Red Vetchling (Lathyrus cicera L.), a Promising Crop for the Sustainable Replacement of Soybean Meal and Reducing the Carbon Footprint of European Aquafeeds
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Design, Diets and Sampling
2.3. Proximate Composition
2.4. Amino Acid Profile
2.5. Profile of Non-Starch Polysaccharide
2.6. Growth Performance
2.7. Digestibility Assay
2.8. Postprandial Analysis
2.9. Histopathological Analysis
2.10. Statistical Analysis
3. Results
3.1. Nutritional Characteristics of Red Vetchling and the Experimental Diets
3.2. Rainbow Trout Growth Performance When Fed Experimental Diets
3.3. Amino Acid Composition of Muscle
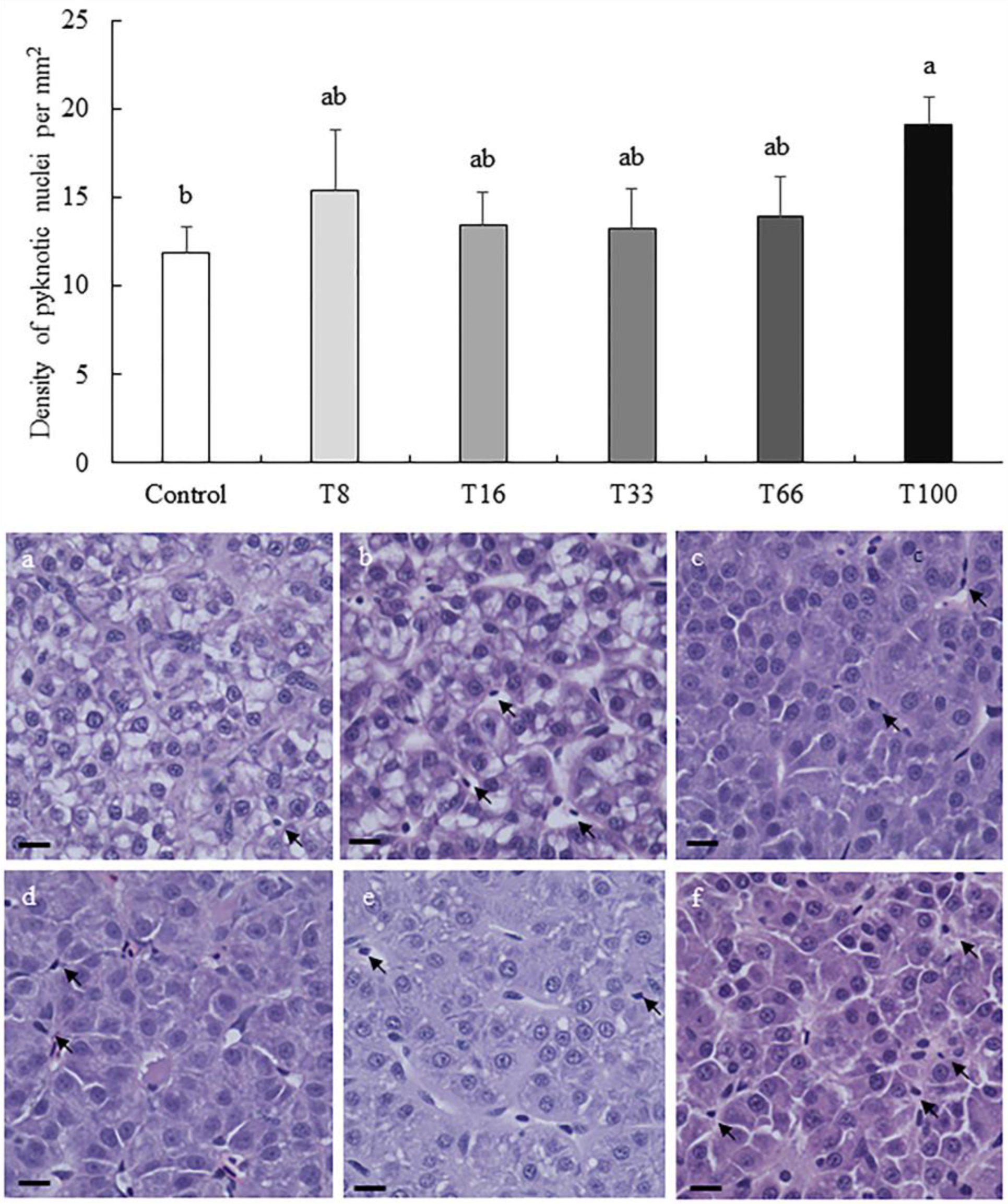
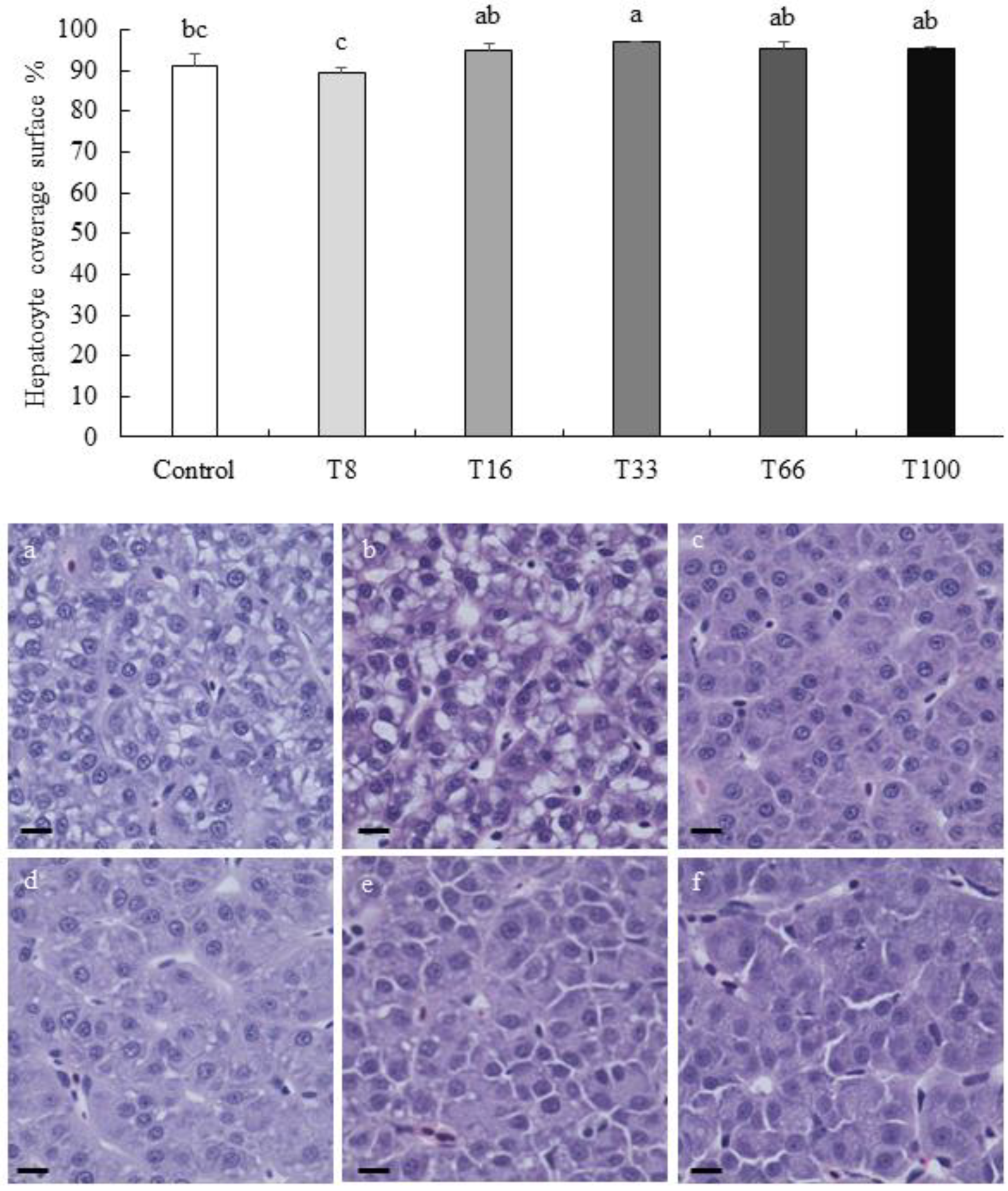
3.4. Histopathological Analysis of Liver and Proximal Intestine
3.5. Postprandial Plasma Levels of Glucose and Triglycerides
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albrektsen, S.; Kortet, R.; Skov, P.V.; Ytteborg, E.; Gitlesen, S.; Kleinegris, D.; Mydland, L.T.; Hansen, J.Ø.; Lock, E.J.; Mørkøre, T.; et al. Future feed resources in sustainable salmonid production: A review. Aquaculture 2022, 14, 1790–1812. [Google Scholar] [CrossRef]
- Turchini, G.M.; Trushenski, J.T.; Glencross, B.D. Thoughts for the future of aquaculture nutrition: Realigning perspectives to reflect contemporary issues related to judicious use of marine resources in aquafeeds. N. Am. J. Aquac. 2019, 81, 13–39. [Google Scholar] [CrossRef]
- Nie, P.; Hallerman, E. Advancing the sustainability of aquaculture. Rev. Aquac. 2021, 13, 781–782. [Google Scholar] [CrossRef]
- Storebakken, T.; Shearer, K.D.; Roem, A.J. Growth, uptake, and retention of nitrogen and phosphorus, and absorption of other minerals in Atlantic salmon Salmo salar fed diets with fish meal and soy-protein concentrate as the main sources of protein. Aquac. Nutr. 2000, 6, 103–108. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Aragão, C.; Gonçalves, A.T.; Costas, B.; Azeredo, R.; Xavier, M.J.; Engrola, S. Alternative proteins for fish diets: Implications beyond growth. Animals 2022, 12, 1211. [Google Scholar] [CrossRef] [PubMed]
- De Visser, C.L.M.; Schreuder, R.; Stoddard, F. The EU’s dependency on soya bean import for the animal feed industry and potential for EU produced alternatives. Oilseeds Fats Crops Lipids 2014, 21, D407. [Google Scholar] [CrossRef]
- Toledo-Solís, F.J.; Hilerio-Ruiz, A.G.; Martinez, F.P.; Barrios, A.; Aznar, M.J.; Larrán, A.M.; Fernández, I.; Moyano, F.J. Selection and improvement of alternative raw materials for rainbow trout (Oncorhynchus mykiss) aquafeeds through a multiparametric screening tool. Anim. Feed. Sci. Technol. 2022, 288, 115284. [Google Scholar] [CrossRef]
- Hammer, K.; Laghetti, G.; Direnzo, P.; Castelli, A.; Mikić, A. Resources and opportunities for re-establishing Lathyrus cicera L. as a multipurpose cultivated plant. Genet. Resour. Crop Evol. 2019, 66, 523–544. [Google Scholar] [CrossRef]
