Assessing the Ecological Conversion Efficiency of Chub Mackerel, Somber japonicus, in Wild Conditions Based on an In Situ Enriched Simulation Method
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Collection and Acclimation
2.2. Experiment Procedure
2.3. The Calculation of Fish Indices
2.4. Data Analysis
3. Results
3.1. Gastric Evacuation Rate
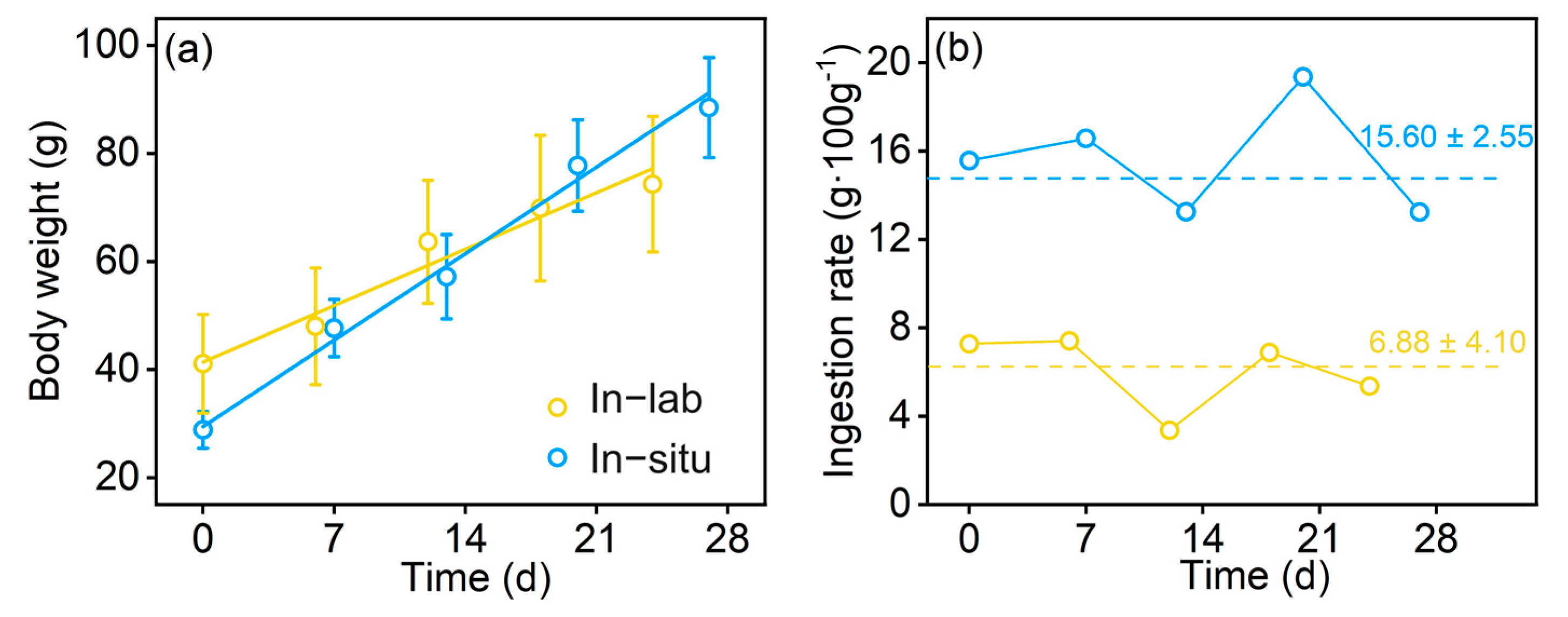
3.2. The Determined Fish Indices
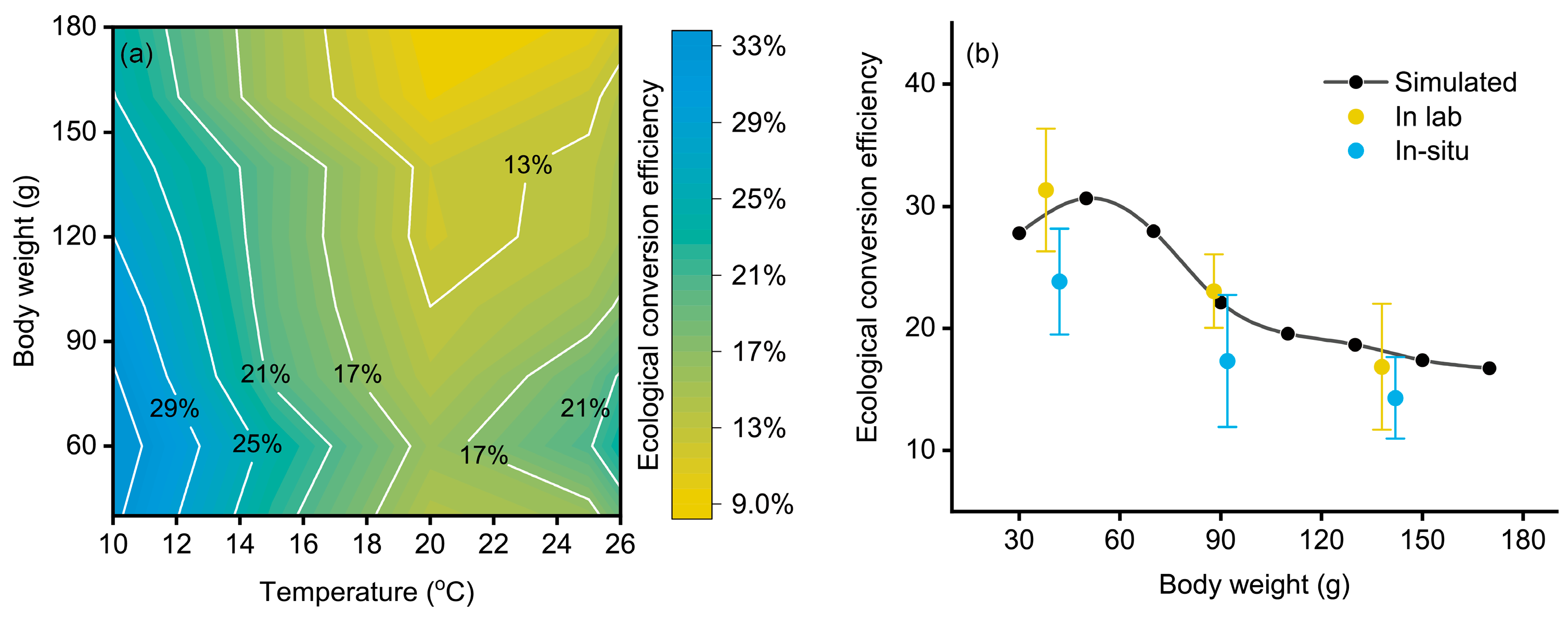
3.3. The Effect of Temperature and Body Weight on the Ecological Conversion Efficiency
4. Discussion
4.1. The Differences in the Indices between In-Lab and In Situ Conditions
4.2. The Ecological Conversion Efficiency of Chub Mackerel
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ríos, M.F.; Venerus, L.A.; Karachle, P.K.; Reid, W.D.; Erzini, K.; Stergiou, K.I.; Galván, D.E. Linking size-based trophodynamics and morphological traits in marine fishes. Fish Fish. 2019, 20, 355–367. [Google Scholar] [CrossRef]
- Bas, M.; Briz, I.G.I.; Alvarez, M.; Vales, D.G.; Crespo, E.A.; Cardona, L. Back to the future? Late Holocene marine food web structure in a warm climatic phase as a predictor of trophodynamics in a warmer South-Western Atlantic Ocean. Glob. Chang. Biol. 2019, 25, 404–419. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Su, J.; Kishi, M.J.; Oh, I.S. An introduction to the Second China–Japan–Korea Joint GLOBEC Symposium on the ecosystem structure, food web trophodynamics and physical–biological processes in the Northwest Pacific. J. Mar. Syst. 2007, 67, 203–204. [Google Scholar] [CrossRef]
- Lawton, J.; May, R. Fisheries Genetics, dynamics and politics. Nature 1984, 309, 744–745. [Google Scholar] [CrossRef]
- Berdnikov, S.; Selyutin, V.; Vasilchenko, V.; Caddy, J. Trophodynamic model of the Black and Azov Sea pelagic ecosystem. Fish. Res. 1999, 42, 261–289. [Google Scholar] [CrossRef]
- Stratton, M.A.; Nesslage, G.M.; Latour, R.J. Multi-decadal climate and fishing predictors of abundance for U.S. South Atlantic coastal fishes and invertebrates. Fish. Oceanogr. 2019, 28, 487–504. [Google Scholar] [CrossRef]
