Fibroblast Growth Factor 21 Has a Diverse Role in Energetic and Reproductive Physiological Functions of Female Beef Cattle
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.1.1. Experiment 1
2.1.2. Experiment 2
2.1.3. Experiment 3
2.1.4. Experiment 4
2.2. Harvest of Serum and Plasma
2.3. Hormone and Metabolite Assays
3. Results
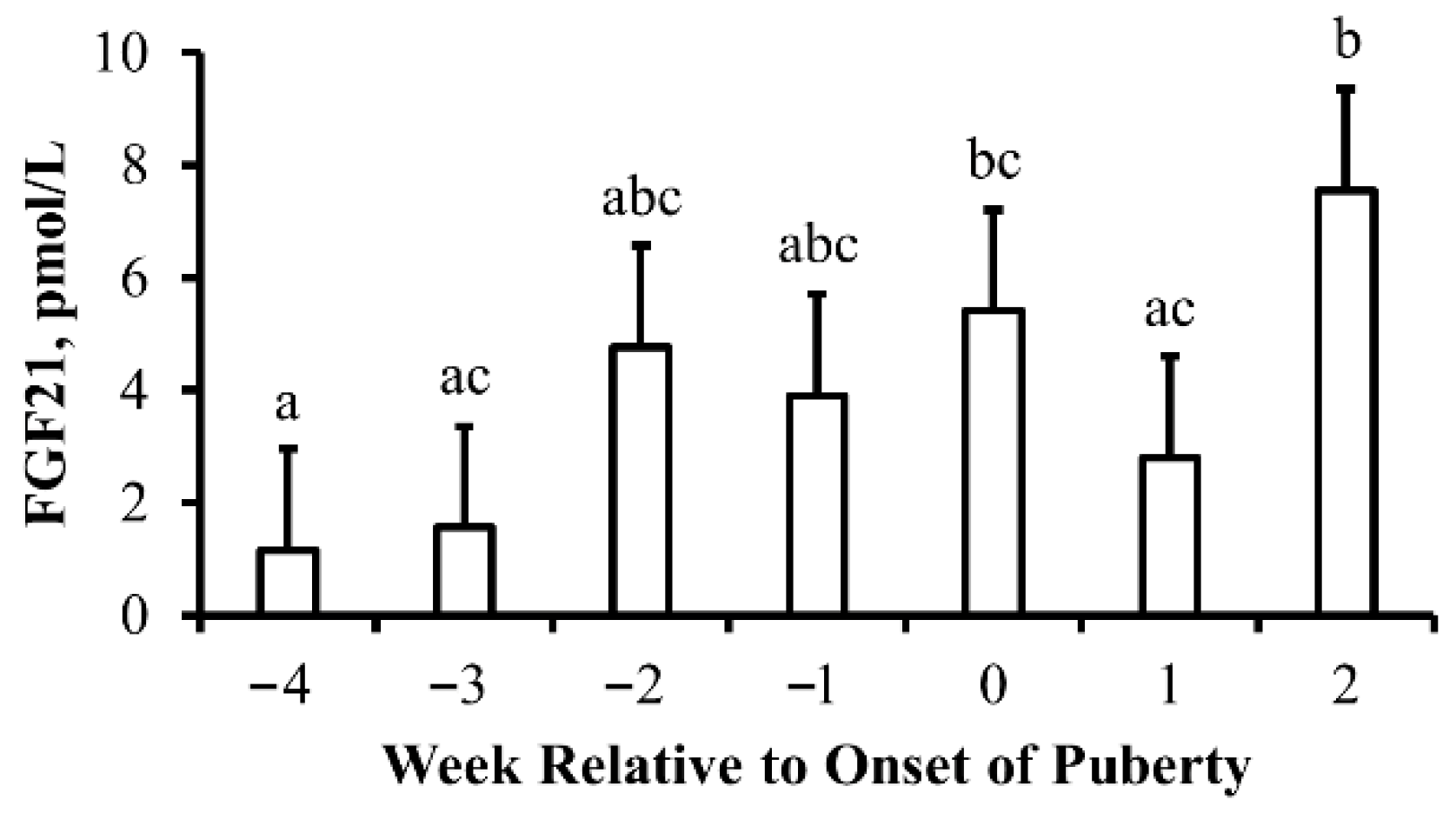
3.1. Experiment 1
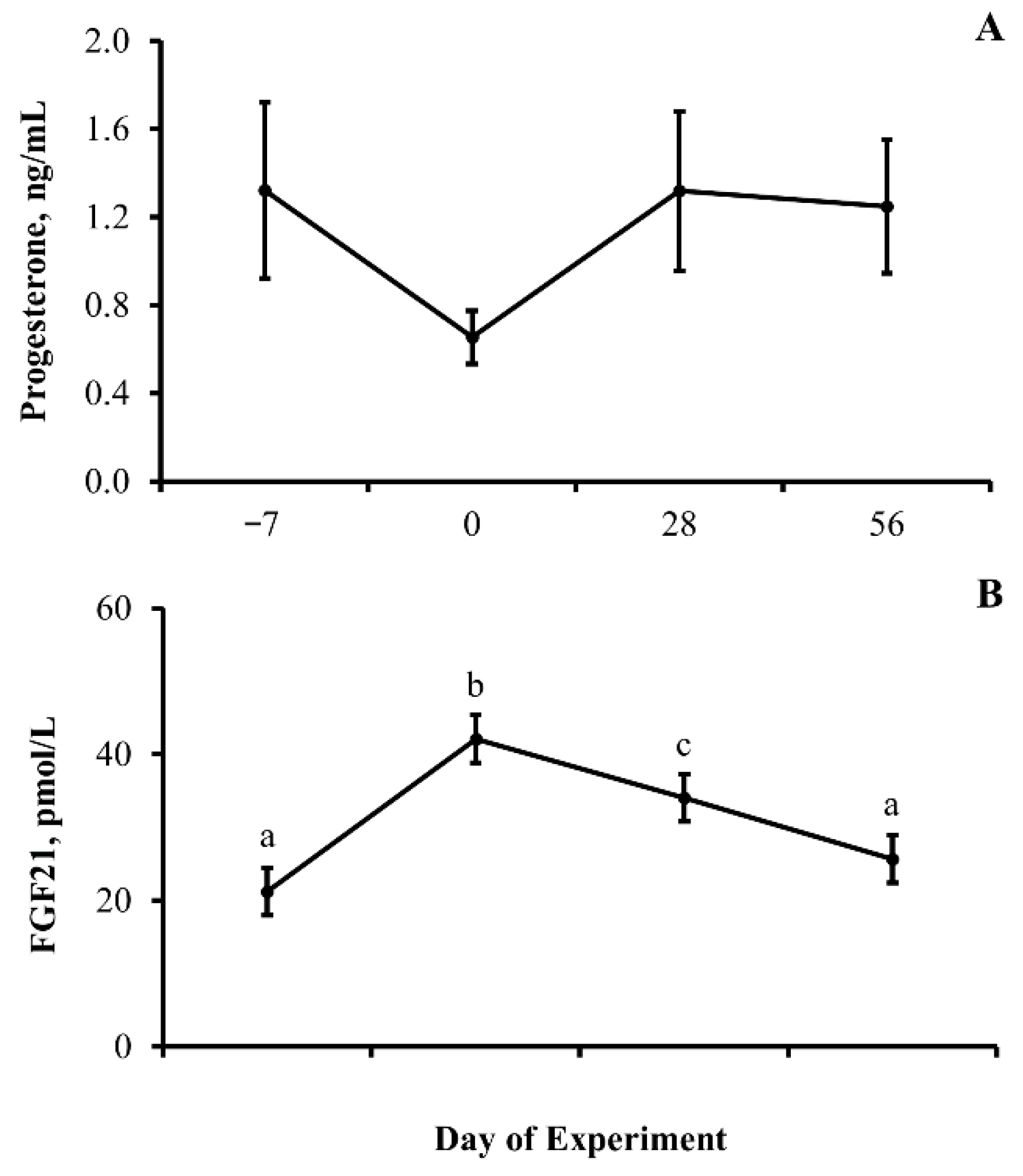
3.2. Experiment 2
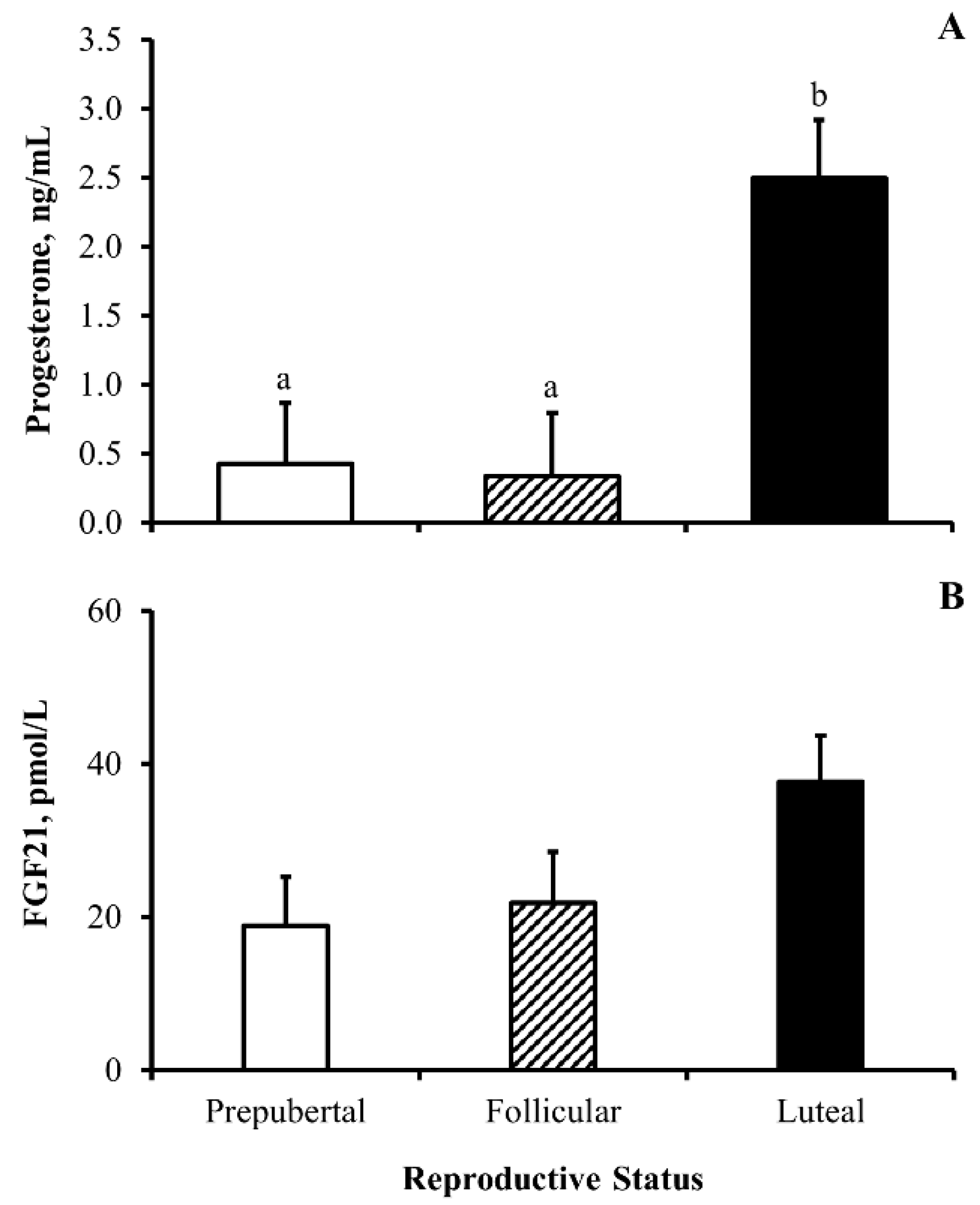
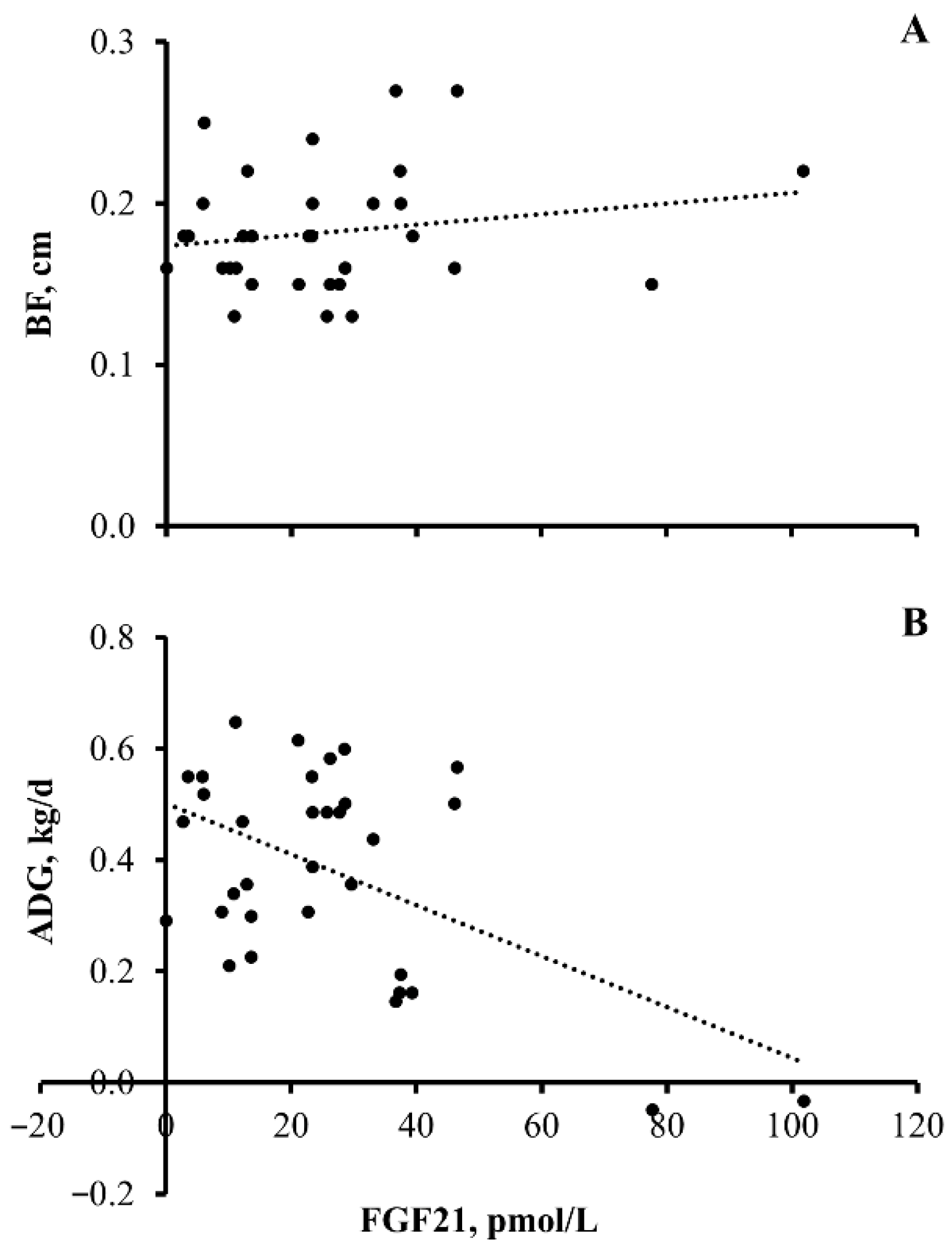
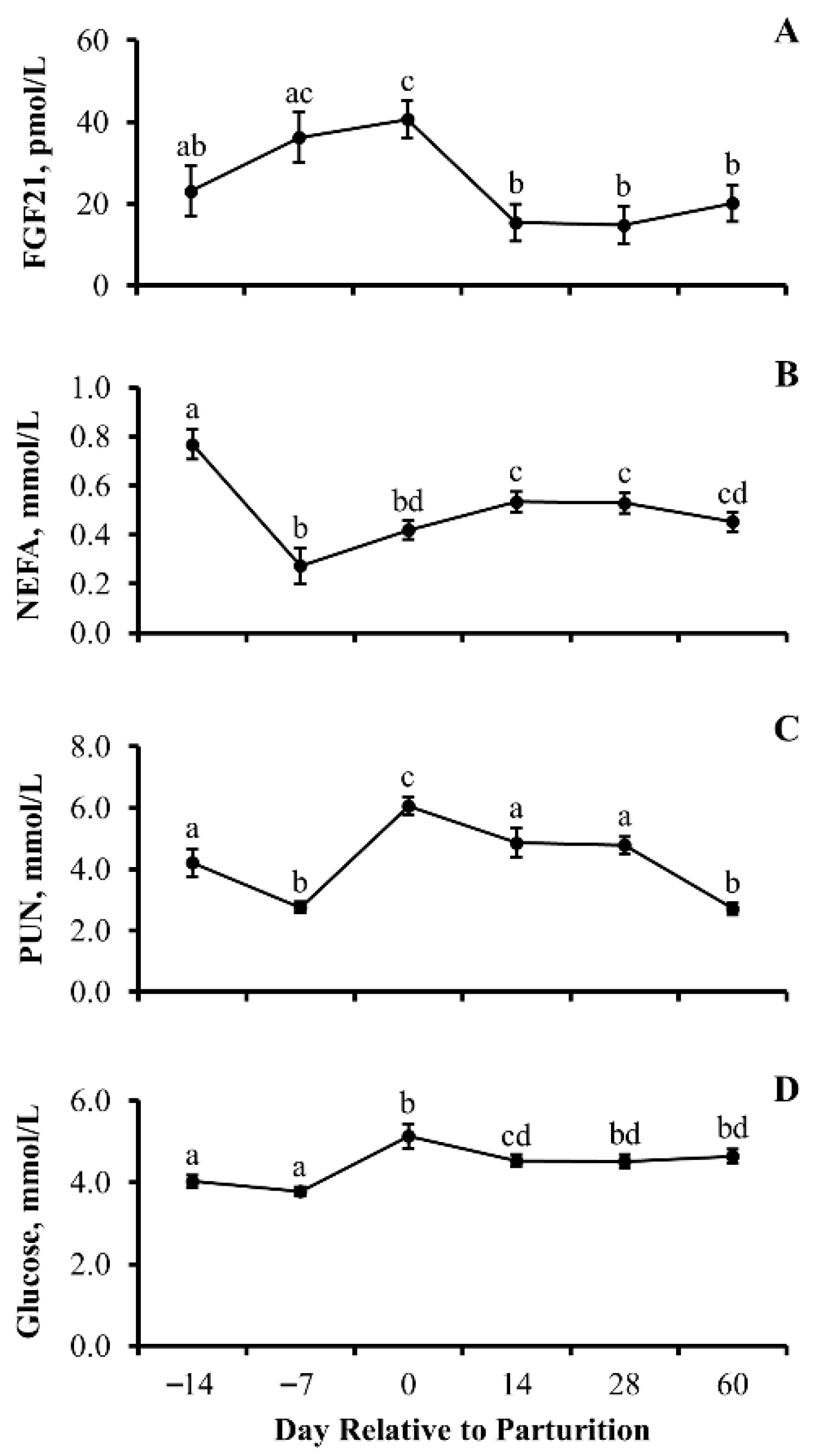
3.3. Experiment 3
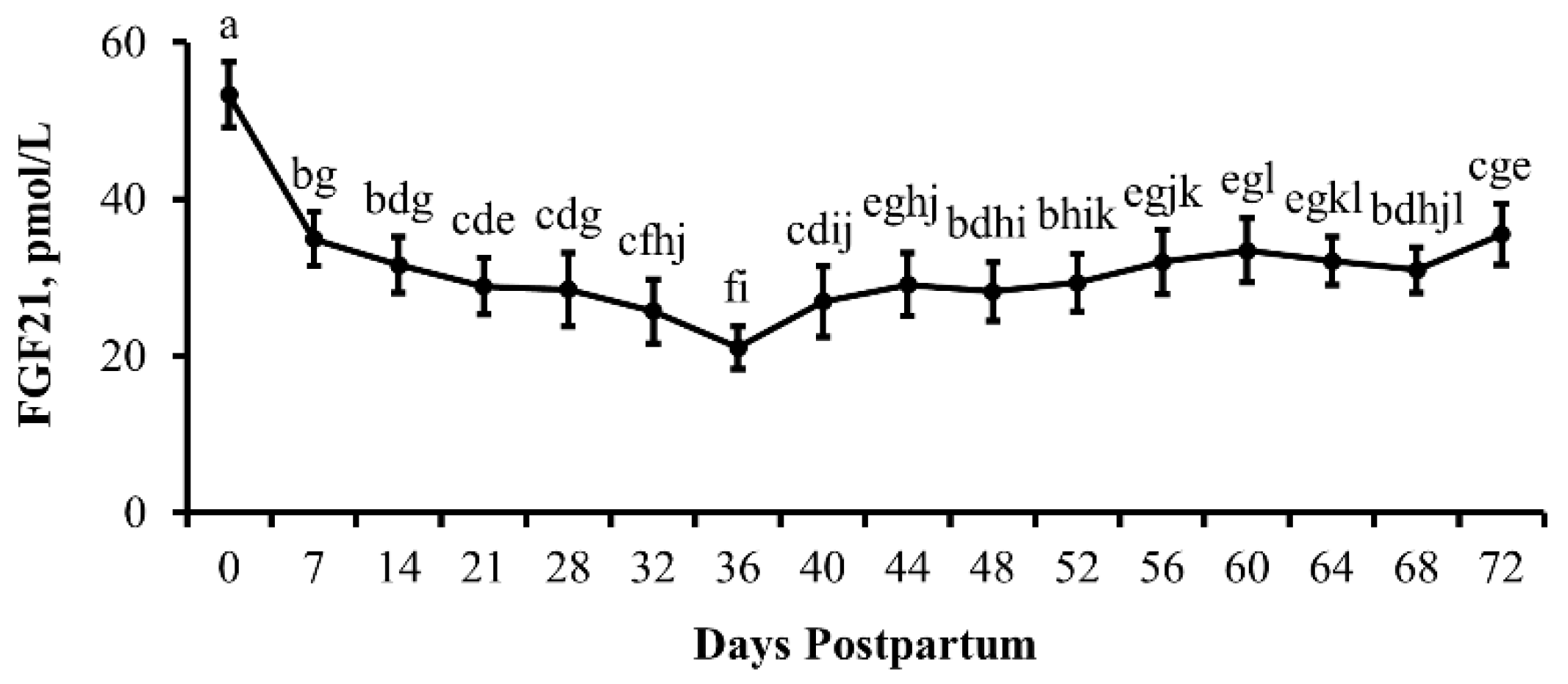
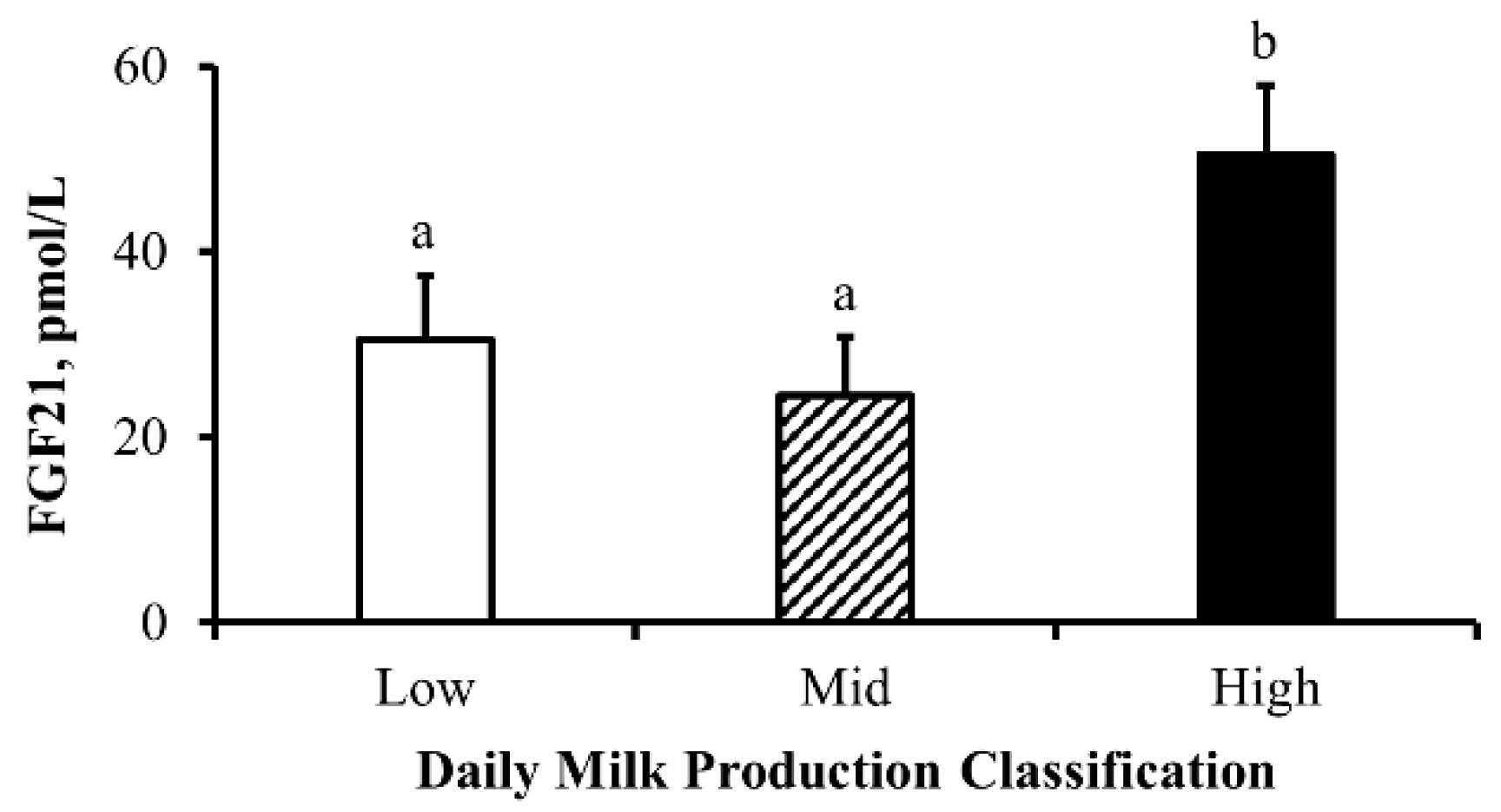
3.4. Experiment 4
4. Discussion
4.1. Pubertal Transition
4.2. Nutritional Transition
4.3. Heifer Reproductive Status
4.4. Heifer Nutrient Utilization
4.5. Periparturient Transitional Metabolites
4.6. Postpartum Resumption of Reproductive Cyclicity
4.7. Lactational Performance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosure
References
- Baldwin, R.L.; Bywater, A.C. Nutritional energetics of animals. Annu. Rev. Nutr. 1984, 4, 101–114. [Google Scholar] [CrossRef]
- Owen, B.M.; Bookout, A.L.; Ding, X.; Lin, V.Y.; Atkin, S.D.; Gautron, L.; Kliewer, S.A.; Mangelsdorf, D.J. FGF21 contributes to neuroendocrine control of female reproduction. Nat. Med. 2013, 19, 1153–1156. [Google Scholar] [CrossRef]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Jacometo, C.B.; Graugnard, D.E.; Corrêa, M.N.; Schmitt, E.; Cardoso, F.; Loor, J.J. Overfeeding dairy cattle during late-pregnancy alters hepatic PPARα-regulated pathways including hepatokines: Impact on metabolism and peripheral insulin sensitivity. Gene Regul. Syst. Bio 2014, 3, 97–111. [Google Scholar] [CrossRef]
