Current State of Conservation Physiology for Amphibians: Major Research Topics and Physiological Parameters
Abstract
:Simple Summary
Abstract
1. Conservation Physiology
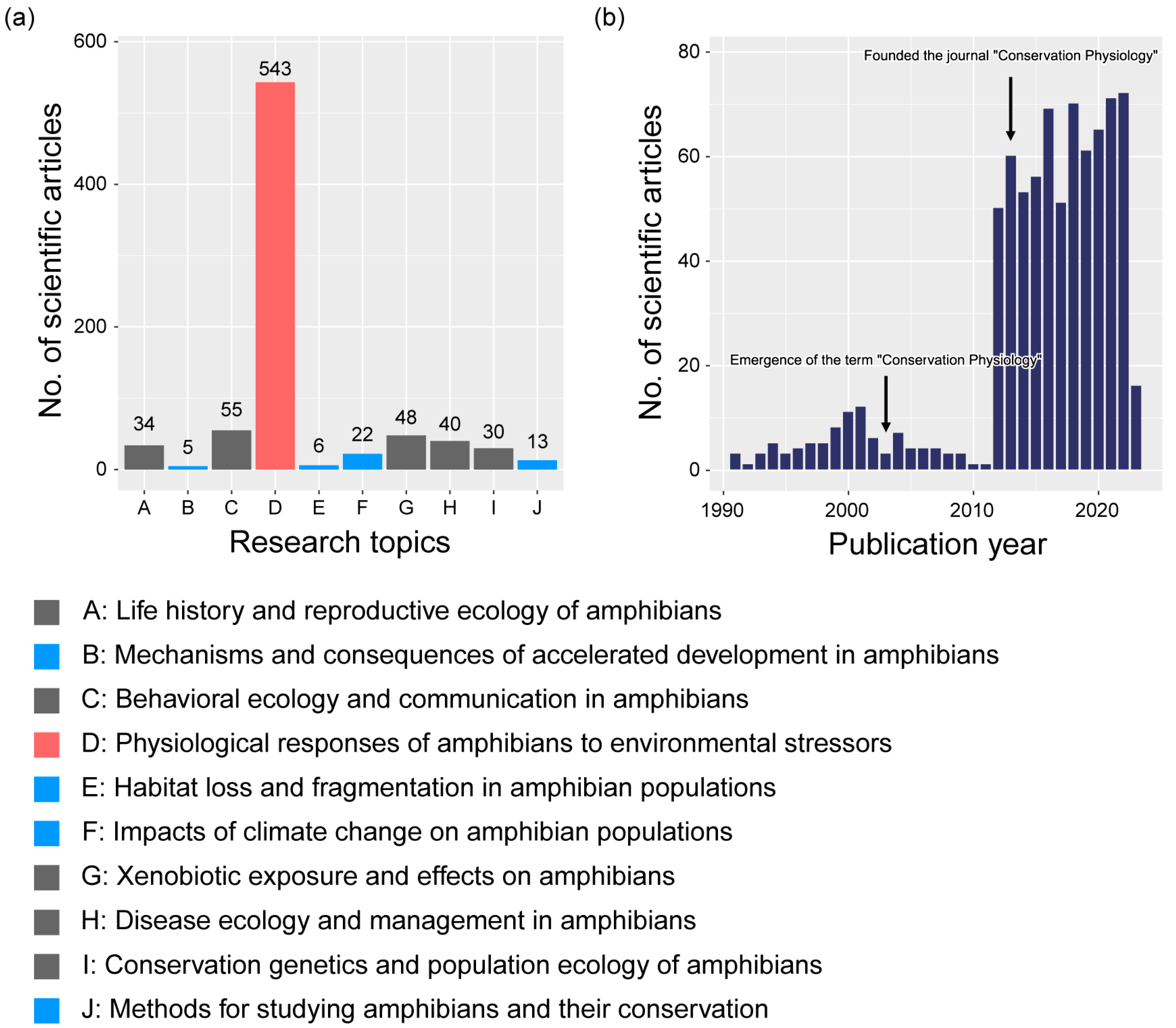
2. Methods and Results of Data Collection
3. Research Topics in Amphibian Conservation Physiology
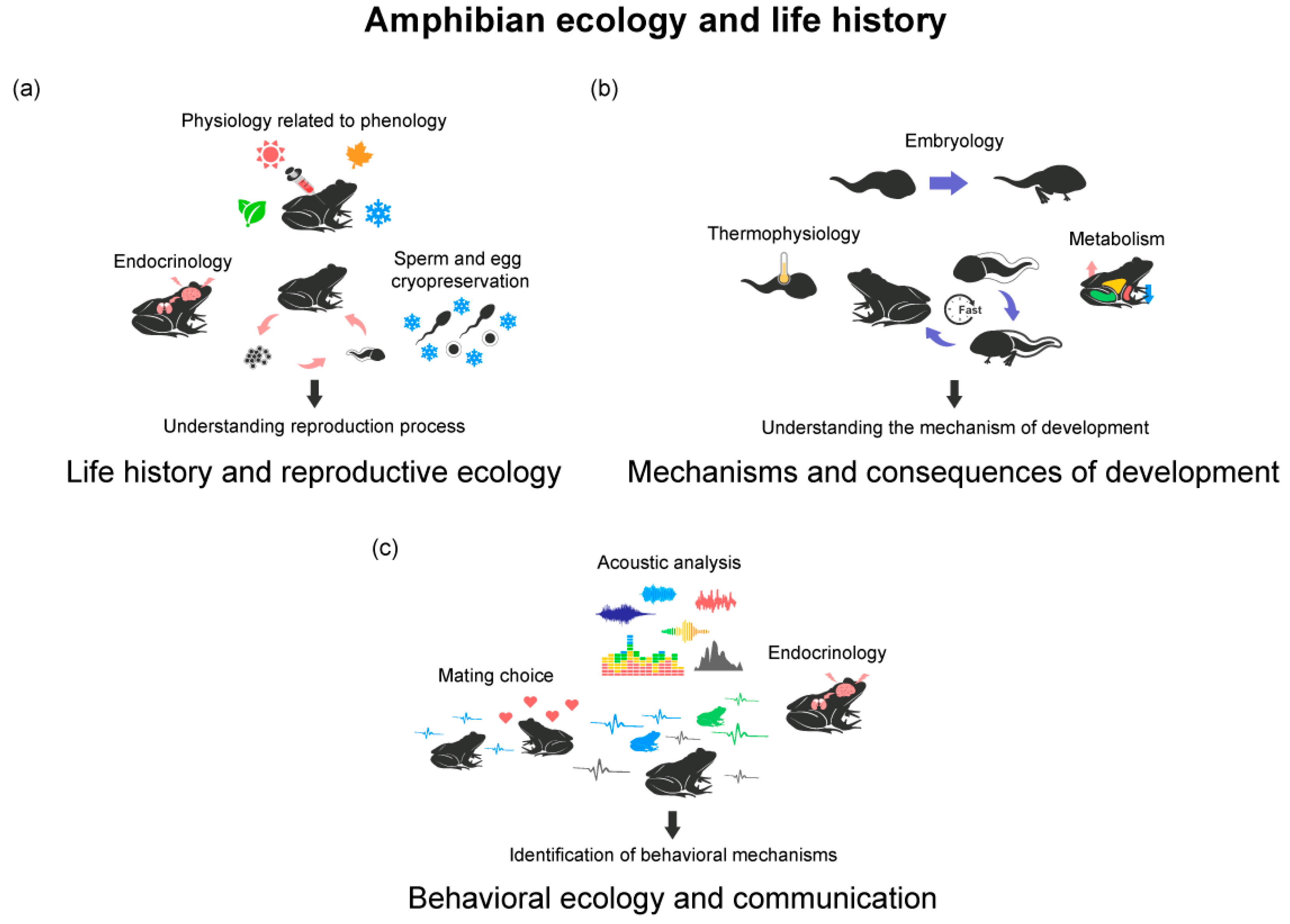
3.1. Amphibian Ecology and Life History
3.1.1. Life History and Reproductive Ecology of Amphibians
3.1.2. Mechanisms and Consequences of Accelerated Development in Amphibians
3.1.3. Behavioral Ecology and Communication in Amphibians
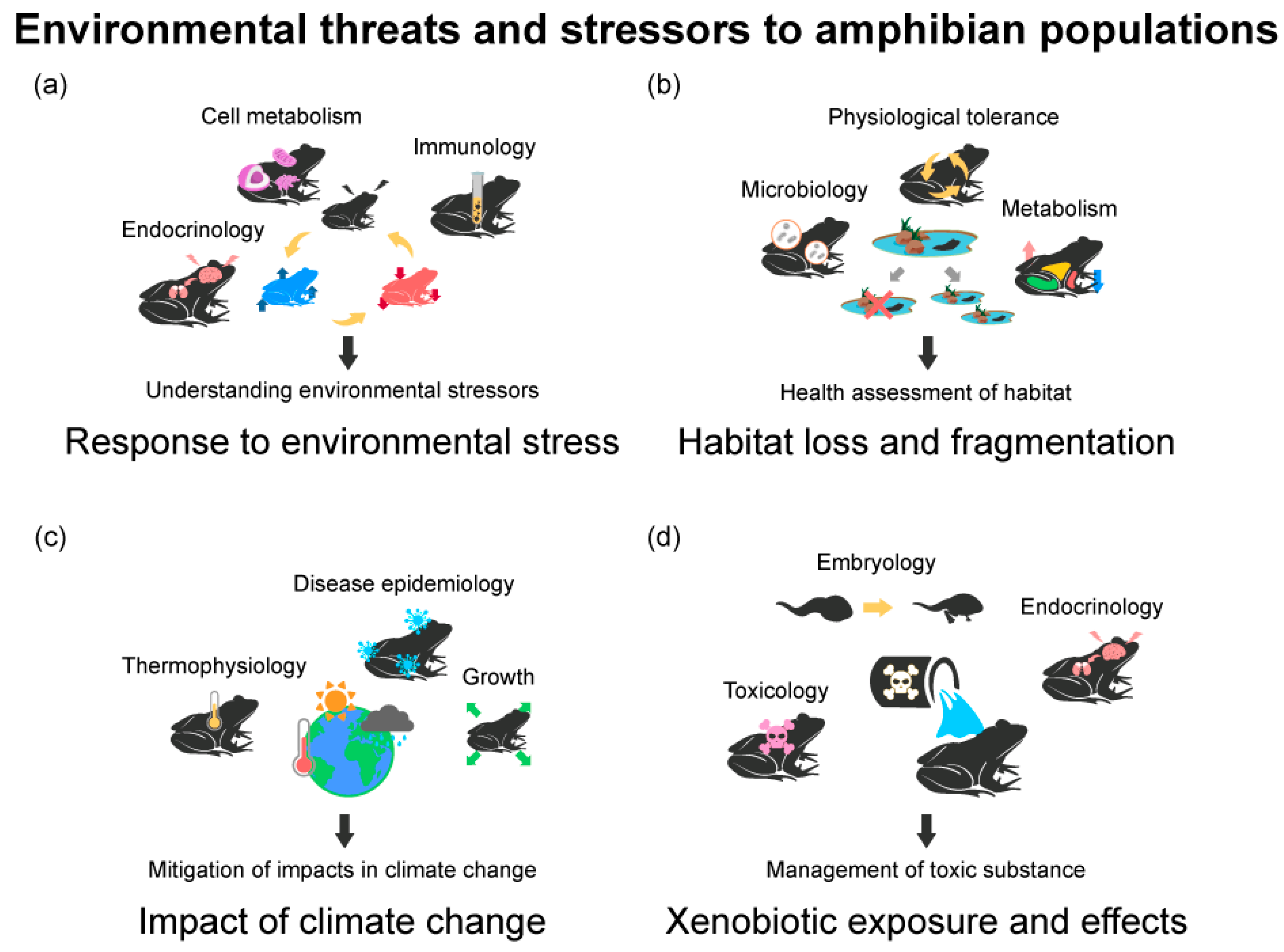
3.2. Environmental Threats and Stressors to Amphibian Populations
3.2.1. Physiological Responses of Amphibians to Environmental Stressors
3.2.2. Habitat Loss and Fragmentation in Amphibian Populations
3.2.3. Impacts of Climate Change on Amphibian Populations
3.2.4. Xenobiotic Exposure and Effects on Amphibians
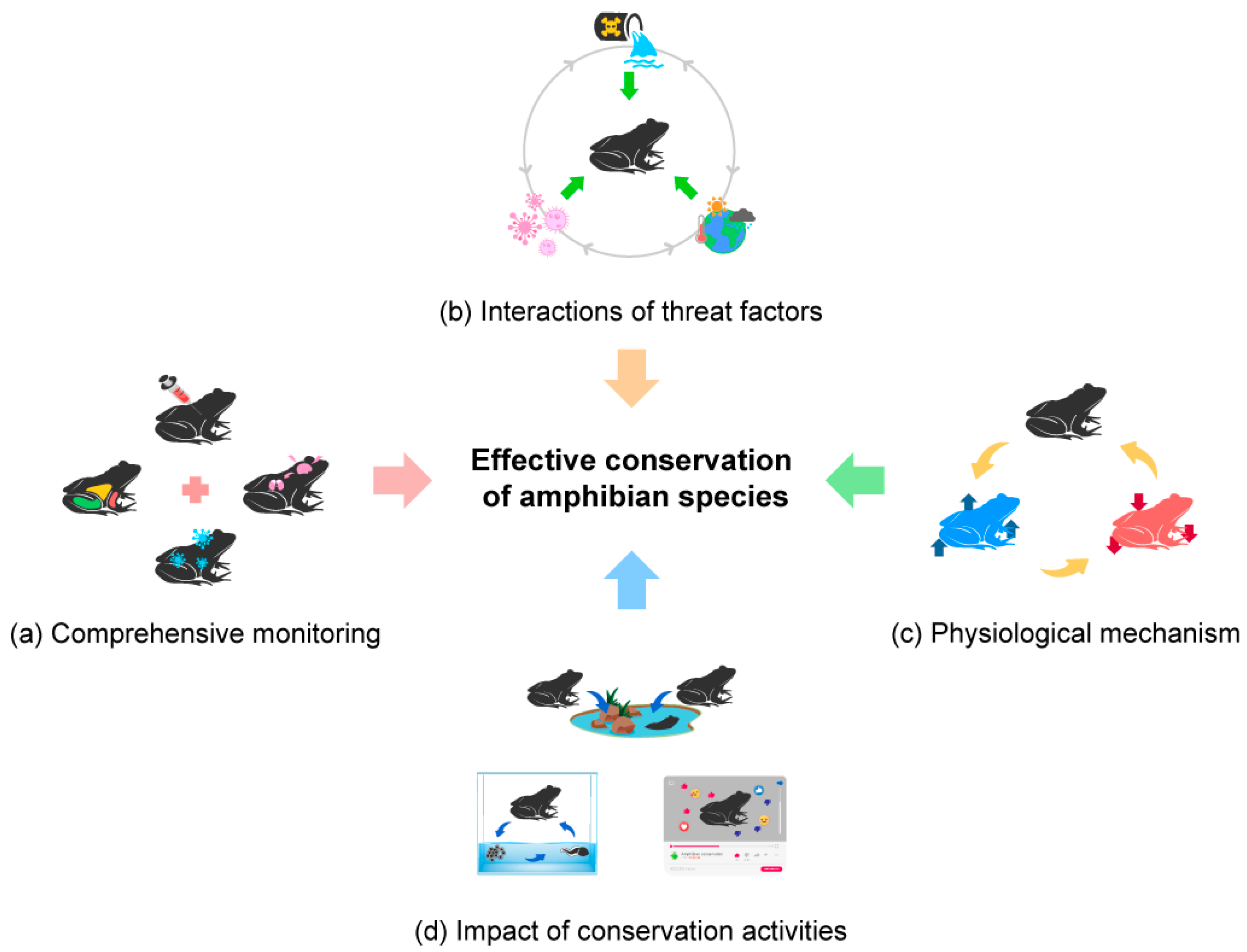
3.3. Amphibian Conservation and Management
3.3.1. Disease Ecology and Management in Amphibians
3.3.2. Conservation Genetics and Population Ecology of Amphibians
3.3.3. Methods for Studying Amphibians and Their Conservation
4. Detailed Subtopics and Physiological Parameters
5. Future Directions of Amphibian Conservation Physiology
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wikelski, M.; Cooke, S.J. Conservation physiology. Trends Ecol. Evol. 2006, 21, 38–46. [Google Scholar] [CrossRef]
- Cooke, S.J.; Sack, L.; Franklin, C.E.; Farrell, A.P.; Beardall, J.; Wikelski, M.; Chown, S.L. What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv. Physiol. 2013, 1, cot001. [Google Scholar] [CrossRef]
- Madliger, C.L.; Love, O.P.; Hultine, K.R.; Cooke, S.J. The conservation physiology toolbox: Status and opportunities. Conserv. Physiol. 2018, 6, coy029. [Google Scholar] [CrossRef]
- Corn, P.S. Climate change and amphibians. Anim. Biodivers. Conserv. 2005, 28, 59–67. [Google Scholar] [CrossRef]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [PubMed]
- Walls, S.C.; Gabor, C.R. Integrating behavior and physiology into strategies for amphibian conservation. Front. Ecol. Evol. 2019, 7, 234. [Google Scholar] [CrossRef]
- Tornabene, B.J.; Hossack, B.R.; Crespi, E.J.; Breuner, C.W. Corticosterone mediates a growth-survival tradeoff for an amphibian exposed to increased salinity. J. Exp. Zool. A Ecol. Integr. Physiol. 2021, 335, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-K.; Kim, J.-B.; Do, Y. Reference intervals in combined veterinary clinical examinations of male black-spotted pond frogs (Pelophylax nigromaculatus). Animals 2021, 11, 1407. [Google Scholar] [CrossRef]
- Park, J.-K.; Do, Y. Wind turbine noise behaviorally and physiologically changes male frogs. Biology 2022, 11, 516. [Google Scholar] [CrossRef]
- Narayan, E.J. Non-invasive reproductive and stress endocrinology in amphibian conservation physiology. Conserv. Physiol. 2013, 1, cot011. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Shu, X.; Pei, E.; Yuan, X.; Wang, T.; Wang, Z. Influence of breeding habitat characteristics and landscape heterogeneity on anuran species richness and abundance in urban parks of Shanghai, China. Urban For. Urban Green. 2018, 32, 56–63. [Google Scholar] [CrossRef]
- Borzée, A.; Purevdorj, Z.; Kim, Y.I.; Kong, S.; Choe, M.; Yi, Y.; Kim, K.; Kim, A.; Jang, Y. Breeding preferences in the Treefrogs Dryophytes japonicus (Hylidae) in Mongolia. J. Nat. Hist. 2019, 53, 2685–2698. [Google Scholar] [CrossRef]