- Natugonza, V.; Ogutu-Ohwayo, R.; Musinguzi, L.; Kashindye, B.; Jónsson, S.; Valtysson, H.T. Exploring the structural and functional properties of the Lake Victoria food web, and the role of fisheries, using a mass balance model. Ecol. Model. 2016, 342, 161–174. [Google Scholar] [CrossRef]
- Jayasinghe, R.P.P.K.; Amarasinghe, U.S.; Newton, A. Evaluation of status of commercial fish stocks in European marine subareas using mean trophic levels of fish landings and spawning stock biomass. Ocean Coast. Manag. 2017, 143, 154–163. [Google Scholar] [CrossRef][Green Version]
- Tang, Q.; Guo, X.; Sun, Y.; Zhang, B. Ecological conversion efficiency and its influencers in twelve species of fish in the Yellow Sea Ecosystem. J. Mar. Syst. 2007, 67, 282–291. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Liu, X.; Tang, Q. The influence of particle size of dietary prey on food consumption and ecological conversion efficiency of young-of-the-year sand lance, Ammodytes personatus. Deep-Sea Res. Part II 2010, 57, 1001–1005. [Google Scholar] [CrossRef]
- Carlucci, R.; Capezzuto, F.; Cipriano, G.; D’Onghia, G.; Fanizza, C.; Libralato, S.; Maglietta, R.; Maiorano, P.; Sion, L.; Tursi, A.; et al. Assessment of cetacean–fishery interactions in the marine food web of the Gulf of Taranto (Northern Ionian Sea, Central Mediterranean Sea). Rev. Fish Biol. Fisher. 2020, 31, 135–156. [Google Scholar] [CrossRef]
- Félix, L.; Vieira, R.; Monteiro, S.M.; Venâncio, C. A meta-analytic review of monoterpene for fish anaesthesia. Fish Fish. 2023, 24, 367–380. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, Y.; Li, Z.; Huang, M.; Li, L.; Gao, Q.; Dong, Y.; Dong, S. Evaluation of the effects of temperature on gastric evacuation and the associated mathematical models in different sizes steelhead trout (Oncorhynchus mykiss). Aquaculture 2022, 549, 737815. [Google Scholar] [CrossRef]
- Shokri, M.; Francesco, C.; Fabio, V.; Marco, B.; Elisabetta, P.; Alberto, B. Metabolic rate and climate change across latitudes: Evidence of mass-dependent responses in aquatic amphipods. J. Exp. Bio. 2022, 225, 22. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, Y.; Yamamoto, S.; Yamaguchi, H.; Ohnishi, T.; Sakamoto, W.; Murata, O. Vertical movement of spawning cultured chub mackerel (Scomber japonicus) in a net-cage. Aquaculture 2014, 422–423, 136–140. [Google Scholar] [CrossRef]