- Schoenberg, K.M.; Giesy, S.L.; Harvatine, K.J.; Waldron, M.R.; Cheng, C.; Kharitonenkov, A.; Boisclair, Y.R. Plasma FGF21 is elevated by the intense lipid mobilization of lactation. Endocrinology 2011, 152, 4652–4661. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, L.; Wang, A.; Eleswarapu, S.; Ge, X.; Chen, D.; Jiang, H. Growth hormone stimulates transcription of the fibroblast growth factor 21 gene in the liver through the signal transducer and activator of transcription 5. Endocrinology 2012, 153, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Moeckli, B.; Pham, T.V.; Slits, F.; Latrille, S.; Peloso, A.; Delaune, V.; Oldani, G.; Lacotte, S.; Toso, C. FGF21 negatively affects long-term female fertility in mice. Heliyon 2022, 8, 11490. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine (NASEM). Nutrient Requirements of Beef Cattle, 8th ed.; The National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- National Research Council (NRC). Nutrient Requirements of Beef Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Beal, W.E.; Notter, D.R.; Akers, R.M. Techniques for estimation of milk yield in beef cows and relationships of milk yield to calf weight gain and postpartum reproduction. J. Anim. Sci. 1990, 68, 937–943. [Google Scholar] [CrossRef]
- Gasser, C.L.; Behlke, E.J.; Grum, D.E.; Day, M.L. Effect of timing of feeding a high-concentrate diet on growth and attainment of puberty in early-weaned heifers. J. Anim. Sci. 2006, 84, 3118–3122. [Google Scholar] [CrossRef]
