The Effects of Anthropogenic Disturbances on the Spatiotemporal Patterns of Medium–Large Mammals in Tropical Volcanic Landscapes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Data Analysis
2.4. Spatial Interaction Analysis
2.5. Temporal Interaction Analysis
3. Results
3.1. Spatial Interaction
3.2. Activity Patterns and Temporal Interaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noroozi, J.; Talebi, A.; Doostmohammadi, M.; Rumpf, S.B.; Linder, H.P.; Schneeweiss, G.M. Hotspots within a Global Biodiversity Hotspot-Areas of Endemism Are Associated with High Mountain Ranges. Sci. Rep. 2018, 8, 10345. [Google Scholar] [CrossRef] [PubMed]
- Rahbek, C.; Borregaard, M.K.; Colwell, R.K.; Dalsgaard, B.; Holt, B.G.; Morueta-Holme, N.; Nogues-Bravo, D.; Whittaker, R.J.; Fjeldså, J. Humboldt’s Enigma: What Causes Global Patterns of Mountain Biodiversity? Science 2019, 365, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Silveira, F.A.O.; Barbosa, M.; Beiroz, W.; Callisto, M.; Macedo, D.R.; Morellato, L.P.C.; Neves, F.S.; Nunes, Y.R.F.; Solar, R.R.; Fernandes, G.W. Tropical Mountains as Natural Laboratories to Study Global Changes: A Long-Term Ecological Research Project in a Megadiverse Biodiversity Hotspot. Perspect. Plant Ecol. Evol. Syst. 2019, 38, 64–73. [Google Scholar] [CrossRef]
- Chakraborty, A. Mountains as Vulnerable Places: A Global Synthesis of Changing Mountain Systems in the Anthropocene. GeoJournal 2019, 86, 585–604. [Google Scholar] [CrossRef]
- Grêt-Regamey, A.; Weibel, B. Global Assessment of Mountain Ecosystem Services Using Earth Observation Data. Ecosyst. Serv. 2020, 46, 101213. [Google Scholar] [CrossRef]
- Referowska-chodak, E. Pressures and Threats to Nature Related to Human Activities in European Urban and Suburban Forests. Forest 2019, 10, 765. [Google Scholar] [CrossRef]
- Preisler, H.K.; Ager, A.A.; Wisdom, M.J. Statistical Methods for Analysing Responses of Wildlife to Human Disturbance. J. Appl. Ecol. 2006, 43, 164–172. [Google Scholar] [CrossRef]
- Gaynor, K.M.; Hojnowski, C.E.; Carter, N.H.; Brashares, J.S. The Influence of Human Disturbance on Wildlife Nocturnality. Science 2018, 360, 1232–1235. [Google Scholar] [CrossRef]
- Wilson, M.W.; Ridlon, A.D.; Gaynor, K.M.; Gaines, S.D.; Stier, A.C.; Halpern, B.S. Ecological Impacts of Human-Induced Animal Behaviour Change. Ecol. Lett. 2020, 23, 1522–1536. [Google Scholar] [CrossRef]
- Wong, B.B.M.; Candolin, U. Behavioral Responses to Changing Environments. Behav. Ecol. 2015, 26, 665–673. [Google Scholar] [CrossRef]
- Santini, L.; González-Suárez, M.; Rondinini, C.; Di Marco, M. Shifting Baseline in Macroecology? Unravelling the Influence of Human Impact on Mammalian Body Mass. Divers. Distrib. 2017, 23, 640–649. [Google Scholar] [CrossRef]
- Andermann, T.; Faurby, S.; Turvey, S.T.; Antonelli, A.; Silvestro, D. The Past and Future Human Impact on Mammalian Diversity. Sci. Adv. 2020, 6, eabb2313. [Google Scholar] [CrossRef] [PubMed]
- Lischka, S.A.; Teel, T.L.; Johnson, H.E.; Reed, S.E.; Breck, S.; Don Carlos, A.; Crooks, K.R. A Conceptual Model for the Integration of Social and Ecological Information to Understand Human-Wildlife Interactions. Biol. Conserv. 2018, 225, 80–87. [Google Scholar] [CrossRef]
- Reilly, M.; Adams, S. Recreation Ecology: The Impact of Hikers on Wildlife. In The Ardeid; Kelly, J., Ed.; Audubon Canyon Ranch: Marshall, CA, USA, 2016; pp. 10–12. [Google Scholar]