- Hugon, J.; Ludolph, A.C.; Spencer, P.S. B-N-oxalylamino-L-alanine. In Experimental and Clinical Neurotoxicology, 2nd ed.; Spencer, P.S., Schaumburg, H., Eds.; Oxford University Press: New York, NY, USA, 2000; pp. 925–938. [Google Scholar]
- Hanbury, C.D.; White, C.L.; Mullan, B.P.; Siddique, K.H.M. A review of the potential of Lathyrus sativus L. and L. cicera L. grain for use as animal feed. Anim. Feed. Sci. Technol. 2000, 87, 1–27. [Google Scholar] [CrossRef]
- Martín-Pedrosa, M.; Varela, A.; Guillamon, E.; Cabellos, B.; Burbano, C.; Gomez-Fernandez, J.; de Mercado, E.; Gomez-Izquierdo, E.; Cuadrado, C.; Muzquiz, M. Biochemical characterization of legume seeds as ingredients in animal feed. Span. J. Agric. Res. 2016, 14, 0901–0915. [Google Scholar] [CrossRef]
- Maas, R.M.; Verdegem, M.C.; Wiegertjes, G.F.; Schrama, J.W. Carbohydrate utilisation by tilapia: A meta-analytical approach. Aquaculture 2020, 12, 1851–1866. [Google Scholar] [CrossRef]
- Sinha, A.K.; Kumar, V.; Makkar, H.P.; De Boeck, G.; Becker, K. Non-starch polysaccharides and their role in fish nutrition—A review. Food Chem. 2011, 127, 1409–1426. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Kostyniuk, D.J.; Marandel, L.; Jubouri, M.; Dias, K.; de Souza, R.F.; Zhang, D.; Martyniuk, C.J.; Panserat, S.; Mennigen, J.A. Profiling the rainbow trout hepatic miRNAome under diet-induced hyperglycemia. Physiol. Genom. 2019, 51, 411–431. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 2020, 40, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- OJEU. Laying down the Methods of Sampling and Analysis for the Offcial Control of Feed, Commision Regulation (EC) No 152/2009. 2009. Available online: http://data.europa.eu/eli/reg/2009/152/oj (accessed on 1 February 2022).
- Tomás-Almenar, C.; Toledo-Solís, F.J.; Larrán, A.M.; de Mercado, E.; Alarcón, F.J.; Rico, D.; Martín-Diana, A.B.; Fernández, I. Effects and safe inclusion of Narbonne vetch (Vicia narbonensis) in rainbow trout (Oncorhynchus mykiss) diets: Towards a more sustainable aquaculture. Animals 2020, 10, 2175. [Google Scholar] [CrossRef]
- Atkinson, J.L.; Hilton, J.W.; Slinger, S.J. Evaluation of acid-insoluble ash as an indicator of feed digestibility in rainbow trout (Salmo gairdneri). Can. J. Fish. Aquat. Sci. 1984, 41, 1384–1386. [Google Scholar] [CrossRef]
- Martoja, R.; Leland, C.G.; Martoja-Pierson, M. Técnicas de Histología Animal; Toray-Masson S.A.: Barcelona, Spain, 1970; pp. 1–350. [Google Scholar]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. (Eds.) Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- White, C.L.; Hanbury, C.D.; Young, P.; Phillips, N.; Wiese, S.C.; Milton, J.B.; Davidson, R.H.; Siddique, K.H.M.; Harris, D. The nutritional value of Lathyrus cicera and Lupinus angustifolius grain for sheep. Anim. Feed. Sci. Technol. 2002, 99, 45–64. [Google Scholar] [CrossRef]
