Mutual Avoidance in the Spectacled Salamander and Centipede: A Discrepancy between Exploratory Field and Laboratory Data
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
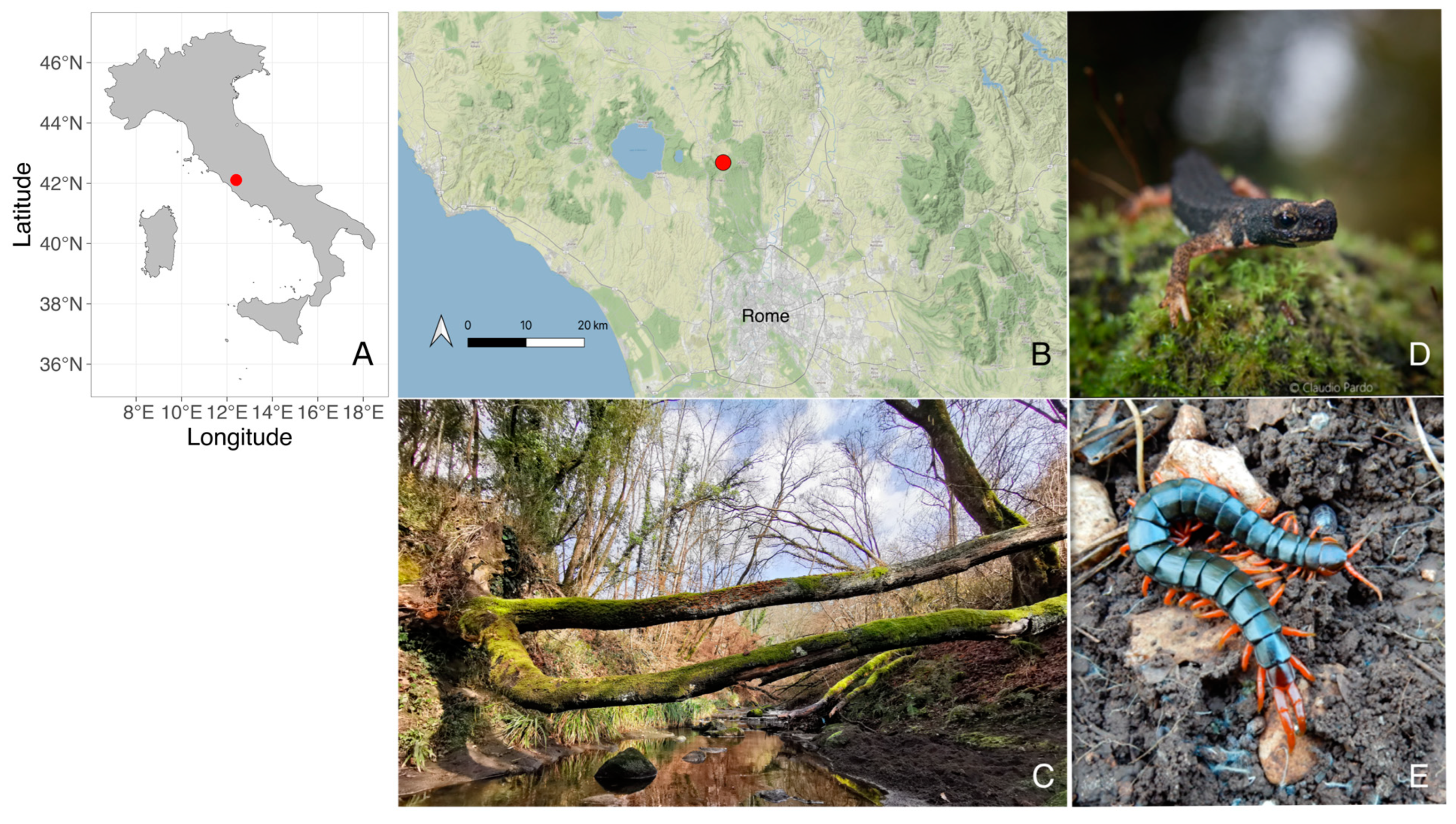
2.1. Study Area and Field Experiments
2.2. Manipulative Experiments
3. Statistical Analysis
4. Results
4.1. Field Experiments
4.2. Manipulative Experiments
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hickerson, C.-A.M.; Anthony, C.D.; Wicknick, J.A. Behavioral Interactions between Salamanders and Centipedes: Competition in Divergent Taxa. Behav. Ecol. 2004, 15, 679–686. [Google Scholar] [CrossRef]
- Hickerson, C.-A.M.; Anthony, C.D.; Figura, A.M. Behavioral Interactions between Terrestrial Salamanders and Spiders: Competition or Intraguild Predation? Ethol. Ecol. Evolut. 2018, 30, 285–296. [Google Scholar] [CrossRef]
- Vignoli, L.; Luiselli, L. Dietary Relationships among Coexisting Anuran Amphibians: A Worldwide Quantitative Review. Oecologia 2012, 169, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, L.; Bissattini, A.M.; Luiselli, L. Food Partitioning and the Evolution of Non-Randomly Structured Communities in Tailed Amphibians: A Worldwide Systematic Review. Biol. J. Linn. Soc. 2017, 120, 489–502. [Google Scholar] [CrossRef]
- McCormick, S.; Polis, G.A. Arthropods That Prey on Vertebrates. Biol. Rev. 1982, 57, 29–58. [Google Scholar] [CrossRef]
- Valdez, J.W. Arthropods as Vertebrate Predators: A Review of Global Patterns. Global Ecol. Biogeogr. 2020, 29, 1691–1703. [Google Scholar] [CrossRef]
- von May, R.; Biggi, E.; Cárdenas, H.; Diaz, M.; Alarcon, C.; Herrera Alva, V.; Santa-Cruz, R.; Tomasinelli, F.; Westeen, E.; Sanchez-Paredes, C.; et al. Ecological Interactions between Arthropods and Small Vertebrates in a Lowland Amazon Rainforest. Amphib. Rept. Conservat. 2019, 13, 65–77. [Google Scholar]
- Toledo, L.F. Predation on Seven South American Anuran Species by Water Bugs (Belostomatidae). Phyllomedusa 2003, 2, 105. [Google Scholar] [CrossRef]
- Oda, F.; Vieira, L.; Guerra Batista, V. Tetracha Brasiliensis Brasiliensis (Kirky, 1818) (Coleoptera: Cicindelidae) as a Predator of Newly-Metamorphosed Anurans. Entomotropica 2014, 29, 183–186. [Google Scholar]
- Chiacchio, M.; Nadolski, B.S.; Suwanwaree, P.; Waengsothorn, S. Centipede, Scolopendra Dawydoffi (Chilopoda: Scolopendridae), Predation on an Egg-Laying Snake, Sibynophis Triangularis (Squamata: Colubridae), in Thailand. J. Insect Behav. 2017, 30, 563–566. [Google Scholar] [CrossRef]