- Alves, B.R.; Cardoso, R.C.; Prezotto, L.D.; Thorson, J.F.; Bedenbaugh, M.; Sharpton, S.M.; Caraty, A.; Keisler, D.H.; Tedeschi, L.O.; Williams, G.L.; et al. Elevated body weight gain during the juvenile period alters neuropeptide Y-gonadotropin-releasing hormone circuitry in prepubertal heifers. Biol. Reprod. 2015, 92, 46. [Google Scholar] [CrossRef]
- Cardoso, R.C.; Alves, B.R.; Prezotto, L.D.; Thorson, J.F.; Tedeschi, L.O.; Keisler, D.H.; Park, C.S.; Amstalden, M.; Williams, G.L. Use of a stair-step compensatory gain nutritional regimen to program the onset of puberty in beef heifers. J. Anim. Sci. 2014, 92, 2942–2949. [Google Scholar] [CrossRef]
- BonDurant, L.D.; Ameka, M.; Naber, M.C.; Markan, K.R.; Idiga, S.O.; Acevedo, M.R.; Walsh, S.A.; Ornitz, D.M.; Potthoff, M.J. FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metab. 2017, 25, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, K.; Takeda, J.; Horikawa, Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Lett. 2009, 583, 2882–2886. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Kim, M.; Doridot, L.; Cunniff, J.C.; Parker, T.S.; Levine, D.M.; Hellerstein, M.K.; Hudgins, L.C.; Maratos-Flier, E.; Herman, M.A. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol. Metab. 2016, 6, 14–21. [Google Scholar] [CrossRef]
- Lundsgaard, A.M.; Fritzen, A.M.; Sjøberg, K.A.; Myrmel, L.S.; Madsen, L.; Wojtaszewski, J.F.P.; Richter, E.A.; Kiens, B. Circulating FGF21 in humans is potently induced by short term overfeeding of carbohydrates. Mol. Metab. 2016, 6, 22–29. [Google Scholar] [CrossRef]
- Pereira, M.P.; Ferreira, L.A.A.; da Silva, F.H.S.; Christoffolete, M.A.; Metsios, G.S.; Chaves, V.E.; de França, S.A.; Damazo, A.S.; Flouris, A.D.; Kawashita, N.H. A low-protein, high-carbohydrate diet increases browning in perirenal adipose tissue but not in inguinal adipose tissue. Nutrition 2017, 42, 37–45. [Google Scholar] [CrossRef]