- Ouboter, D.A.; Kadosoe, V.S.; Ouboter, P.E. Impact of Ecotourism on Abundance, Diversity and Activity Patterns of Medium-Large Terrestrial Mammals at Brownsberg Nature Park, Suriname. PLoS ONE 2021, 16, e0250390. [Google Scholar] [CrossRef] [PubMed]
- Patana, P.; Alikodra, H.S.; Mawengkang, H.; Hamdani Harahap, R. State of Human Tiger Conflict around Gunung Leuser National Park in Langkat Landscape, North Sumatra, Indonesia. Biodiversitas 2023, 24, 837–846. [Google Scholar] [CrossRef]
- Qomariah, I.N.; Rahmi, T.; Said, Z.; Wijaya, A. Conflict between Human and Wild Sumatran Elephant (Elephas Maximus Sumatranus Temminck, 1847) in Aceh Province, Indonesia. Biodiversitas 2019, 20, 77–84. [Google Scholar] [CrossRef]
- Gunawan, H.; Iskandar, S.; Sihombing, V.S.; Wienanto, R. Conflict between Humans and Leopards (Panthera Pardus Melas Cuvier, 1809) in Western Java, Indonesia. Biodiversitas 2017, 18, 652–658. [Google Scholar] [CrossRef]
- Maskulino; Harianja, A.H.; Kuswanda, W. Mitigation of Human-Orangutan Conflict in Orangutan Reintroduction Area at Suo-Suo Village, Buffer Zone of Bukit Tigapuluh National Park. IOP Conf. Ser. Earth Environ. Sci. 2021, 715, 012076. [Google Scholar] [CrossRef]
- Md-Zain, B.M.; Ruslin, F.; Idris, W.M.R. Human-Macaque Conflict at the Main Campus of Universiti Kebangsaan Malaysia. Pertanika J. Trop. Agric. Sci. 2014, 37, 73–85. [Google Scholar]
- Akinsorotan, O.A.; Odelola, V.A.; Olaniyi, O.E.; Oguntuase, B.G. Human-Wildlife Conflicts and Rural Livelihood in Okomu National Park, Edo State, Nigeria. IOP Conf. Ser. Earth Environ. Sci. 2021, 655, 012097. [Google Scholar] [CrossRef]
- Ji, Y.; Wei, X.; Liu, F.; Li, D.; Li, J. Spatial-Temporal Patterns of Human-Wildlife Conflicts under Coupled Impact of Natural and Anthropogenic Factors in Mt. Gaoligong, Western Yunnan, China. Glob. Ecol. Conserv. 2022, 40, e02329. [Google Scholar] [CrossRef]
- Nyhus, P.J. Human-Wildlife Conflict and Coexistence. Annu. Rev. Environ. Resour. 2016, 41, 143–171. [Google Scholar] [CrossRef]
- Anand, S.; Radhakrishna, S. Investigating Trends in Human-Wildlife Conflict: Is Conflict Escalation Real or Imagined? J. Asia-Pac. Biodivers. 2017, 10, 154–161. [Google Scholar] [CrossRef]
- Waters, C.N.; Zalasiewicz, J.; Summerhayes, C.; Barnosky, A.D.; Poirier, C.; Gałuszka, A.; Cearreta, A.; Edgeworth, M.; Ellis, E.C.; Ellis, M.; et al. The Anthropocene Is Functionally and Stratigraphically Distinct from the Holocene. Science 2016, 351, 1304–1307. [Google Scholar] [CrossRef] [PubMed]
- Rahman, D.A.; Mardiastuti, A. Factors Influencing the Activity Patterns of Two Deer Species and Their Response to Predators in Two Protected Areas in Indonesia. Therya 2021, 12, 149–161. [Google Scholar] [CrossRef]
- Tarjuelo, R.; Barja, I.; Morales, M.B.; Traba, J.; Benítez-López, A.; Casas, F.; Arroyo, B.; Delgado, M.P.; Mougeot, F. Effects of Human Activity on Physiological and Behavioral Responses of an Endangered Steppe Bird. Behav. Ecol. 2015, 26, 828–838. [Google Scholar] [CrossRef]
- Cremonesi, G.; Bisi, F.; Gaffi, L.; Zaw, T.; Naing, H.; Moe, K.; Aung, Z.; Gagliardi, A.; Wauters, L.A.; Preatoni, D.G.; et al. Evaluation of Human Disturbance on the Activity of Medium–Large Mammals in Myanmar Tropical Forests. Forests 2021, 12, 290. [Google Scholar] [CrossRef]