- Grela, E.R.; Rybiński, W.; Matras, J.; Sobolewska, S. Variability of phenotypic and morphological characteristics of some Lathyrus sativus L. and Lathyrus cicera L. accessions and nutritional traits of their seeds. Genet. Resour. Crop Evol. 2012, 59, 1687–1703. [Google Scholar] [CrossRef]
- Hua, K.; Bureau, D.P. Estimating changes in essential amino acid requirements of rainbow trout and Atlantic salmon as a function of body weight or diet composition using a novel factorial requirement model. Aquaculture 2019, 513, 734440. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Ortega-Barrales, P.; Zengin, G.; Mocan, A.; Simirgiotis, M.J.; Ceylan, R.; Uysal, S.; Aktumsek, A. Evaluation of antioxidant potential, enzyme inhibition activity and phenolic profile of Lathyrus cicera and Lathyrus digitatus: Potential sources of bioactive compounds for the food industry. Food Chem. Toxicol. 2017, 107, 609–619. [Google Scholar] [CrossRef]
- Sacristán, M.; Varela, A.; Pedrosa, M.M.; Burbano, C.; Cuadrado, C.; Legaz, M.E.; Muzquiz, M. Determination of β-n-oxalyl-l-α, β-diaminopropionic acid and homoarginine in Lathyrus sativus and Lathyrus cicera by capillary zone electrophoresis. J. Sci. Food Agric. 2015, 95, 1414–1420. [Google Scholar] [CrossRef]
- Suman; Ahmad, Y.; Nain, V. A convenient and robust protocol for preparation of ODAP-free Lathyrus sativus protein. Anal. Biochem. 2020, 591, 113544. [Google Scholar] [CrossRef]
- Barse, A.V.; Srivastava, P.P.; Jadhao, S.B.; Jain, K.K. Toxicity and histopathology studies of BOAA in Labeo rohita. Toxicol. Sci. 2002, 66, 27–28. [Google Scholar]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; De Boeck, G.; Becker, K. Phytate and phytase in fish nutrition. J. Anim. Physiol. Anim. Nutr. 2011, 96, 335–364. [Google Scholar] [CrossRef] [PubMed]
- Morales, G.A.; Denstadli, V.; Collins, S.A.; Mydland, L.T.; Moyano, F.J.; Øverland, M. Phytase and sodium diformate supplementation in a plant-based diet improves protein and mineral utilization in rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2016, 22, 1301–1311. [Google Scholar] [CrossRef]
- Barse, A.V.; Jadhao, S.B.; Sahu, N.P.; Srivastava, P.P.; Jain, K.K.; Pal, A.K. Responses of Labeo rohita to dietary Lathyrus sativus seeds. Asian Australas. J. Anim. Sci. 2004, 17, 127–130. [Google Scholar] [CrossRef]
- Magalhaes, S.C.Q.; Cabrita, A.R.J.; Valentao, P.; Andrade, P.B.; Rema, P.; Maia, M.R.G.; Valente, L.M.P.; Fonseca, A.J.M. Apparent digestibility coefficients of European grain legumes in rainbow trout (Oncorhynchus mykiss) and Nile tilapia (Oreochromis niloticus). Aquac. Nutr. 2018, 24, 332–340. [Google Scholar] [CrossRef]
- Ramachandran, S.; Bairagi, A.; Ray, A.K. Improvement of nutritive value of grass pea (Lathyrus sativus) seed meal in the formulated diets for rohu, Labeo rohita (Hamilton) fingerlings after fermentation with a fish gut bacterium. Bioresour. Technol. 2005, 96, 1465–1472. [Google Scholar] [CrossRef]