- Nielsen, M.S.; Ritz, C.; Chenchar, A.; Bredie, W.L.P.; Gillum, M.P.; Sjödin, A. Does FGF21 mediate the potential decrease in sweet food intake and preference following bariatric surgery? Nutrients 2021, 13, 3840. [Google Scholar] [CrossRef]
- Buell-Acosta, J.D.; Garces, M.F.; Parada-Baños, A.J.; Angel-Muller, E.; Paez, M.C.; Eslava-Schmalbach, J.; Escobar-Cordoba, F.; Caminos-Cepeda, S.A.; Lacunza, E.; Castaño, J.P.; et al. Maternal fibroblast growth factor 21 levels decrease during early pregnancy in normotensive pregnant women but are higher in preeclamptic women-A longitudinal study. Cells 2022, 11, 2251. [Google Scholar] [CrossRef]
- Allard, C.; Bonnet, F.; Xu, B.; Coons, L.; Albarado, D.; Hill, C.; Fagherazzi, G.; Korach, K.S.; Levin, E.R.; Lefante, J.; et al. Activation of hepatic estrogen receptor-α increases energy expenditure by stimulating the production of fibroblast growth factor 21 in female mice. Mol. Metab. 2019, 22, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhao, C.; Tan, P.; Liu, S.; Huang, Y.; Zeng, F.; Ma, P.; Guo, Y.; Zhao, B.; Wang, J. FGF21 reduces lipid accumulation in bovine hepatocytes by enhancing lipid oxidation and reducing lipogenesis via AMPK signaling. Animals 2022, 12, 939. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Hou, L.; Wu, W.; Zhao, S.; Xiong, Y. Fibroblast growth factor 21 suppresses adipogenesis in pig intramuscular fat cells. Int. J. Mol. Sci. 2015, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Morrison, C.D.; Stephens, J.M.; Redman, L.M. Fibroblast growth factor 21, adiposity, and macronutrient balance in a healthy, pregnant population with overweight and obesity. Endocr. Res. 2018, 43, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Chui, P.C.; Antonellis, P.J.; Bina, H.A.