.34 ± 1.23 | 0.311 | 6.18 ± 1.20 | 6.57 ± 0.83 | 5.13 ± 1.15 | 0.598 |
| Lynf (103) | /µL | 5.70 ± 0.58 | 5.39 ± 0.42 | 7.05 ± 0.63 | 0.098 | 7.86 ± 1.11 | 9.63 ± 0.80 | 6.60 ± 1.11 | 0.087 |
| Mon (102) | /µL | 7.48 ± 1.42 a | 3.36 ± 1.03 b | 3.26 ± 1.58 b | 0.056 | 2.73 ± 1.16 B | 6.33 ± 1.16 A | 2.42 ± 1.46 B | 0.058 |
| Seg (103) | /µL | 8.77 ± 0.60 | 8.71 ± 0.44 | 9.79 ± 0.67 | 0.379 | 7.81 ± 0.52 | 7.59 ± 0.38 | 7.26 ± 0.52 | 0.758 |
| Seg:Lynf | - | 1.61 ± 0.20 | 1.77 ± 0.14 | 1.61 ± 0.22 | 0.741 | 1.03 ± 1.12 | 0.96 ± 0.08 | 1.20 ± 0.12 | 0.260 |
| Cortisol | µg | - | - | - | - | 0.05 ± 0.002 | 0.05 ± 0.001 | 0.05 ± 0.002 | 0.847 |
| Item | Dominance Sociomatrix | |||
|---|---|---|---|---|
| SUB | INT | DOM | p-Value | |
| 1 One-Way ANOVA | ||||
| Duration (s) | 1.16 ± 0.18 | 1.16 ± 0.13 | 0.75 ± 0.18 | 0.142 |
| 2 Mann–Whitney: W-Test | ||||
| Reactivity (n) | 1 | 1 | 1 | - |
| Vocalization (n) | 0 (0–0) | 0 (0–1) | 0 (0–3) | - |
| 3 Kendall’s Tau b | ||||
| Defecate (%) | 10.42 | 27.08 | 10.42 | - |
| Item | Period | Dominance Sociomatrix | |||
|---|---|---|---|---|---|
| SUB | INT | DOM | p-Value | ||
| 1 One-Way ANOVA | |||||
| IBW (Kg) | I | 25.63 ± 0.94 | 25.57 ± 0.68 | 26.92 ± 0.94 | 0.478 |
| II | 40.72 ± 0.95 | 41.23 ± 0.69 | 42.19 ± 0.95 | 0.542 | |
| III | 73.09 ± 1.65 | 75.64 ± 1.19 | 75.96 ± 1.65 | 0.384 | |
| FBW (Kg) | I | 40.72 ± 0.95 | 41.23 ± 0.69 | 42.19 ± 0.95 | 0.542 |
| II | 73.09 ± 1.65 | 75.64 ± 1.19 | 75.96 ± 1.65 | 0.384 | |
| III | 124.20 ± 3.01 | 131.91 ± 2.17 | 130.75 ± 3.01 | 0.118 | |
| ADG (Kg) | I | 0.79 ± 0.02 | 0.82 ± 0.02 | 0.80 ± 0.02 | 0.530 |
| II | 0.79 ± 0.02 | 0.84 ± 0.02 | 0.82 ± 0.02 | 0.154 | |
| III | 0.97 ± 0.04 | 1.06 ± 0.03 | 1.03 ± 0.04 | 0.113 | |
| ADFI (Kg) | I | 1.56 ± 0.09 | 1.52 ± 0.07 | 1.54 ± 0.09 | 0.942 |
| II | 2.32 ± 0.13 | 2.50 ± 0.10 | 2.30 ± 0.13 | 0.366 | |
| III | 2.94 ± 0.15 | 3.13 ± 0.11 | 3.27 ± 0.15 | 0.302 | |
| F:G | I | 2.01 ± 0.14 | 1.87 ± 0.10 | 1.95 ± 0.14 | 0.726 |
| II | 2.94 ± 0.15 | 2.98 ± 0.11 | 2.82 ± 0.15 | 0.708 | |
| III | 3.08 ± 0.13 | 2.96 ± 0.09 | 3.15 ± 0.13 | 0.451 | |
| ATES (h) | I | 23.28 ± 1.30 | 25.21 ± 0.94 | 22.68 ± 1.31 | 0.240 |
| II | 45.42 ± 3.70 ab | 54.51 ± 2.67 a | 44.86 ± 3.70 b | 0.053 | |
| III | 44.97 ± 2.99 | 50.92 ± 2.16 | 50.65 ± 2.99 | 0.250 | |
| 2 Mann–Whitney: W-Test | |||||
| NVF (n) | I | 396 (245–610) | 328 (160–660) | 324 (226–718) | - |
| II | 468.5 (255–719) | 535 (189–638) | 497.5 (152–916) | - | |
| III | 325.5 (255–676) B | 392.0 (201–577) B | 437.5 (369–871) A | - | |