- Ramachandran, S.; Ray, A. Effect of different processing techniques on the nutritive value of grass pea, Lathyrus sativus L., seed meal in compound diets for Indian major carp rohu, (Hamilton), Fingerlings. Fish. Aquat. Life 2008, 16, 189–202. [Google Scholar] [CrossRef][Green Version]
- Glencross, B.; Rutherford, N.; Bourne, N. The influence of various starch and non-starch polysaccharides on the digestibility of diets fed to rainbow trout (Oncorhynchus mykiss). Aquaculture 2012, 356, 141–146. [Google Scholar] [CrossRef]
- Lannuzel, C.; Smith, A.; Mary, A.L.; Della Pia, E.A.; Kabel, M.A.; de Vries, S. Improving fiber utilization from rapeseed and sunflower seed meals to substitute soybean meal in pig and chicken diets: A review. Anim. Feed. Sci. Technol. 2022, 285, 115213. [Google Scholar] [CrossRef]
- Toledo-Solís, F.J.; Larrán, A.M.; Martín, B.; López de la Cuesta, P.; Mateos-Aparicio, I.; Pérez, V.; Moyano, F.J.; Fernández, I. Uncovering the physiological impacts of soybean meal replacement by Narbonne vetch (Vicia narbonensis) meal in rainbow trout (Oncorhynchus mykiss) diets: Towards the future and sustainable European aquaculture. Anim. Feed. Sci. Technol. 2023, 296, 115555. [Google Scholar] [CrossRef]
- Leenhouwers, J.I.; Adjei-Boateng, D.; Verreth, J.A.J.; Schrama, J.W. Digesta viscosity, nutrient digestibility and organ weights in African catfish (Clarias gariepinus) fed diets supplemented with different levels of a soluble non-starch polysaccharide. Aquac. Nutr. 2006, 12, 111–116. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, X.; Sun, Y.; Mi, H.; Zhang, L. Effects of different types of non-starch polysaccharides on growth, digestive enzyme activity, intestinal barrier function and antioxidant activity of rainbow trout (Oncorhynchus mykiss). Aquac. Rep. 2021, 21, 100864. [Google Scholar] [CrossRef]
- Staessen, T.W.O.; Verdegem, M.C.J.; Schrama, J.W. Effect of dietary NSP level and bile acid supplementation on nutrient digestibility and the bile acid metabolism in rainbow trout (Oncorhynchus mykiss). Aquaculture 2022, 561, 738724. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Campbell, C.G. Genotype variation in BOAA, condensed tannins, phenolics and enzyme inhibitors of grass pea (Lathyrus sativus). Can. J. Plant Sci. 1992, 72, 1037–1047. [Google Scholar] [CrossRef]
- Liu, D.; Gao, H.; Tang, W.; Nie, S. Plant non-starch polysaccharides that inhibit key enzymes linked to type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2017, 1401, 28–36. [Google Scholar] [CrossRef]
- Ren, S.; Cai, C.; Cui, G.; Ni, Q.; Jiang, R.; Su, X.; Wang, Q.; Chen, W.; Zhang, J.; Wu, P.; et al. High dosages of pectin and cellulose cause different degrees of damage to the livers and intestines of Pelteobagrus fulvidraco. Aquaculture 2020, 514, 734445. [Google Scholar] [CrossRef]