- Carpenter, C.; Gillingham, J. Giant Centipede (Scolopendra Alternans) Attacks Marine Toad (Bufo Marinus). Caribbean J. Sci. 1984, 20, 71–72. [Google Scholar]
- Heyer, W.R.; McDiarmid, R.W.; Weigmann, D.L. Tadpoles, Predation and Pond Habitats in the Tropics. Biotropica 1975, 7, 100–111. [Google Scholar] [CrossRef]
- Undheim, E.A.B.; King, G.F. On the Venom System of Centipedes (Chilopoda), a Neglected Group of Venomous Animals. Toxicon 2011, 57, 512–524. [Google Scholar] [CrossRef]
- Zimić, A.; Jelić, D. Interspecific Illusions: Underestimation of the Power of the Mediterranean Banded Centipede. Hyla 2014, 2014, 27–29. [Google Scholar]
- Deimezis-Tsikoutas, A.; Kapsalas, G.; Pafilis, P. A Rare Case of Saurophagy by Scolopendra Cingulata (Chilopoda: Scolopendridae) in the Central Aegean Archipelago: A Role for Insularity? Zool. Ecol. 2020, 30, 48–51. [Google Scholar] [CrossRef]
- Della Rocca, F.; Bottari, V.; Filippi, E.; Luiselli, L.; Utzeri, C. Modelling Aspects of Terrestrial Ecology in an Italian Endemic Salamander, Salamandrina Perspicillata. Rev. Ecol. Terre Vie 2008, 63, 261–270. [Google Scholar] [CrossRef]
- Angelini, C.; Antonelli, D.; Utzeri, C. Capture-Mark-Recapture Analysis Reveals Survival Correlates in Salamandrina Perspicillata (Savi, 1821). Amphibia-Reptilia 2010, 31, 21–26. [Google Scholar] [CrossRef]
- Angelini, C.; Vanni, S.; Vignoli, L. Salamandrina Terdigitata (Bonnaterre, 1789) Salamandrina Perspicillata (Savi, 1821). Fauna Ital. Amphib. 2007, 42, 228–237. [Google Scholar]
- Kaltsas, D.; Simaiakis, S. Seasonal Patterns of Activity of Scolopendra cretica and S. Cingulata (Chilopoda, Scolopendromorpha) in East Mediterranean Maquis Ecosystem. IJM 2012, 7, 1–14. [Google Scholar] [CrossRef]
- Bradley, J.G.; Eason, P.K. Novel Interaction between a Pisaurid Spider (Araneae: Pisauridae) and an Adult Eurycea Lucifuga (Caudata: Plethodontidae). Phyllomedusa 2017, 16, 279. [Google Scholar] [CrossRef]
- Crane, A.; Mathis, A. Observation of Predation by a Lycosid Spider on a Captive-Reared Salamander Larva (Ambystoma Annulatum). Herpetol. Notes 2015, 8, 455–457. [Google Scholar]
- Ficetola, G.F.; Pennati, R.; Manenti, R. Spatial Segregation among Age Classes in Cave Salamanders: Habitat Selection or Social Interactions? Popul. Ecol. 2013, 55, 217–226. [Google Scholar] [CrossRef]
- Romano, A.; Ruggiero, A. Olfactory Recognition of Terrestrial Shelters in Female Northern Spectacled Salamanders Salamandrina Perspicillata (Caudata, Salamandridae). Phyllomedusa 2008, 7, 3–10. [Google Scholar] [CrossRef][Green Version]