- Bradfield, K.S.; Tapley, B.; Johnson, K. Amphibians and conservation breeding programmes: How do we determine who should be on the ark? Biodivers. Conserv. 2023, 32, 885–898. [Google Scholar] [CrossRef]
- Crump, M.L. Anuran reproductive modes: Evolving perspectives. J. Herpetol. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Chen, W.; Guan, T.; Ren, L.; He, D.; Wang, Y.; Lu, X. Prehibernation energy storage in Heilongjiang brown frogs (Rana amurensis) from five populations in North China. Asian. Herpetol. Res. 2015, 6, 45–50. [Google Scholar]
- Woodhams, D.C.; Bell, S.C.; Bigler, L.; Caprioli, R.M.; Chaurand, P.; Lam, B.A.; Reinert, L.K.; Stalder, U.; Vazquez, V.M.; Schliep, K. Life history linked to immune investment in developing amphibians. Conserv. Physiol. 2016, 4, cow025. [Google Scholar] [CrossRef]
- Borah, B.K.; Renthlei, Z.; Trivedi, A.K. Seasonality in terai tree frog (Polypedates teraiensis): Role of light and temperature in regulation of seasonal breeding. J. Photochem. Photobiol. B Biol. 2019, 191, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, G.; Sinsch, U.; Pelster, B. Migratory behaviour during autumn and hibernation site selection in common frogs (Rana temporaria) at high altitude. Herpetol. J. 2013, 23, 121–124. [Google Scholar]
- Graham, K.M.; Langhorne, C.J.; Vance, C.K.; Willard, S.T.; Kouba, A.J. Ultrasound imaging improves hormone therapy strategies for induction of ovulation and in vitro fertilization in the endangered dusky gopher frog (Lithobates sevosa). Conserv. Physiol. 2018, 6, coy020. [Google Scholar] [CrossRef]
- Guy, E.L.; Martin, M.W.; Kouba, A.J.; Cole, J.A.; Kouba, C.K. Evaluation of different temporal periods between hormone-induced ovulation attempts in the female Fowler’s toad Anaxyrus fowleri. Conserv. Physiol. 2020, 8, coz113. [Google Scholar] [CrossRef]
- Arav, A. Cryopreservation by directional freezing and vitrification focusing on large tissues and organs. Cells 2022, 11, 1072. [Google Scholar] [CrossRef] [PubMed]
- Guy, E.L.; Gillis, A.B.; Kouba, A.J.; Barber, D.; Poole, V.; Marcec-Greaves, R.M.; Kouba, C.K. Sperm collection and cryopreservation for threatened newt species. Cryobiology 2020, 94, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Brun, C.; Exbrayat, J.-M.; Raquet, M. Localization of receptors for sex steroids and pituitary hormones in the female genital duct throughout the reproductive cycle of a viviparous gymnophiona amphibian, Typhlonectes compressicauda. Animals 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Vu, M.; Trudeau, V.L. Neuroendocrine control of spawning in amphibians and its practical applications. Gen. Comp. Endocrinol. 2016, 234, 28–39. [Google Scholar] [CrossRef]
- Gilbert, D.J.; Magrath, M.J.; Byrne, P.G. Warmer temperature and provision of natural substrate enable earlier metamorphosis in the critically endangered Baw Baw frog. Conserv. Physiol. 2020, 8, coaa030. [Google Scholar] [CrossRef]
- Mueller, C.A.; Augustine, S.; Kooijman, S.A.; Kearney, M.R.; Seymour, R.S. The trade-off between maturation and growth during accelerated development in frogs. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 163, 95–102. [Google Scholar] [CrossRef]
- Townsend, D.S. Sexual selection, natural selection, and a fitness trade-off in a tropical frog with male parental care. Am. Nat. 1989, 133, 266–272. [Google Scholar] [CrossRef]
- Giacoma, C.; Zugolaro, C.; Beani, L. The advertisement calls of the green toad (Bufo viridis): Variability and role in mate choice. Herpetologica 1997, 53, 454–464. [Google Scholar]