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 2010, 59, 2781–2789. [Google Scholar] [CrossRef]
- Badman, M.K.; Pissios, P.; Kennedy, A.R.; Koukos, G.; Flier, J.S.; Maratos-Flier, E. Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007, 5, 426–437. [Google Scholar] [CrossRef]
- Douglas, G.N.; Drackley, J.K.; Overton, T.R.; Bateman, H.G. Lipid metabolism and production by Holstein cows fed control or high fat diets at restricted or ad libitum intakes during the dry period. J. Dairy Sci. 1998, 81 (Suppl. S1), 295. [Google Scholar]
- Weber, C.; Hametner, C.; Tuchscherer, A.; Losand, B.; Kanitz, E.; Otten, W.; Singh, S.P.; Bruckmaier, R.M.; Becker, F.; Kanitz, W.; et al. Variation in fat mobilization during early lactation differently affects feed intake, body condition, and lipid and glucose metabolism in high-yielding dairy cows. J. Dairy Sci. 2013, 96, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Piepenbrink, M.S.; Marr, A.L.; Waldron, M.R.; Butler, W.R.; Overton, T.R.; Vazquez-Anon, M.; Holt, M.D. Feeding 2-hydroxy-4-(methylthio)-butanoic acid to periparturient dairy cows improves milk production but not hepatic metabolism. J. Dairy Sci. 2004, 87, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Lacetera, N.; Scalia, D.; Bernabucci, U.; Ronchi, B.; Pirazzi, D.; Nardone, A. Lymphocyte functions in overconditioned cows around parturition. J. Dairy Sci. 2005, 88, 2010–2016. [Google Scholar] [CrossRef] [PubMed]