- Vlachostergios, D.N.; Lithourgidis, A.S.; Dordas, C.A. Agronomic, forage quality and economic advantages of red pea (Lathyrus cicera L.) intercropping with wheat and oat under low-input farming. Grass Forage Sci. 2018, 73, 777–788. [Google Scholar] [CrossRef]

| Ingredients (g/100 g) | Diets | |||||
|---|---|---|---|---|---|---|
| Control | T8 | T16 | T33 | T66 | T100 | |
| Fishmeal LT | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| Red vetchling | 0.00 | 2.50 | 5.00 | 10.00 | 20.00 | 30.00 |
| Soybean meal | 30.00 | 27.50 | 25.00 | 20.00 | 10.00 | 0.00 |
| Wheat gluten | 12.02 | 13.02 | 14.01 | 16.01 | 19.99 | 23.98 |
| Wheat meal | 13.46 | 12.37 | 11.27 | 9.27 | 5.13 | 0.99 |
| Fish oil | 6.75 | 6.75 | 6.75 | 6.75 | 6.75 | 6.75 |
| Vegetable oil | 6.75 | 6.75 | 6.75 | 6.75 | 6.75 | 6.75 |
| Soy lecithin | 1.45 | 1.43 | 1.40 | 1.35 | 1.25 | 1.14 |
| Premix a | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Binder b | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Methionine | 0.52 | 0.54 | 0.54 | 0.56 | 0.56 | 0.56 |
| Lysine | 0.05 | 0.12 | 0.18 | 0.31 | 0.57 | 0.83 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Proximate composition (% on dry matter) | ||||||
| Moisture | 5.56 ± 0.33 | 3.98 ± 0.15 | 4.10 ± 0.25 | 4.79 ± 0.35 | 4.93 ± 0.30 | 5.27 ± 0.11 |
| Crude protein | 42.67 ± 0.92 | 42.34 ± 0.38 | 42.34 ± 0.47 | 42.61 ± 1.60 | 42.43 ± 1.26 | 42.78 ± 0.63 |
| Crude fat | 20.82 ± 0.18 | 22.01 ± 0.33 | 21.06 ± 0.71 | 20.86 ± 0.20 | 20.85 ± 0.16 | 20.21 ± 0.31 |
| Crude fiber | 1.91 ± 0.16 | 1.81 ± 0.20 | 1.69 ± 0.15 | 1.98 ± 0.14 | 1.91 ± 0.00 | 1.91 ± 0.18 |
| Ash | 8.17 ± 0.05 | 7.63 ± 0.25 | 8.46 ± 0.17 | 7.30 ± 1.05 | 7.12 ± 0.29 | 8.37 ± 0.25 |
| Proximate Composition (% on Dry Matter) | |
|---|---|
| Moisture | 3.62 ± 0.22 |
| Crude protein | 23.82 ± 0.47 |
| Crude fat | 2.03 ± 0.06 |
| Ash | 3.78 ± 0.07 |
| Nfe * | 70.37 ± 0.39 |
| Amino acid content (g/100 g ingredient) | |
| Essential amino acid | |
| Arginine | 1.88 ± 0.12 |
| Histidine | 0.38 ± 0.02 |
| Isoleucine | 0.72 ± 0.12 |
| Leucine | 1.45 ± 0.09 |
| Lysine | 1.75 ± 0.10 |
| Phenylalanine | 0.88 ± 0.04 |
| Valine | 1.07 ± 0.11 |
| Methionine | 0.24 ± 0.01 |
| Threonine | 0.91 ± 0.05 |
| Non-essential amino acid | |
| Alanine | 1.21 ± 0.05 |
| Aspartic acid | 2.75 ± 0.06 |
| Glutamic acid | 4.19 ± 0.12 |
| Glycine | 0.97 ± 0.04 |
| Serine | 1.26 ± 0.02 |
| Tyrosine | 0.71 ± 0.02 |
| Cysteine | 0.15 ± 0.01 |
| Proline | 1.89 ± 0.20 |
| NSPs (mg/g Ingredient) | Soluble | Insoluble | Total |
|---|---|---|---|
| Rhamnose | nd | nd | nd |
| Fucose | nd | nd | nd |
| Arabinose | 0.84 ± 0.18 | 1.98 ± 0.18 | 2.79 ± 0.33 |
| Xylose | 0.31 ± 0.13 | 0.68 ± 0.12 | 1.03 ± 0.17 |
| Mannose | 0.26 ± 0.05 | nd | 0.26 ± 0.05 |
| Galactose | 0.14 ± 0.04 | 0.24 ± 0.05 | 0.38 ± 0.05 |
| Glucose | 0.79 ± 0.18 | 3.15 ± 0.32 | 3.82 ± 0.36 |
| Galacturonic acid | 0.46 ± 0.22 | 1.71 ± 0.20 | 2.28 ± 0.46 |
| Total | 2.78 ± 0.46 | 7.68 ± 0.66 | 10.40 ± 0.84 |