- Rudh, A.; Rogell, B.; Håstad, O.; Qvarnström, A. Rapid population divergence linked with co-variation between coloration and sexual display in strawberry poison frogs. Evolution 2011, 65, 1271–1282. [Google Scholar] [CrossRef]
- Szuroczki, D.; Richardson, J.M. The behavioral response of larval amphibians (Ranidae) to threats from predators and parasites. PLoS ONE 2012, 7, e49592. [Google Scholar] [CrossRef]
- Carr, J.A.; Brown, C.L.; Mansouri, R.; Venkatesan, S. Neuropeptides and amphibian prey-catching behavior. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 132, 151–162. [Google Scholar] [CrossRef]
- Boyd, S.K. Effects of intracerebroventricular arginine vasotocin on a female amphibian proceptive behavior. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2019, 205, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Ten Eyck, G.R.; Ten Eyck, L.M. Mammalian nonapeptides activate territorial behavior in an amphibian. Physiol. Behav. 2017, 179, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Schulte, L.M.; Summers, K. Searching for hormonal facilitators: Are vasotocin and mesotocin involved in parental care behaviors in poison frogs? Physiol. Behav. 2017, 174, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Stückler, S.; Fuxjager, M.J.; Preininger, D. Evidence that catecholaminergic systems mediate dynamic colour change during explosive breeding events in toads. Biol. Lett. 2022, 18, 20220337. [Google Scholar] [CrossRef] [PubMed]
- Marketon, J.I.W.; Glaser, R. Stress hormones and immune function. Cell. Immunol. 2008, 252, 16–26. [Google Scholar] [CrossRef]
- Araspin, L.; Martinez, A.S.; Wagener, C.; Courant, J.; Louppe, V.; Padilla, P.; Measey, J.; Herrel, A. Rapid shifts in the temperature dependence of locomotor performance in an invasive frog, Xenopus laevis, implications for conservation. Integr. Comp. Biol. 2020, 60, 456–466. [Google Scholar] [CrossRef]
- Bielby, J.; Sausor, C.; Monsalve-Carcaño, C.; Bosch, J. Temperature and duration of exposure drive infection intensity with the amphibian pathogen Batrachochytrium dendrobatidis. PeerJ 2022, 10, e12889. [Google Scholar] [CrossRef]
- Zaffaroni-Caorsi, V.; Both, C.; Márquez, R.; Llusia, D.; Narins, P.; Debon, M.; Borges-Martins, M. Effects of anthropogenic noise on anuran amphibians. Bioacoustics 2023, 32, 90–120. [Google Scholar] [CrossRef]
- Ruthsatz, K.; Dausmann, K.H.; Paesler, K.; Babos, P.; Sabatino, N.M.; Peck, M.A.; Glos, J. Shifts in sensitivity of amphibian metamorphosis to endocrine disruption: The common frog (Rana temporaria) as a case study. Conserv. Physiol. 2020, 8, coaa100. [Google Scholar] [CrossRef]
- Scheun, J.; Greeff, D.; Medger, K.; Ganswindt, A. Validating the use of dermal secretion as a matrix for monitoring glucocorticoid concentrations in African amphibian species. Conserv. Physiol. 2019, 7, coz022. [Google Scholar] [CrossRef] [PubMed]
- Woodley, S.K.; Freeman, P.; Ricciardella, L.F. Environmental acidification is not associated with altered plasma corticosterone levels in the stream-side salamander, Desmognathus ochrophaeus. Gen. Comp. Endocrinol. 2014, 201, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Santymire, R.; Manjerovic, M.; Sacerdote-Velat, A. A novel method for the measurement of glucocorticoids in dermal secretions of amphibians. Conserv. Physiol. 2018, 6, coy008. [Google Scholar] [CrossRef] [PubMed]