- Bergen, W.G.; Mersmann, H.J. Comparative aspects of lipid metabolism: Impact on contemporary research and use of animal models. J. Nutr. 2005, 135, 2499–2502. [Google Scholar] [CrossRef] [PubMed]
- Oost, L.J.; Kustermann, M.; Armani, A.; Blaauw, B.; Romanello, V. Fibroblast growth factor 21 controls mitophagy and muscle mass. J. Cachexia Sarcopenia Muscle 2019, 10, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W.; Burhans, W.S.; Overton, T.R. Protein nutrition in late pregnancy, maternal protein reserves and lactation performance in dairy cows. Proc. Nutr. Soc. 2000, 59, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.M.; Albarado, D.C.; Coco, L.G.; Spann, R.A.; Khan, M.S.; Qualls-Creekmore, E.; Burk, D.H.; Burke, S.J.; Collier, J.J.; Yu, S.; et al. FGF21 is required for protein restriction to extend lifespan and improve metabolic health in male mice. Nat. Commun. 2022, 13, 1897. [Google Scholar] [CrossRef] [PubMed]
- Leiva, T.; Cooke, R.F.; Brandão, A.P.; Marques, R.S.; Vasconcelos, J.L. Effects of rumen-protected choline supplementation on metabolic and performance responses of transition dairy cows. J. Anim. Sci. 2015, 93, 1896–1904. [Google Scholar] [CrossRef] [PubMed]
- Knob, D.A.; Thaler Neto, A.; Schweizer, H.; Weigand, A.C.; Kappes, R.; Scholz, A.M. Energy balance indicators during the transition period and early lactation of purebred Holstein and Simmental cows and their crosses. Animals 2021, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Prezotto, L.D.; Thorson, J.F. Effect of dietary urea in gestating beef cows: Circulating metabolites, morphometrics, and mammary secretions. Animals 2022, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Stanislaus, S.; Chinookoswong, N.; Lau, Y.Y.; Hager, T.; Patel, J.; Ge, H.; Weiszmann, J.; Lu, S.C.; Graham, M.; et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models—Association with liver and adipose tissue effects. Am. J. Physiol. Endocrinol. Metab. 