- Vignoli, L.; Silici, R.; Bissattini, A.M.; Bologna, M.A. Aspects of Olfactory Mediated Orientation and Communication in Salamandrina Perspicillata (Amphibia Caudata): An Experimental Approach. Ethol. Ecol. Evolut. 2012, 24, 165–173. [Google Scholar] [CrossRef]
- Burgett, A.A.; Smith, G.R. Differential Responses of Eastern Red-Backed Salamanders (Plethodon Cinereus) to Conspecifics and Centipedes. Curr. Herpetol. 2012, 31, 78–86. [Google Scholar] [CrossRef]
- Ibáñez, A.; Caspers, B.A.; López, P.; Martín, J.; Krause, E.T. Is the Reaction to Chemical Cues of Predators Affected by Age or Experience in Fire Salamanders (Salamandra Salamandra)? Amphibia-Reptilia 2014, 35, 189–196. [Google Scholar] [CrossRef]
- Della Rocca, F.; Vignoli, L.; Bologna, M. The Reproductive Biology of Salamandrina Terdigitata (Caudata Salamandridae). Herpetol. J. 2005, 15, 273–278. [Google Scholar]
- Jolley, D.B.; Ditchkoff, S.S.; Sparklin, B.D.; Hanson, L.B.; Mitchell, M.S.; Grand, J.B. Estimate of Herpetofauna Depredation by a Population of Wild Pigs. J. Mammal. 2010, 91, 519–524. [Google Scholar] [CrossRef]
- Hammond, J.I.; Luttbeg, B.; Sih, A. Predator and Prey Space Use: Dragonflies and Tadpoles in an Interactive Game. Ecology 2007, 88, 1525–1535. [Google Scholar] [CrossRef]
- Galindo-Aguilar, R.E.; Luna-Olivera, B.C.; Ramírez-Ibáñez, M.; Lavariega, M.C. Spatiotemporal Co-Occurrence of Predators and Prey in a Neotropical Mammal Community in Southern Mexico. J. Trop. Ecol. 2022, 38, 285–294. [Google Scholar] [CrossRef]
- Vignoli, L.; Silici, R.; Brizzi, R.; Bologna, M.A. In Vivo Sexual Discrimination in Salamandrina Perspicillata: A Cross-Check Analysis of Annual Changes in External Cloacal Morphology and Spermic Urine Release. Herpetol. J. 2010, 20, 17–24. [Google Scholar]
- Veech, J.A. A Probabilistic Model for Analysing Species Co-Occurrence. Global Ecol. Biogeogr. 2013, 22, 252–260. [Google Scholar] [CrossRef]
- Griffith, D.M.; Veech, J.A.; Marsh, C.J. Cooccur: Probabilistic Species Co-Occurrence Analysis in R. J. Stat. Soft. 2016, 69, 1–17. [Google Scholar] [CrossRef]
- Murray, D.L.; Roth, J.D.; Wirsing, A.J. Predation Risk Avoidance by Terrestrial Amphibians: The Role of Prey Experience and Vulnerability to Native and Exotic Predators. Ethology 2004, 110, 635–647. [Google Scholar] [CrossRef]
- Ferrari, M.C.O.; Brown, G.E.; Messier, F.; Chivers, D.P. Threat-Sensitive Generalization of Predator Recognition by Larval Amphibians. Behav. Ecol. Sociobiol. 2009, 63, 1369–1375. [Google Scholar] [CrossRef]