- Titon, S.C.M.; Assis, V.R.; Titon Junior, B.; Cassettari, B.d.O.; Fernandes, P.A.C.M.; Gomes, F.R. Captivity effects on immune response and steroid plasma levels of a Brazilian toad (Rhinella schneideri). J. Exp. Zool. A Ecol. Integr. Physiol. 2017, 327, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.K. Brain vasotocin pathways and the control of sexual behaviors in the bullfrog. Brain Res. Bull. 1997, 44, 345–350. [Google Scholar] [CrossRef]
- Moore, F.L.; Lowry, C.A. Comparative neuroanatomy of vasotocin and vasopressin in amphibians and other vertebrates. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 1998, 119, 251–260. [Google Scholar] [CrossRef]
- Prater, C.M.; Harris, B.N.; Carr, J.A. Tectal CRFR1 receptor involvement in avoidance and approach behaviors in the South African clawed frog, Xenopus laevis. Horm. Behav. 2020, 120, 104707. [Google Scholar] [CrossRef]
- Chen, D.M.; Moore, M.G.; Willis, E.L.; Kouba, A.J.; Kouba, C.K. The impact of time and environmental factors on the mitochondrial vesicle and subsequent motility of amphibian sperm. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2022, 268, 111191. [Google Scholar] [CrossRef]
- Demori, I.; El Rashed, Z.; Corradino, V.; Catalano, A.; Rovegno, L.; Queirolo, L.; Salvidio, S.; Biggi, E.; Zanotti-Russo, M.; Canesi, L. Peptides for skin protection and healing in amphibians. Molecules 2019, 24, 347. [Google Scholar] [CrossRef]
- Lundsgaard, N.U.; Cramp, R.L.; Franklin, C.E. Effects of ultraviolet-B radiation on physiology, immune function and survival is dependent on temperature: Implications for amphibian declines. Conserv. Physiol. 2020, 8, coaa002. [Google Scholar] [CrossRef]
- Le Crom, S.; Sugamori, K.S.; Sidhu, A.; Niznik, H.B.; Vernier, P. Delineation of the conserved functional properties of D1A, D1B and D1C dopamine receptor subtypes in vertebrates. Biol. Cell. 2004, 96, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Segan, D.B.; Murray, K.A.; Watson, J.E. A global assessment of current and future biodiversity vulnerability to habitat loss–climate change interactions. Glob. Ecol. Conserv. 2016, 5, 12–21. [Google Scholar] [CrossRef]
- Watling, J.I.; Braga, L. Desiccation resistance explains amphibian distributions in a fragmented tropical forest landscape. Landsc. Ecol. 2015, 30, 1449–1459. [Google Scholar] [CrossRef]
- Wu, J. The hazard and unsureness of reducing habitat ranges in response to climate warming for 91 amphibian species in China. Acta Oecol. 2020, 108, 103640. [Google Scholar] [CrossRef]
- Goff, C.B.; Walls, S.C.; Rodriguez, D.; Gabor, C.R. Changes in physiology and microbial diversity in larval ornate chorus frogs are associated with habitat quality. Conserv. Physiol. 2020, 8, coaa047. [Google Scholar] [CrossRef]
- Löfvenhaft, K.; Runborg, S.; Sjögren-Gulve, P. Biotope patterns and amphibian distribution as assessment tools in urban landscape planning. Landsc. Urban Plan. 2004, 68, 403–427. [Google Scholar] [CrossRef]
- Holmes, I.; McLaren, K.; Wilson, B. Precipitation constrains amphibian chytrid fungus infection rates in a terrestrial frog assemblage in Jamaica, West Indies. Biotropica 2014, 46, 219–228. [Google Scholar] [CrossRef]
- Catenazzi, A.; Lehr, E.; Vredenburg, V.T. Thermal physiology, disease, and amphibian declines on the eastern slopes of the Andes. Conserv. Biol. 2014, 28, 509–517. [Google Scholar] [CrossRef]
- De Pous, P.; Montori, A.; Amat, F.; Sanuy, D. Range contraction and loss of genetic variation of the Pyrenean endemic newt Calotriton asper due to climate change. Reg. Environ. Chang. 2016, 16, 995–1009. [Google Scholar] [CrossRef]