| Amino Acid Content (g/100 g Ingredient) | Diets | |||||
|---|---|---|---|---|---|---|
| Control | T8 | T16 | T33 | T66 | T100 | |
| Essential amino acid | ||||||
| Arginine | 2.10 ± 0.01 | 2.12 ± 0.05 | 1.97 ± 0.01 | 1.99 ± 0.01 | 1.92 ± 0.01 | 1.87 ± 0.02 |
| Histidine | 1.03 ± 0.38 | 1.09 ± 0.44 | 1.52 ± 0.32 | 1.21 ± 0.08 | 1.31 ± 0.08 | 1.27 ± 0.09 |
| Isoleucine | 1.77 ± 0.01 | 1.80 ± 0.10 | 1.65 ± 0.03 | 1.62 ± 0.06 | 1.65 ± 0.01 | 1.63 ± 0.13 |
| Leucine | 2.89 ± 0.07 a | 2.92 ± 0.03 a | 2.63 ± 0.05 b | 2.63 ± 0.07 b | 2.64 ± 0.00 b | 2.73 ± 0.05 ab |
| Lysine | 2.81 ± 0.06 | 2.84 ± 0.02 | 2.87 ± 0.12 | 2.73 ± 0.11 | 2.87 ± 0.05 | 2.95 ± 0.08 |
| Phenylalanine | 1.96 ± 0.15 | 1.95 ± 0.20 | 1.98 ± 0.10 | 1.93 ± 0.02 | 1.96 ± 0.04 | 2.00 ± 0.02 |
| Valine | 2.42 ± 0.04 | 2.36 ± 0.05 | 2.19 ± 0.10 | 2.20 ± 0.09 | 2.24 ± 0.05 | 2.21 ± 0.13 |
| Methionine | 1.13 ± 0.10 | 1.16 ± 0.10 | 1.03 ± 0.11 | 1.08 ± 0.04 | 1.17 ± 0.09 | 1.12 ± 0.03 |
| Threonine | 1.28 ± 0.11 | 1.35 ± 0.12 | 1.13 ± 0.07 | 1.14 ± 0.12 | 1.11 ± 0.05 | 1.24 ± 0.12 |
| Non-essential amino acid | ||||||
| Alanine | 2.07 ± 0.01 a | 2.07 ± 0.00 a | 1.93 ± 0.11 ab | 1.93 ± 0.03 ab | 1.88 ± 0.07 ab | 1.86 ± 0.02 b |
| Aspartic acid | 3.40 ± 0.06 a | 3.37 ± 0.03 a | 2.96 ± 0.11 b | 2.93 ± 0.06 b | 2.78 ± 0.00 bc | 2.69 ± 0.04 c |
| Glutamic acid | 8.21 ± 0.11 bc | 8.25 ± 0.08 bc | 7.65 ± 0.25 c | 8.07 ± 0.22 bc | 8.54 ± 0.09 b | 9.20 ± 0.08 a |
| Glycine | 1.91 ± 0.04 ab | 1.90 ± 0.01 ab | 1.95 ± 0.10 ab | 2.02 ± 0.06 a | 1.98 ± 0.02 ab | 1.77 ± 0.05 b |
| Serine | 1.38 ± 0.31 | 1.57 ± 0.31 | 1.26 ± 0.28 | 1.28 ± 0.36 | 1.23 ± 0.10 | 1.59 ± 0.29 |
| Tyrosine | 1.02 ± 0.03 | 1.12 ± 0.01 | 1.12 ± 0.19 | 1.10 ± 0.09 | 1.08 ± 0.02 | 1.16 ± 0.06 |
| Cysteine | 0.34 ± 0.02 | 0.35 ± 0.02 | 0.32 ± 0.01 | 0.33 ± 0.00 | 0.34 ± 0.00 | 0.35 ± 0.00 |
| Proline | 1.08 ± 0.01 | 1.13 ± 0.02 | 1.07 ± 0.00 | 1.19 ± 0.05 | 1.17 ± 0.04 | 1.26 ± 0.03 |
| Day | Parameter | Diets | |||||
|---|---|---|---|---|---|---|---|
| Control | T8 | T16 | T33 | T66 | T100 | ||
| 0 | IBW (g) | 10.36 ± 0.16 | 10.25 ± 0.05 | 10.37 ± 0.08 | 10.34 ± 0.04 | 10.37 ± 0.13 | 10.34 ± 0.05 |
| IFL (cm) | 9.49 ± 0.08 | 9.44 ± 0.07 | 9.47 ± 0.05 | 9.45 ± 0.04 | 9.4 ± 0.06 | 9.45 ± 0.03 | |
| 90 | FBW (g) | 150.66 ± 2.27 a | 144.90 ± 13.20 a | 146.05 ± 8.28 a | 142.14 ± 3.60 a | 140.38 ± 8.74 a | 100.52 ± 5.78 b |
| FFL (cm) | 22.69 ± 0.16 a | 22.39 ± 0.62 a | 22.34 ± 0.42 a | 22.03 ± 0.14 a | 22.11 ± 0.28 a | 20.15 ± 0.35 b | |
| WG (%) | 1357.79 ± 18.21 a | 1313.70 ± 136.29 a | 1308.58 ± 87.12 a | 1285.39 ± 36.59 a | 1254.58 ± 97.69 a | 871.87 ± 60.07 b | |
| SGR (%/day) | 2.98 ± 0.01 a | 2.94 ± 0.11 a | 2.94 ± 0.07 a | 2.91 ± 0.04 a | 2.89 ± 0.08 a | 2.53 ± 0.07 b | |
| FCR | 0.79 ± 0.01 a | 0.80 ± 0.03 a | 0.80 ± 0.02 a | 0.81 ± 0.01 a | 0.83 ± 0.03 a | 1.00 ± 0.04 b | |
| CF | 1.29 ± 0.02 a | 1.29 ± 0.02 a | 1.31 ± 0.01 a | 1.33 ± 0.01 a | 1.30 ± 0.03 a | 1.23 ± 0.01 b | |