- Johann, F.; Handschuh, M.; Linderoth, P.; Dormann, C.F.; Arnold, J. Adaptation of Wild Boar (Sus scrofa) Activity in a Human-Dominated Landscape. BMC Ecol. 2020, 20, 4. [Google Scholar] [CrossRef]
- Wang, Y.; Smith, J.A.; Wilmers, C.C. Residential Development Alters Behavior, Movement, and Energetics in a Top Carnivore. PLoS ONE 2017, 12, e0184687. [Google Scholar] [CrossRef]
- Kays, R.; Parsons, A.W.; Baker, M.C.; Kalies, E.L.; Forrester, T.; Costello, R.; Rota, C.T.; Millspaugh, J.J.; McShea, W.J. Does Hunting or Hiking Affect Wildlife Communities in Protected Areas? J. Appl. Ecol. 2017, 54, 242–252. [Google Scholar] [CrossRef]
- Parsons, A.W.; Bland, C.; Forrester, T.; Baker-Whatton, M.C.; Schuttler, S.G.; McShea, W.J.; Costello, R.; Kays, R. The Ecological Impact of Humans and Dogs on Wildlife in Protected Areas in Eastern North America. Biol. Conserv. 2016, 203, 75–88. [Google Scholar] [CrossRef]
- Zimbres, B.; Peres, C.A.; Machado, R.B. Terrestrial Mammal Responses to Habitat Structure and Quality of Remnant Riparian Forests in an Amazonian Cattle-Ranching Landscape. Biol. Conserv. 2017, 206, 283–292. [Google Scholar] [CrossRef]
- Ohashi, H.; Saito, M.; Horie, R.; Tsunoda, H.; Noba, H.; Ishii, H.; Kuwabara, T.; Hiroshige, Y.; Koike, S.; Hoshino, Y.; et al. Differences in the Activity Pattern of the Wild Boar Sus scrofa Related to Human Disturbance. Eur. J. Wildl. Res. 2013, 59, 167–177. [Google Scholar] [CrossRef]
- Ducatez, S.; Sol, D.; Sayol, F.; Lefebvre, L. Behavioural Plasticity Is Associated with Reduced Extinction Risk in Birds. Nat. Ecol. Evol. 2020, 4, 788–793. [Google Scholar] [CrossRef]
- Mekonen, S. Coexistence between Human and Wildlife: The Nature, Causes and Mitigations of Human Wildlife Conflict around Bale Mountains National Park, Southeast Ethiopia. BMC Ecol. 2020, 20, 51. [Google Scholar] [CrossRef]
- Dos Santos, C.L.A.; Le Pendu, Y.; Giné, G.A.F.; Dickman, C.R.; Newsome, T.M.; Cassano, C.R. Human Behaviors Determine the Direct and Indirect Impacts of Free-Ranging Dogs on Wildlife. J. Mammal. 2018, 99, 1261–1269. [Google Scholar] [CrossRef]
- Muhly, T.B.; Semeniuk, C.; Massolo, A.; Hickman, L.; Musiani, M. Human Activity Helps Prey Win the Predator-Prey Space Race. PLoS ONE 2011, 6, e17050. [Google Scholar] [CrossRef] [PubMed]
- Soga, M.; Gaston, K.J. The Ecology of Human-Nature Interactions. Proc. R. Soc. B Biol. Sci. 2020, 287, 20191882. [Google Scholar] [CrossRef]
- Widodo, F.A.; Imron, M.A.; Sunarto, S.; Giordano, A.J. Carnivores and Their Prey in Sumatra: Occupancy and Activity in Human-Dominated Forests. PLoS ONE 2022, 17, e0265440. [Google Scholar] [CrossRef]
- Larson, K.L.; Rosales Chavez, J.B.; Brown, J.A.; Morales-Guerrero, J.; Avilez, D. Human–Wildlife Interactions and Coexistence in an Urban Desert Environment. Sustainability 2023, 15, 3307. [Google Scholar] [CrossRef]
- Ma, B.; Xie, Y.; Zhang, T.; Zeng, W.; Xue, Y. Construction of a Human-Wildlife Spatial Interaction Index in the Three-River Source Region, China. Ecol. Indic. 2021, 129, 107986. [Google Scholar] [CrossRef]
- Sulaksono, N.; Pudyatmoko, S.; Soemardi; Wardhana, W.; Hadiyan, Y.; Nurvianto, S. Response of Terrestrial Mammals to Various Types of Disturbance in the Gunung Merapi National Park, Indonesia. Biodiversitas 2022, 23, 1635–1647. [Google Scholar] [CrossRef]