| Item | Dominance Sociomatrix | p-Value | ||
|---|---|---|---|---|
| SUB | INT | DOM | ||
| 1 One-Way ANOVA | ||||
| Activity (s) | 44.92 ± 6.65 | 51.58 ± 4.70 | 64.00 ± 6.65 | 0.128 |
| 2 Mann–Whitney: W-Test | ||||
| Reactivity (n) | 0 (0–0) | 0 (0–4) | 0 (0–1) | - |
| QO–1 (n) | 3.0 (1–5) | 3.0 (0–7) | 3.5 (0–11) | - |
| QO–2 (n) | 5.5 (2–9) | 6.0 (0–14) | 5.5 (2–19) | - |
| QO–3 (n) | 3.0 (0–4) | 2.0 (0–6) | 3.0 (1–7) | - |
| Vocalization (n) | 7.5 (0–49) | 13.5 (0–49) | 6.0 (0–93) | - |
| 3 Kendall’s Tau b | ||||
| Defecate (%) | 12.50 | 37.50 | 20.83 | - |
| Urinate (%) | 6.25 ab | 14.58 a | 0 b | - |
| Item | Dominance Sociomatrix | |||
|---|---|---|---|---|
| SUB | INT | DOM | p-Value | |
| 1 One-Way ANOVA | ||||
| Latency (s) | 49.50 ± 13.26 | 75.52 ± 9.58 | 54.00 ± 13.26 | 0.213 |
| Duration (s) | 59.08 ± 11.39 | 30.78 ± 8.22 | 38.92 ± 11.39 | 0.142 |
| 2 Mann–Whitney: W-Test | ||||
| Reactivity (n) | 0 (0–0) | 0 (0–2) | 0 (0–1) | - |
| QO-1 (n) | 1 (0–3) | 1 (0–4) | 1 (0–6) | - |
| QO-2 (n) | 3.5 (1–6) | 3 (1–7) | 3 (1–8) | - |
| QO-3 (n) | 1.5 (0–4) | 2 (0–5) | 2 (0–5) | - |
| Vocalization (n) | 7.5 (0–24) | 9.0 (0–54) | 7.0 (1–22) | - |
| 3 Kendall’s Tau b | ||||
| Defecate (%) | 8.51 | 25.53 | 17.02 | - |
| Urinate (%) | 2.13 | 8.51 | 4.26 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Fonseca de Oliveira, A.C.; Webber, S.H.; Ramayo-Caldas, Y.; Dalmau, A.; Costa, L.B. Hierarchy Establishment in Growing Finishing Pigs: Impacts on Behavior, Growth Performance, and Physiological Parameters. Animals 2023, 13, 292. https://doi.org/10.3390/ani13020292
da Fonseca de Oliveira AC, Webber SH, Ramayo-Caldas Y, Dalmau A, Costa LB. Hierarchy Establishment in Growing Finishing Pigs: Impacts on Behavior, Growth Performance, and Physiological Parameters. Animals. 2023; 13(2):292. https://doi.org/10.3390/ani13020292
Chicago/Turabian Styleda Fonseca de Oliveira, Angela Cristina, Saulo Henrique Webber, Yuliaxis Ramayo-Caldas, Antoni Dalmau, and Leandro Batista Costa. 2023. "Hierarchy Establishment in Growing Finishing Pigs: Impacts on Behavior, Growth Performance, and Physiological Parameters" Animals 13, no. 2: 292. https://doi.org/10.3390/ani13020292
APA Styleda Fonseca de Oliveira, A. C., Webber, S. H., Ramayo-Caldas, Y., Dalmau, A., & Costa, L. B. (2023). Hierarchy Establishment in Growing Finishing Pigs: Impacts on Behavior, Growth Performance, and Physiological Parameters. Animals, 13(2), 292. https://doi.org/10.3390/ani13020292