| HSI (%) | 1.42 ± 0.12 a | 1.35 ± 0.04 ab | 1.30 ± 0.08 ab | 1.20 ± 0.10 ab | 1.13 ± 0.02 b | 1.29 ± 0.09 ab | |
| VSI (%) | 12.00 ± 0.90 | 11.81 ± 0.58 | 11.16 ± 0.28 | 10.87 ± 0.25 | 10.19 ± 2.62 | 11.69 ± 1.52 | |
| Apparent digestibility of the protein and proximate composition of the fish fillet (%) | |||||||
| ADCprotein 1 | 92.89 ± 0.18 b | 91.66 ± 0.18 b | 94.38 ± 0.52 a | 92.44 ± 0.47 b | 93.88 ± 0.38 a | 90.03 ± 0.84 c | |
| Moisture | 1.74 ± 0.15 ab | 1.18 ± 0.13 b | 1.04 ± 0.06 b | 2.41 ± 0.88 a | 1.74 ± 0.35 ab | 0.68 ± 0.28 b | |
| Crude protein | 15.56 ± 0.21 a | 16.31 ± 0.54 a | 13.06 ± 0.04 b | 12.5 ± 0.50 b | 11.44 ± 0.19 c | 11.06 ± 0.31 c | |
| Ash | 4.69 ± 0.06 a | 4.48 ± 0.05 a | 4.62 ± 0.38 a | 4.58 ± 0.43 a | 3.25 ± 0.15 b | 2.66 ± 0.15 b | |
| Amino Acid Content (g/100 g Dry Ingredient) | Diets | |||||
|---|---|---|---|---|---|---|
| Control | T8 | T16 | T33 | T66 | T100 | |
| Essential amino acid | ||||||
| Arginine | 3.33 ± 0.24 | 3.73 ± 0.05 | 3.83 ± 0.14 | 3.80 ± 0.31 | 3.86 ± 0.34 | 4.06 ± 0.44 |
| Histidine | 2.90 ± 0.33 | 2.79 ± 0.47 | 2.81 ± 0.49 | 2.86 ± 0.40 | 3.05 ± 0.50 | 2.13 ± 0.72 |
| Isoleucine | 4.31 ± 0.32 | 4.38 ± 0.09 | 4.58 ± 0.16 | 4.32 ± 0.32 | 4.37 ± 0.13 | 4.68 ± 0.42 |
| Leucine | 7.62 ± 0.44 | 7.24 ± 0.34 | 7.51 ± 0.67 | 7.22 ± 0.37 | 7.69 ± 0.36 | 7.69 ± 0.57 |
| Lysine | 6.82 ± 0.59 | 6.91 ± 0.76 | 7.08 ± 0.31 | 6.96 ± 0.45 | 7.12 ± 0.81 | 8.08 ± 0.64 |
| Phenylalanine | 3.55 ± 0.13 | 3.56 ± 0.34 | 3.70 ± 0.14 | 3.53 ± 0.19 | 3.69 ± 0.30 | 4.12 ± 0.50 |
| Valine | 3.55 ± 0.18 | 3.36 ± 0.15 | 3.52 ± 0.33 | 3.31 ± 0.17 | 3.53 ± 0.15 | 3.53 ± 0.28 |
| Methionine | 2.72 ± 0.14 | 2.75 ± 0.36 | 2.86 ± 0.09 | 2.63 ± 0.19 | 2.76 ± 0.30 | 3.23 ± 0.54 |
| Threonine | 2.28 ± 0.17 | 2.16 ± 0.04 | 2.26 ± 0.22 | 2.31 ± 0.16 | 2.50 ± 0.22 | 2.41 ± 0.33 |
| Non-essential amino acid | ||||||
| Alanine | 3.52 ± 0.11 | 3.38 ± 0.42 | 3.46 ± 0.26 | 3.37 ± 0.20 | 3.47 ± 0.37 | 3.09 ± 0.12 |
| Aspartic acid | 6.60 ± 1.18 | 6.67 ± 0.78 | 6.65 ± 0.21 | 7.16 ± 0.46 | 7.50 ± 0.61 | 7.06 ± 0.47 |
| Glutamic acid | 9.55 ± 0.72 | 9.84 ± 0.54 | 9.97 ± 0.17 | 10.27 ± 0.74 | 10.47 ± 0.51 | 10.51 ± 0.69 |
| Glycine | 2.91 ± 0.28 | 2.95 ± 0.26 | 3.02 ± 0.12 | 3.06 ± 0.23 | 3.17 ± 0.29 | 2.89 ± 0.01 |
| Serine | 4.21 ± 0.80 | 3.81 ± 0.32 | 3.96 ± 0.64 | 4.08 ± 0.31 | 4.33 ± 0.46 | 3.73 ± 0.30 |
| Tyrosine | 1.75 ± 0.63 b | 2.36 ± 0.40 ab | 2.49 ± 0.02 ab | 2.05 ± 0.58 ab | 1.83 ± 0.85 b | 2.89 ± 0.24 a |
| Cysteine | 0.21 ± 0.03 | 0.22 ± 0.02 | 0.26 ± 0.00 | 0.28 ± 0.09 | 0.28 ± 0.10 | 0.36 ± 0.11 |
| Proline | 2.22 ± 0.10 | 2.06 ± 0.02 | 2.15 ± 0.29 | 2.00 ± 0.19 | 2.05 ± 0.11 | 2.01 ± 0.13 |
| Parameter | Diets | |||||
|---|---|---|---|---|---|---|
| Control | T8 | T16 | T33 | T66 | T100 | |
| Width of submucosa layer (µm) | 8.82 ± 0.72 | 8.17 ± 0.54 | 8.90 ± 0.66 | 7.93 ± 0.81 | 6.99 ± 1.50 | 6.06 ± 1.86 |