- Cobos, M.E.; Bosch, R.A. Recent and future threats to the Endangered Cuban toad Peltophryne longinasus: Potential additive impacts of climate change and habitat loss. Oryx 2018, 52, 116–125. [Google Scholar] [CrossRef]
- Adams, E.; Leeb, C.; Roodt, A.P.; Brühl, C.A. Interspecific sensitivity of European amphibians towards two pesticides and comparison to standard test species. Environ. Sci. Eur. 2021, 33, 49. [Google Scholar] [CrossRef]
- Katzenberger, M.; Hammond, J.; Duarte, H.; Tejedo, M.; Calabuig, C.; Relyea, R.A. Swimming with predators and pesticides: How environmental stressors affect the thermal physiology of tadpoles. PLoS ONE 2014, 9, e98265. [Google Scholar] [CrossRef]
- Gabor, C.R.; Perkins, H.R.; Heitmann, A.T.; Forsburg, Z.R.; Aspbury, A.S. Roundup™ with corticosterone functions as an infodisruptor to antipredator response in tadpoles. Front. Ecol. Evol. 2019, 7, 114. [Google Scholar] [CrossRef]
- Pochini, K.M.; Hoverman, J.T. Immediate and lag effects of pesticide exposure on parasite resistance in larval amphibians. Parasitology 2017, 144, 817–822. [Google Scholar] [CrossRef]
- Weeks, D.M.; Parris, M.J. A Bacillus thuringiensis kurstaki biopesticide does not reduce hatching success or tadpole survival at environmentally relevant concentrations in Southern leopard frogs (Lithobates sphenocephalus). Environ. Toxicol. Chem. 2020, 39, 155–161. [Google Scholar] [CrossRef]
- Wagner, N.; Mingo, V.; Schulte, U.; Lötters, S. Risk evaluation of pesticide use to protected European reptile species. Biol. Conserv. 2015, 191, 667–673. [Google Scholar] [CrossRef]
- Pinto-Vidal, F.A.; dos Santos Carvalho, C.; Abdalla, F.C.; Ceschi-Bertoli, L.; Utsunomiya, H.S.M.; da Silva, R.H.; Salla, R.F.; Jones-Costa, M. Metabolic, immunologic, and histopathologic responses on premetamorphic American bullfrog (Lithobates catesbeianus) following exposure to lithium and selenium. Environ. Pollut. 2021, 270, 116086. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Lv, Y.; Zhang, Q.-D.; Chang, L.-M.; Chen, Q.-H.; Wang, B.; Jiang, J.-P. Cascading effects of Pb on the environmental and symbiotic microbiota and tadpoles’ physiology based on field data and laboratory validation. Sci. Total Environ. 2023, 862, 160817. [Google Scholar] [CrossRef]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. 2001, 78, 103–116. [Google Scholar] [CrossRef]
- DuRant, S.E.; Hopkins, W.A.; Davis, A.K.; Romero, L.M. Evidence of ectoparasite-induced endocrine disruption in an imperiled giant salamander, the eastern hellbender (Cryptobranchus alleganiensis). J. Exp. Biol. 2015, 218, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Orlofske, S.A.; Belden, L.K.; Hopkins, W.A. Effects of Echinostoma trivolvis metacercariae infection during development and metamorphosis of the wood frog (Lithobates sylvaticus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 203, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.; Bower, D.S.; Clulow, S.; Stockwell, M.; Clulow, J.; Mahony, M. Interaction between temperature and sublethal infection with the amphibian chytrid fungus impacts a susceptible frog species. Sci. Rep. 2019, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Friday, B.; Holzheuser, C.; Lips, K.R.; Longo, A.V. Preparing for invasion: Assessing risk of infection by chytrid fungi in southeastern plethodontid salamanders. J. Exp. Zool. A Ecol. Integr. Physiol. 2020, 333, 829–840. [Google Scholar] [CrossRef]