- Petranka, J.W.; Kats, L.B.; Sih, A. Predator-Prey Interactions among Fish and Larval Amphibians: Use of Chemical Cues to Detect Predatory Fish. Anim. Behav. 1987, 35, 420–425. [Google Scholar] [CrossRef]
- Rubbo, M.J.; Mirza, R.S.; Belden, L.K.; Falkenbach, J.J.; Storrs, S.I.; Kiesecker, J.M. Evaluating a Predator–Prey Interaction in the Field: The Interaction between Beetle Larvae (Predator) and Tadpoles (Prey). J. Zool. 2006, 269, 1–5. [Google Scholar] [CrossRef]
- Gall, B.G.; Mathis, A. Innate Predator Recognition and the Problem of Introduced Trout. Ethology 2010, 116, 47–58. [Google Scholar] [CrossRef]
- Anthony, C.D.; Hickerson, C.A.M.; Venesky, M.D. Responses of Juvenile Terrestrial Salamanders to Introduced (Lithobius Forficatus) and Native Centipedes (Scolopocryptops Sexspinosus). J. Zool. 2007, 271, 54–62. [Google Scholar] [CrossRef]
- Melotto, A.; Ficetola, G.F.; Alari, E.; Romagnoli, S.; Manenti, R. Visual Recognition and Coevolutionary History Drive Responses of Amphibians to an Invasive Predator. Behav. Ecol. 2021, 32, 1352–1362. [Google Scholar] [CrossRef]
- Cerini, F.; Bologna, M.A.; Vignoli, L. Dragonflies Community Assembly in Artificial Habitats: Glimpses from Field and Manipulative Experiments. PLoS ONE 2019, 14, e0214127. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Salvidio, S.; Posillico, M.; Matteucci, G.; De Cinti, B.; Romano, A. Generalisation within Specialization: Inter-Individual Diet Variation in the Only Specialized Salamander in the World. Sci. Rep. 2015, 5, 13260. [Google Scholar] [CrossRef]
- Boets, P.; Lock, K.; Messiaen, M.; Goethals, P.L.M. Combining Data-Driven Methods and Lab Studies to Analyse the Ecology of Dikerogammarus Villosus. Ecol. Informat. 2010, 5, 133–139. [Google Scholar] [CrossRef]
- Silbernagel, J.J.; Sorensen, P.W. Direct Field and Laboratory Evidence That a Combination of Egg and Larval Predation Controls Recruitment of Invasive Common Carp in Many Lakes of the Upper Mississippi River Basin. Transact. Am. Fish. Soc. 2013, 142, 1134–1140. [Google Scholar] [CrossRef]
- Blanchet, S.; Loot, G.; Grenouillet, G.; Brosse, S. Competitive Interactions between Native and Exotic Salmonids: A Combined Field and Laboratory Demonstration. Ecol. Freshwater Fish 2007, 16, 133–143. [Google Scholar] [CrossRef]
- Morin, P.J.; Johnson, E.A. Experimental Studies of Asymmetric Competition among Anurans. Oikos 1988, 53, 398. [Google Scholar] [CrossRef]