- Jansen, T.; Post, S.; Olafsdottir, A.H.; Reynisson, P.; Óskarsson, G.J.; Arendt, K.E. Diel vertical feeding behaviour of Atlantic mackerel (Scomber scombrus) in the Irminger current. Fish. Res. 2019, 214, 25–34. [Google Scholar] [CrossRef]
- Varela, J.L.; Spares, A.D.; Stokesbury, M.J.W. Feeding ecology of Atlantic bluefin tuna (Thunnus thynnus) in the Gulf of Saint Lawrence, Canada. Mar. Environ. Res. 2020, 161, 105087. [Google Scholar] [CrossRef]
- Takahashi, M.; Tamura, T.; Bando, T.; Konishi, K. Feeding habits of Bryde’s and sei whales in the western North Pacific inferred from stomach contents and skin stable isotope ratios. J. Sea Res. 2022, 184, 102204. [Google Scholar] [CrossRef]
- Wiujamson, C.E. Laboratory and field experiments on the feeding ecology of the cyclopoid copepod, Mesocyclops edax. Freshwater Biol. 1984, 14, 575–585. [Google Scholar] [CrossRef]
- Cathcart, K.; Shin, S.Y.; Milton, J.; Ellerby, D. Field swimming performance of bluegill sunfish, Lepomis macrochirus: Implications for field activity cost estimates and laboratory measures of swimming performance. Ecol. Evol. 2017, 7, 8657–8666. [Google Scholar] [CrossRef]
- Huang, M.; Ding, L.; Wang, J.; Ding, C.; Tao, J. The impacts of climate change on fish growth: A summary of conducted studies and current knowledge. Ecol. Indic. 2021, 121, 106976. [Google Scholar] [CrossRef]
- Egger, D. Factors in interpretion data obtained by diel sampling of fish stomachs. Fish. Res. B. Can. 1977, 34, 290–294. [Google Scholar] [CrossRef]
- Rezende, E.L.; Bozinovic, F. Thermal performance across levels of biological organization. Biol. Sci. 2019, 374, 20180549. [Google Scholar] [CrossRef] [PubMed]
- Shokri, M.; Ciotti, M.; Gjoni, V.; Basset, A. Components of standard metabolic rate variability in three species of gammarids. Web Eco 2019, 19, 1–13. [Google Scholar] [CrossRef]
- Riche, M.; Haley, D.I.; Oetker, M.; Garbrecht, S.; Garling, D.L. Effect of feeding frequency on gastric evacuation and the return of appetite in tilapia Oreochromis niloticus (L.). Aquaculture 2004, 234, 657–673. [Google Scholar] [CrossRef]