- Surono, N.; Jousset, P.; Pallister, J.; Boichu, M.; Buongiorno, M.F.; Budisantoso, A.; Costa, F.; Andreastuti, S.; Prata, F.; Schneider, D.; et al. The 2010 Explosive Eruption of Java’s Merapi Volcano—A ‘100-Year’ Event. J. Volcanol. Geotherm. Res. 2012, 241–242, 121–135. [Google Scholar] [CrossRef]
- Sutomo. Ecological Succession on Volcanic Ecosystem of Mount Merapi Indonesia and Its Implication for Restoration; Biotrop: Bogor, Indonesia, 2013. [Google Scholar]
- Yang, J.; Langford, F.; Kiddie, J. Risk Factors for Aggressive Behaviour in Domestic Dogs (Canis familiaris), as Reported by Owners in Mainland China. Appl. Anim. Behav. Sci. 2021, 234, 105211. [Google Scholar] [CrossRef]
- Paschoal, A.M.O.; Massara, R.L.; Bailey, L.L.; Doherty, P.F.; Santos, P.M.; Paglia, A.P.; Hirsch, A.; Chiarello, A.G. Anthropogenic Disturbances Drive Domestic Dog Use of Atlantic Forest Protected Areas. Trop. Conserv. Sci. 2018, 11, 1–14. [Google Scholar] [CrossRef]
- Sutherland, W.J. Future Directions in Disturbance Research. Ibis 2007, 149, 120–124. [Google Scholar] [CrossRef]
- Carter, N.; Jasny, M.; Gurung, B.; Liu, J. Impacts of People and Tigers on Leopard Spatiotemporal Activity Patterns in a Global Biodiversity Hotspot. Glob. Ecol. Conserv. 2015, 3, 149–162. [Google Scholar] [CrossRef]
- Carter, N.H.; Shrestha, B.K.; Karki, J.B.; Pradhan, N.M.B.; Liu, J. Coexistence between Wildlife and Humans at Fine Spatial Scales. Proc. Natl. Acad. Sci. USA 2012, 109, 15360–15365. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Krishnamurthy, R.; Sathyakumar, S. Avoidance or Coexistence? The Spatiotemporal Patterns of Wild Mammals in a Human-Dominated Landscape in the Western Himalaya. Mt. Res. Dev. 2020, 40, R20–R31. [Google Scholar] [CrossRef]
- Lavigne, F.; Thouret, J.C.; Voight, B.; Suwa, H.; Sumaryono, A. Lahars at Merapi Volcano, Central Java: An Overview. J. Volcanol. Geotherm. Res. 2000, 100, 423–456. [Google Scholar] [CrossRef]
- Thouret, J.-C.; Lavigne, F.; Kelfoun, K.; Bronto, S. Toward a Revised Hazard Assessment at Merapi Volcano, Central Java. J. Volcanol. Geotherm. Res. 2000, 100, 479–502. [Google Scholar] [CrossRef]
- Umaya, R.; Hardjanto; Soekmadi, R.; Sunito, S. Direct Economic Benefits and Human Dependence toward Gunung Merapi National Park, Indonesia. Biodiversitas 2020, 21, 982–993. [Google Scholar] [CrossRef]
- Jones, T.; Bamford, A.J.; Ferrol-Schulte, D.; Hieronimo, P.; Mcwilliam, N.; Rovero, F. Vanishing Wildlife Corridors and Options for Restoration: A Case Study from Tanzania. Trop. Conserv. Sci. 2012, 5, 463–474. [Google Scholar] [CrossRef]
- Tobler, M.W.; Carrillo-Percastegui, S.E.; Leite Pitman, R.; Mares, R.; Powell, G. An Evaluation of Camera Traps for Inventorying Large- and Medium-Sized Terrestrial Rainforest Mammals. Anim. Conserv. 2008, 11, 169–178. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.D.; Royle, A.; Pollock, K.H.; Bailey, L.L.; Hines, J.E. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence; Maragioglio, N., Ed.; Academic Press Elsevier: London, UK, 2006; Volume 12, ISBN 9780120887668. [Google Scholar]
- Mackenzie, D.I.; Nichols, J.D.; Lachman, G.B.; Droege, S.; Royle, J.A.; Langtimm, C.A. Estimating Site Occupancy Rates When Detection Probabilities Are Less Than One. Ecology 2002, 83, 2248–2255. [Google Scholar] [CrossRef]