- Richter-Boix, A.; Garriga, N.; Montori, A.; Franch, M.; San Sebastián, O.; Villero, D.; Llorente, G.A. Effects of the Non-Native Amphibian Species Discoglossus Pictus on the Recipient Amphibian Community: Niche Overlap, Competition and Community Organization. Biol. Invasions 2013, 15, 799–815. [Google Scholar] [CrossRef]
- Brown, G.P.; Phillips, B.L.; Shine, R. The Ecological Impact of Invasive Cane Toads on Tropical Snakes: Field Data Do Not Support Laboratory-Based Predictions. Ecology 2011, 92, 422–431. [Google Scholar] [CrossRef]
- Calisi, R.M.; Bentley, G.E. Lab and Field Experiments: Are They the Same Animal? Hormones Behav. 2009, 56, 1–10. [Google Scholar] [CrossRef]


| Sampling Date | n Stones | S. perspicillata | S. cingulata | Obs Co-oc | Exp Co-oc | p_lt | Classification |
|---|---|---|---|---|---|---|---|
| 15 October 2009 | 19 | 11 | 12 | 0.366 | 6.9 | 0.006 | Negative |
| 29 October 2009 | 12 | 4 | 8 | 0 | 2.7 | 0.002 | Negative |
| 7 November 2009 | 16 | 11 | 6 | 1 | 4.1 | 0.001 | Negative |
| 26 November 2019 | 6 | 2 | 4 | 0 | NA | NA | Unclassifiable |
| 29 November 2019 | 13 | 6 | 8 | 1 | 3.7 | 0.004 | Negative |
| Experiment | Humidification | Total Trials | Success Trials | Avoidance | Frequency |
|---|---|---|---|---|---|
| 1 | uniform | 18 | 11 | 4 | 0.363 |
| 2 | under tiles | 23 | 23 | 12 | 0.521 |
| Coefficients | Estimate | Std. Error | z Value | p |
|---|---|---|---|---|
| Intercept | −0.087 | 0.417 | −0.208 | 0.835 |
| Design_uniform | 0.646 | 0.753 | 0.859 | 0.391 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerini, F.; Pardo, C.; Taurozzi, D.; Gambioli, B.; Vignoli, L. Mutual Avoidance in the Spectacled Salamander and Centipede: A Discrepancy between Exploratory Field and Laboratory Data. Animals 2023, 13, 3214. https://doi.org/10.3390/ani13203214
Cerini F, Pardo C, Taurozzi D, Gambioli B, Vignoli L. Mutual Avoidance in the Spectacled Salamander and Centipede: A Discrepancy between Exploratory Field and Laboratory Data. Animals. 2023; 13(20):3214. https://doi.org/10.3390/ani13203214
Chicago/Turabian StyleCerini, Francesco, Claudio Pardo, Davide Taurozzi, Benedetta Gambioli, and Leonardo Vignoli. 2023. "Mutual Avoidance in the Spectacled Salamander and Centipede: A Discrepancy between Exploratory Field and Laboratory Data" Animals 13, no. 20: 3214. https://doi.org/10.3390/ani13203214
APA StyleCerini, F., Pardo, C., Taurozzi, D., Gambioli, B., & Vignoli, L. (2023). Mutual Avoidance in the Spectacled Salamander and Centipede: A Discrepancy between Exploratory Field and Laboratory Data. Animals, 13(20), 3214. https://doi.org/10.3390/ani13203214