2009, 297, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.T.; Pelton, S.H.; Butler, W.R. Energy balance, metabolic status, and the first postpartum ovarian follicle wave in cows administered propylene glycol. J. Dairy Sci. 2006, 89, 2938–2951. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Wente, W.; Efanov, A.M.; Brenner, M.; Kharitonenkov, A.; Köster, A.; Sandusky, G.E.; Sewing, S.; Treinies, I.; Zitzer, H.; Gromada, J. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 2006, 55, 2470–2478. [Google Scholar] [CrossRef]
- Wang, Z.; Song, Y.; Zhang, F.; Zhao, C.; Fu, S.; Xia, C.; Bai, Y. Early warning for inactive ovaries based on liver function index, serum MDA, IL-6, FGF21 and ANGPTL8 in dairy cows. Ital. J. Anim. Sci. 2022, 21, 113–122. [Google Scholar] [CrossRef]
- Crowe, M.A. Resumption of ovarian cyclicity in post-partum beef and dairy cows. Reprod. Domest. Anim. 2008, 43 (Suppl. S5), 20–28. [Google Scholar] [CrossRef] [PubMed]
- Caixeta, L.S.; Giesy, S.L.; Krumm, C.S.; Perfield, J.W., 2nd; Butterfield, A.; Boisclair, Y.R. Fibroblast growth factor-21 (FGF21) administration to early-lactating dairy cows. II. Pharmacokinetics, whole-animal performance, and lipid metabolism. J. Dairy Sci. 2019, 102, 11597–11608. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, G.; Ringseis, R.; Keller, J.; Schwarz, F.J.; Windisch, W.; Eder, K. Expression of fibroblast growth factor 21 in the liver of dairy cows in the transition period and during lactation. J. Anim. Physiol. Anim. Nutr. 