- Riley, K.; Berry, O.F.; Roberts, J.D. Do global models predicting environmental suitability for the amphibian fungus, B atrachochytrium dendrobatidis, have local value to conservation managers? J. Appl. Ecol. 2013, 50, 713–720. [Google Scholar] [CrossRef]
- O’hanlon, S.J.; Rieux, A.; Farrer, R.A.; Rosa, G.M.; Waldman, B.; Bataille, A.; Kosch, T.A.; Murray, K.A.; Brankovics, B.; Fumagalli, M. Recent Asian origin of chytrid fungi causing global amphibian declines. Science 2018, 360, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Waldman, B. Ancestral chytrid pathogen remains hypervirulent following its long coevolution with amphibian hosts. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190833. [Google Scholar] [CrossRef]
- Arroyo-Lambaer, D.; Chapman, H.; Hale, M.; Blackburn, D. Conservation genetics of two threatened frogs from the Mambilla highlands, Nigeria. PLoS ONE 2018, 13, e0202010. [Google Scholar] [CrossRef]
- Ball, S.E.; Bovero, S.; Sotgiu, G.; Tessa, G.; Angelini, C.; Bielby, J.; Durrant, C.; Favelli, M.; Gazzaniga, E.; Garner, T.W. Islands within an island: Population genetic structure of the endemic Sardinian newt, Euproctus platycephalus. Ecol. Evol. 2017, 7, 1190–1211. [Google Scholar] [CrossRef]
- Turner, A.; Heard, G.; Hall, A.; Wassens, S. Age structure of amphibian populations with endemic chytridiomycosis, across climatic regions with markedly different infection risk. Ecol. Evol. 2022, 12, e9123. [Google Scholar] [CrossRef]
- Murphy, R.W.; Fu, J.; Upton, D.E.; De Lema, T.; Zhao, E.M. Genetic variability among endangered Chinese giant salamanders, Andrias davidianus. Mol. Ecol. 2000, 9, 1539–1547. [Google Scholar] [CrossRef]
- Allentoft, M.E.; Siegismund, H.R.; Briggs, L.; Andersen, L.W. Microsatellite analysis of the natterjack toad (Bufo calamita) in Denmark: Populations are islands in a fragmented landscape. Conserv. Genet. 2009, 10, 15–28. [Google Scholar] [CrossRef]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Ray, N.; Lehmann, A.; Joly, P. Modeling spatial distribution of amphibian populations: A GIS approach based on habitat matrix permeability. Biodivers. Conserv. 2002, 11, 2143–2165. [Google Scholar] [CrossRef]
- Gerick, A.A.; Munshaw, R.G.; Palen, W.J.; Combes, S.A.; O’Regan, S.M. Thermal physiology and species distribution models reveal climate vulnerability of temperate amphibians. J. Biogeogr. 2014, 41, 713–723. [Google Scholar] [CrossRef]
- Carey, C. How physiological methods and concepts can be useful in conservation biology. Integr. Comp. Biol. 2005, 45, 4–11. [Google Scholar] [CrossRef]
- Naguib, M. Veterinary approach to the amphibian patient. Practice 2022, 44, 91–99. [Google Scholar] [CrossRef]
- Park, J.-K.; Do, Y. Evaluating the physical condition of Hyla japonica using radiographic techniques. Sci. Total Environ. 2020, 726, 138596. [Google Scholar] [CrossRef]
- Chen, L.-D.; Santos-Rivera, M.; Burger, I.J.; Kouba, A.J.; Barber, D.M.; Vance, C.K. Near-infrared spectroscopy (NIRS) as a method for biological sex discrimination in the endangered Houston toad (Anaxyrus houstonensis). Methods Protoc. 2022, 5, 4. [Google Scholar] [CrossRef]
- Lazcano, I.; Cisneros-Mejorado, A.; Concha, L.; Ortiz-Retana, J.J.; Garza-Villarreal, E.A.; Orozco, A. MRI-and histologically derived neuroanatomical atlas of the Ambystoma mexicanum (axolotl). Sci. Rep. 2021, 11, 9850. [Google Scholar] [CrossRef]
- Park, J.-K.; Chung, K.W.; Kim, J.Y.; Do, Y. Population structure and morphological pattern of the black-spotted pond frog (Pelophylax nigromaculatus) inhabiting watershed areas of the Geum river in South Korea. Sustainability 2022, 14, 16530. [Google Scholar] [CrossRef]