| Width of serosa layer (µm) | 13.44 ± 2.62 | 16.54 ± 1.79 | 15.83 ± 3.28 | 15.66 ± 0.63 | 13.46 ± 0.65 | 12.47 ± 1.80 |
| Height of villi (µm) | 343.63 ± 69.91 | 357.68 ± 35.12 | 354.80 ± 15.05 | 338.31± 27.69 | 341.42 ± 28.35 | 339.13 ± 16.04 |
| Height of enterocytes (µm) | 14.46 ± 1.49 | 15.47 ± 0.26 | 15.05 ± 0.74 | 15.23 ± 0.38 | 15.20 ± 1.65 | 14.54 ± 0.94 |
| Density of goblet cells (cells/mm) | 59.11 ± 5.32 | 58.20 ± 7.30 | 61.82 ± 18.04 | 56.79 ± 8.56 | 50.33 ± 5.75 | 61.61 ± 8.30 |
| Parameter | Diet | Time Post-Feeding | ||
|---|---|---|---|---|
| 3 h | 6 h | 24 h | ||
| Glucose (mg/dL) | Control | 100.37 ± 10.22 ab | 79.90 ± 2.41 ab | 100.18 ± 13.18 a |
| T8 | 117.31 ± 12.14 a | 71.35 ± 5.23 bc | 93.54 ± 11.97 a | |
| T16 | 90.53 ± 7.77 bc | 86.26 ± 4.57 a | 81.71 ± 2.94 ab | |
| T33 | 80.47 ± 3.87 bc | 71.48 ± 8.70 bc | 81.13 ± 8.46 ab | |
| T66 | 73.59 ± 9.97 c | 69.55 ± 6.33 bc | 66.91 ± 7.23 b | |
| T100 | 73.82 ± 7.46 c | 62.45 ± 3.11 c | 67.82 ± 5.33 b | |
| Triglycerides (mg/dL) | Control | 377.78 ± 48.02 a | 369.62 ± 52.25 a | 273.61 ± 85.61 |
| T8 | 229.72 ± 36.48 b | 314.78 ± 31.31 ab | 225.63 ± 29.77 | |
| T16 | 213.31 ± 49.73 bc | 29255 ± 12.06 b | 260.10 ± 43.93 | |
| T33 | 223.00 ± 20.75 bc | 180.26 ± 14.58 c | 225.63 ± 68.79 | |
| T66 | 203.75 ± 48.33 bc | 177.30 ± 35.47 c | 260.10 ± 43.93 | |
| T100 | 134.75 ± 24.68 c | 219.27 ± 12.28 c | 225.63 ± 68.79 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledo-Solís, F.J.; Mokhles Abadi Farahani, A.; Yagüe, S.; Mateos-Aparicio, I.; Pérez, V.; Larrán, A.M.; Moyano, F.J.; Fernández, I. Red Vetchling (Lathyrus cicera L.), a Promising Crop for the Sustainable Replacement of Soybean Meal and Reducing the Carbon Footprint of European Aquafeeds. Animals 2023, 13, 3178. https://doi.org/10.3390/ani13203178
Toledo-Solís FJ, Mokhles Abadi Farahani A, Yagüe S, Mateos-Aparicio I, Pérez V, Larrán AM, Moyano FJ, Fernández I. Red Vetchling (Lathyrus cicera L.), a Promising Crop for the Sustainable Replacement of Soybean Meal and Reducing the Carbon Footprint of European Aquafeeds. Animals. 2023; 13(20):3178. https://doi.org/10.3390/ani13203178
Chicago/Turabian StyleToledo-Solís, Francisco Javier, Amin Mokhles Abadi Farahani, Sara Yagüe, Inmaculada Mateos-Aparicio, Valentín Pérez, Ana María Larrán, Francisco Javier Moyano, and Ignacio Fernández. 2023. "Red Vetchling (Lathyrus cicera L.), a Promising Crop for the Sustainable Replacement of Soybean Meal and Reducing the Carbon Footprint of European Aquafeeds" Animals 13, no. 20: 3178. https://doi.org/10.3390/ani13203178
APA StyleToledo-Solís, F. J., Mokhles Abadi Farahani, A., Yagüe, S., Mateos-Aparicio, I., Pérez, V., Larrán, A. M., Moyano, F. J., & Fernández, I. (2023). Red Vetchling (Lathyrus cicera L.), a Promising Crop for the Sustainable Replacement of Soybean Meal and Reducing the Carbon Footprint of European Aquafeeds. Animals, 13(20), 3178. https://doi.org/10.3390/ani13203178