2012, 97, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Carriquiry, M.; Weber, W.J.; Fahrenkrug, S.C.; Crooker, B.A. Hepatic gene expression in multiparous Holstein cows treated with bovine somatotropin and fed n-3 fatty acids in early lactation. J. Dairy Sci. 2009, 92, 4889–4900. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, G.; Keller, J.; Hirche, F.; Geissler, S.; Schwarz, F.J.; Ringseis, R.; Stangl, G.I.; Eder, K. Expression of genes involved in hepatic carnitine synthesis and uptake in dairy cows in the transition period and at different stages of lactation. BMC Vet. Res. 2012, 8, 28. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prezotto, L.D.; Keane, J.A.; Cupp, A.S.; Thorson, J.F. Fibroblast Growth Factor 21 Has a Diverse Role in Energetic and Reproductive Physiological Functions of Female Beef Cattle. Animals 2023, 13, 3185. https://doi.org/10.3390/ani13203185
Prezotto LD, Keane JA, Cupp AS, Thorson JF. Fibroblast Growth Factor 21 Has a Diverse Role in Energetic and Reproductive Physiological Functions of Female Beef Cattle. Animals. 2023; 13(20):3185. https://doi.org/10.3390/ani13203185
Chicago/Turabian StylePrezotto, Ligia D., Jessica A. Keane, Andrea S. Cupp, and Jennifer F. Thorson. 2023. "Fibroblast Growth Factor 21 Has a Diverse Role in Energetic and Reproductive Physiological Functions of Female Beef Cattle" Animals 13, no. 20: 3185. https://doi.org/10.3390/ani13203185
APA StylePrezotto, L. D., Keane, J. A., Cupp, A. S., & Thorson, J. F. (2023). Fibroblast Growth Factor 21 Has a Diverse Role in Energetic and Reproductive Physiological Functions of Female Beef Cattle. Animals, 13(20), 3185. https://doi.org/10.3390/ani13203185