- Dürrani, Ö.; Seyhan, K. Gastric evacuation rates in farmed brook trout subjected to a range of feeding conditions fed commercial pellets. Aquaculture 2019, 513, 734390. [Google Scholar] [CrossRef]
- Moran, C.J.; Ferry, L.A.; Gibb, A.C. Why does Gila elegans have a bony tail? A study of swimming morphology convergence. Zoology 2016, 119, 175–181. [Google Scholar] [CrossRef]
- Stavrakidis-Zachou, O.; Papandroulakis, N.; Lika, K. A DEB model for European sea bass (Dicentrarchus labrax): Parameterisation and application in aquaculture. J. Sea Res. 2019, 143, 262–271. [Google Scholar] [CrossRef]
- Sadoul, B.; Geffroy, B.; Lallement, S.; Kearney, M. Multiple working hypotheses for hyperallometric reproduction in fishes under metabolic theory. Ecol. Model. 2020, 433, 1. [Google Scholar] [CrossRef]
- Sigourney, D.B.; Letcher, B.H.; Obedzinski, M.; Cunjak, R.A. Size-independent growth in fishes: Patterns, models and metrics. J. Fish Biol. 2008, 72, 2435–2455. [Google Scholar] [CrossRef]
- Denderen, D.; Gislason, H.; Heuvel, J.; Andersen, K.H.; Leprieur, F. Global analysis of fish growth rates shows weaker responses to temperature than metabolic predictions. Global Ecol. Biogeogr. 2020, 29, 2203–2213. [Google Scholar] [CrossRef]
- Kooijman, S.A.L.M.; Andersen, T.; Kooi, B.W. Dynamic Energy Budget Representations of Stoichiometric Constraints on Population Dynamics. Ecology 2004, 85, 1230–1243. [Google Scholar] [CrossRef]
- Go, S.; Lee, K.; Jung, S. A Temperature-Dependent Growth Equation for Larval Chub Mackerel (Scomber japonicus). Ocean Sci. J. 2020, 55, 157–164. [Google Scholar] [CrossRef]
- Tyler, A. Rates of gastric emptying in young cod. Fish. Res. B. Can. 1970, 27, 1177–1789. [Google Scholar] [CrossRef]
- Bernreuther, M.; Temming, A.; Herrmann, J.P. Effect of temperature on the gastric evacuation in sprat Sprattus sprattus. J. Fish Biol. 2009, 75, 1525–1541. [Google Scholar] [CrossRef] [PubMed]
- Beltramino, L.E.; Venerus, L.A.; Trobbiani, G.A.; Wilson, R.P.; Ciancio, J.E. Activity budgets for the sedentary Argentine sea bass Acanthistius patachonicus inferred from accelerometer data loggers. Austral Ecol. 2019, 44, 397–408. [Google Scholar] [CrossRef]