- Neto, J.G.D.S.; Sutton, W.B.; Spear, S.F.; Freake, M.J.; Kéry, M.; Schmidt, B.R. Integrating species distribution and occupancy modeling to study hellbender (Cryptobranchus alleganiensis) occurrence based on eDNA surveys. Biol. Conserv. 2020, 251, 108787. [Google Scholar]
- Rowley, J.J.; Callaghan, C.T. The FrogID dataset: Expert-validated occurrence records of Australia’s frogs collected by citizen scientists. ZooKeys 2020, 912, 139. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.J.; Callaghan, C.T.; Cutajar, T.; Portway, C.; Potter, K.; Mahony, S.; Trembath, D.F.; Flemons, P.; Woods, A. FrogID: Citizen scientists provide validated biodiversity data on frogs of Australia. Herpetol. Conserv. Biol. 2019, 14, 155–170. [Google Scholar]
- Thompson, M.M.; Rowley, J.J.; Poore, A.G.; Callaghan, C.T. Citizen science reveals meteorological determinants of frog calling at a continental scale. Divers. Distrib. 2022, 28, 2375–2387. [Google Scholar] [CrossRef]
- Hoffmann, A.; Plötner, J.; Pruvost, N.B.; Christiansen, D.G.; Röthlisberger, S.; Choleva, L.; Mikulíček, P.; Cogălniceanu, D.; Sas-Kovács, I.; Shabanov, D.; et al. Genetic diversity and distribution patterns of diploid and polyploid hybrid water frog populations (Pelophylax esculentus complex) across Europe. Mol. Ecol. 2015, 24, 4371–4391. [Google Scholar] [CrossRef] [PubMed]
- Dedukh, D.; Litvinchuk, S.; Rosanov, J.; Mazepa, G.; Saifitdinova, A.; Shabanov, D.; Krasikova, A. Optional endoreplication and selective elimination of parental genomes during oogenesis in diploid and triploid hybrid European water frogs. PLoS ONE 2015, 10, e0123304. [Google Scholar] [CrossRef]
- Liu, K.; Wang, F.; Chen, W.; Tu, L.; Min, M.S.; Bi, K.; Fu, J. Rampant historical mitochondrial genome introgression between two species of green pond frogs, Pelophylax nigromaculatus and P. plancyi. BMC Evol. Biol. 2010, 10, 201. [Google Scholar] [CrossRef] [PubMed]
- Komaki, S.; Igawa, T.; Lin, S.M.; Tojo, K.; Min, M.S.; Sumida, M. Robust molecular phylogeny and palaeodistribution modelling resolve a complex evolutionary history: Glacial cycling drove recurrent mt DNA introgression among Pelophylax frogs in East Asia. J. Biogeogr. 2015, 42, 2159–2171. [Google Scholar] [CrossRef]
- Fayzulin, A.; Chikhlyaev, I.; Mineev, A.; Kuzovenko, A.; Mihaylov, R.; Zaripova, F.; Popov, A.; Ermakov, O. New data on the anomalies of tailless amphibians of the Volga Basin. KnE Life Sci. 2018, 2018, 29–35. [Google Scholar] [CrossRef]
- Korzikov, V.; Faizulin, A.; Ermakov, O.; Alekseev, S.; Aleksanov, V. New occurrences of anomalous specimens of anuran amphibians in northwest upper Poochye. KnE Life Sci. 2018, 2018, 80–86. [Google Scholar] [CrossRef]
- Denoël, M.; Bichot, M.; Ficetola, G.F.; Delcourt, J.; Ylieff, M.; Kestemont, P.; Poncin, P. Cumulative effects of road de-icing salt on amphibian behavior. Aquat. Toxicol. 2010, 99, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Karraker, N.E.; Gibbs, J.P. Road deicing salt irreversibly disrupts osmoregulation of salamander egg clutches. Environ. Pollut. 2011, 159, 833–835. [Google Scholar] [CrossRef]
- Collins, S.J.; Russell, R.W. Toxicity of road salt to Nova Scotia amphibians. Environ. Pollut. 2009, 157, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, G.R.; Brodie, E.D., Jr. Occurrence of amphibians in saline habitats: A review and evolutionary perspective. Herpetol. Monogr. 2015, 29, 1–27. [Google Scholar] [CrossRef]