- Guillen, A.C.; Borges, M.E.; Herrerias, T.; Kandalski, P.K.; de Souza, M.; Donatti, L. Gradual increase of temperature trigger metabolic and oxidative responses in plasma and body tissues in the Antarctic fish Notothenia rossii. Fish Physiol. Biochem. 2022, 48, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Brownscombe, J.W.; Raby, G.D.; Murchie, K.J.; Danylchuk, A.J.; Cooke, S.J. An energetics-performance framework for wild fishes. J. Fish Biol. 2022, 101, 4–12. [Google Scholar] [CrossRef]


| Body Weight | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 g | 50 g | 70 g | 90 g | 110 g | 130 g | 150 g | 170 g | ||
| Temperature | 10 °C | 33.70 | 35.01 | 33.56 | 31.29 | 29.06 | 26.93 | 25.15 | 24.02 |
| 14 °C | 20.52 | 22.06 | 20.25 | 19.33 | 18.01 | 17.40 | 14.98 | 14.11 | |
| 18 °C | 14.2 | 16.03 | 14.11 | 12.97 | 12.31 | 12.19 | 9.72 | 8.97 | |
| 22 °C | 19.48 | 21.50 | 19.12 | 15.92 | 14.29 | 14.14 | 12.61 | 11.69 | |
| 26 °C | 31.8 | 34.22 | 31.76 | 24.33 | 21.50 | 20.23 | 20.44 | 19.21 | |
| 26 °C | 18 °C | 10 °C | |
|---|---|---|---|
| Gd | 22.36 ± 3.23 a | 18.80 ± 4.98 a | 7.34 ± 0.94 c |
| Cd | 110.54 ± 6.23 a | 119.78 ± 7.60 a | 22.39 ± 1.92 c |
| Eg | 20.23 ± 2.03% a | 15.86 ± 5.17% b | 33.07 ± 7.01% c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Yu, M.; Tang, Q.; Sun, Y. Assessing the Ecological Conversion Efficiency of Chub Mackerel, Somber japonicus, in Wild Conditions Based on an In Situ Enriched Simulation Method. Animals 2023, 13, 3159. https://doi.org/10.3390/ani13203159
Sun X, Yu M, Tang Q, Sun Y. Assessing the Ecological Conversion Efficiency of Chub Mackerel, Somber japonicus, in Wild Conditions Based on an In Situ Enriched Simulation Method. Animals. 2023; 13(20):3159. https://doi.org/10.3390/ani13203159
Chicago/Turabian StyleSun, Xin, Miao Yu, Qisheng Tang, and Yao Sun. 2023. "Assessing the Ecological Conversion Efficiency of Chub Mackerel, Somber japonicus, in Wild Conditions Based on an In Situ Enriched Simulation Method" Animals 13, no. 20: 3159. https://doi.org/10.3390/ani13203159
APA StyleSun, X., Yu, M., Tang, Q., & Sun, Y. (2023). Assessing the Ecological Conversion Efficiency of Chub Mackerel, Somber japonicus, in Wild Conditions Based on an In Situ Enriched Simulation Method. Animals, 13(20), 3159. https://doi.org/10.3390/ani13203159