- Park, J.K.; Do, Y. Physiological response of Pelophylax nigromaculatus adults to salinity exposure. Animals 2020, 10, 1698. [Google Scholar] [CrossRef] [PubMed]


| Category | Physiological Parameters (Experimental Items) |
|---|---|
| Survival rates and mortality | Annual survival rate; cause-specific mortality rate; age-specific mortality rate; mortality due to predation, disease, or environmental stressors; survival and mortality of different life stages (e.g., eggs, larvae, juveniles, adults) |
| Reproductive success and fertility | Clutch size; hatching success; fertilization success; brood size; nesting success; incubation period; mating success; reproductive lifespan |
| Growth rates and size | Growth rate (length or mass); age-specific growth rate; size at metamorphosis; size at maturity; growth plasticity in response to environmental conditions |
| Hormone levels and effects | Hormone levels (e.g., testosterone, estrogen, corticosterone); hormone receptor density; behavioral effects of hormones (e.g., territoriality, courtship, aggression) |
| Behavior and activity patterns | Activity budget (e.g., proportion of time spent foraging, resting, moving); circadian rhythm; social behavior (e.g., mating, parental care, territoriality); response to stimuli (e.g., predator avoidance, conspecific recognition) |
| Feeding ecology and diet | Diet composition; feeding rate; prey selection; digestive efficiency; nutrient absorption and allocation |
| Disease prevalence and resistance | Disease prevalence (e.g., prevalence of chytrid fungus in amphibians); resistance to diseases; immune response (e.g., cytokine levels, leukocyte counts) |
| Habitat use and distribution | Habitat selection; home range size; migration patterns; habitat fragmentation effects on population dynamics |
| Physiological responses to environmental stressors | Thermal tolerance; response to temperature fluctuations; resistance to pollutants; detoxification enzyme activity |
| Genetic diversity and population structure | Genetic diversity (e.g., heterozygosity, allelic richness); effective population size; gene flow; population structure (e.g., subpopulations, metapopulations) |
| Energetics and metabolic rates | Basal metabolic rate; maximum metabolic rate; energy intake and expenditure; respiration rate; energy allocation to growth, reproduction, and maintenance |
| Chemical cues and olfactory responses | Detection threshold for chemical cues; discrimination ability between different chemical cues; behavioral response to chemical cues (e.g., foraging, predator avoidance, mate choice) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-K.; Do, Y. Current State of Conservation Physiology for Amphibians: Major Research Topics and Physiological Parameters. Animals 2023, 13, 3162. https://doi.org/10.3390/ani13203162
Park J-K, Do Y. Current State of Conservation Physiology for Amphibians: Major Research Topics and Physiological Parameters. Animals. 2023; 13(20):3162. https://doi.org/10.3390/ani13203162
Chicago/Turabian StylePark, Jun-Kyu, and Yuno Do. 2023. "Current State of Conservation Physiology for Amphibians: Major Research Topics and Physiological Parameters" Animals 13, no. 20: 3162. https://doi.org/10.3390/ani13203162
APA StylePark, J.-K., & Do, Y. (2023). Current State of Conservation Physiology for Amphibians: Major Research Topics and Physiological Parameters. Animals, 13(20), 3162. https://doi.org/10.3390/ani13203162