- Crego, R.D.; Stabach, J.A.; Connette, G. Implementation of Species Distribution Models in Google Earth Engine. Divers. Distrib. 2022, 28, 904–916. [Google Scholar] [CrossRef]
- Olaya, V. A Gentle Introduction to SAGA GIS; The SAGA User Group eV: Gottingen, Germany, 2004; ISBN 9788578110796. [Google Scholar]
- Henrich, M.; Hartig, F.; Dormann, C.F.; Kühl, H.S.; Peters, W.; Franke, F.; Peterka, T.; Šustr, P.; Heurich, M. Deer Behavior Affects Density Estimates With Camera Traps, but Is Outweighed by Spatial Variability. Front. Ecol. Evol. 2022, 10, 881502. [Google Scholar] [CrossRef]
- Niedballa, J.; Sollmann, R.; Courtiol, A.; Wilting, A. CamtrapR: An R Package for Efficient Camera Trap Data Management. Methods Ecol. Evol. 2016, 7, 1457–1462. [Google Scholar] [CrossRef]
- Meredith, M.; Ridout, M. Overview of the Overlap Package. Available online: https://cran.r-project.org/web/packages/overlap/overlap.pdf (accessed on 18 January 2022).
- Courtiol, A.; Sollmann, R.; Mathai, J.; Timothy, S.; Wilting, A. Package ‘CamtrapR’; R Package Version; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Richmond, O.M.W.; Hines, J.E.; Beissinger, S.R. Two-Species Occupancy Models: A New Paramaterization Applied to Co-Occurence of Secretive Rails. Ecol. Appl. 2010, 20, 2036–2046. [Google Scholar] [CrossRef]
- Pudyatmoko, S. Spatiotemporal Inter-Predator and Predator—Prey Interactions of Mammalian Species in a Tropical Savanna and Deciduous Forest in Indonesia. Mammal Res. 2018, 18, 191–202. [Google Scholar] [CrossRef]
- Robinson, Q.H.; Bustos, D.; Roemer, G.W. The Application of Occupancy Modeling to Evaluate Intraguild Predation in a Model Carnivore System. Ecology 2014, 95, 3112–3123. [Google Scholar] [CrossRef]
- Mackenzie, D.I.; Bailey, L.L.; Nichols, J.D. Investigating Species Co-Occurrence Patterns When Species Are Detected Imperfectly. J. Anim. Ecol. 2004, 73, 546–555. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2002; ISBN 0387953647. [Google Scholar]
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC Model Selection and Multimodel Inference in Behavioral Ecology: Some Background, Observations, and Comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Schuette, P.; Wagner, A.P.; Wagner, M.E.; Creel, S. Occupancy Patterns and Niche Partitioning within a Diverse Carnivore Community Exposed to Anthropogenic Pressures. Biol. Conserv. 2013, 158, 301–312. [Google Scholar] [CrossRef]
- Mazerolle, M.M.J. R Package ‘AICcmodavg’; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Richard, A.; Kellner, K.; Fiske, I.; Miller, D.; Hutchinson, R.; Smith, A.; Kery, M.; Meredith, M. Package ‘Unmarked’; R Package Version 1.1.1; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Fiske, I.J.; Chandler, R.B. Unmarked: An R Package for Fitting Hierarchical Models of Wildlife Occurrence and Abundance. J. Stat. Softw. 2011, 43, 1–23. [Google Scholar] [CrossRef]
- Meredith, A.M.; Ridout, M.; Meredith, M.M. Package ‘Overlap’; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Linkie, M.; Ridout, M.S. Assessing Tiger-Prey Interactions in Sumatran Rainforests. J. Zool. 2011, 284, 224–229. [Google Scholar] [CrossRef]
- Ridout, M.S.; Linkie, M. Estimating Overlap of Daily Activity Patterns from Camera Trap Data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Ulric, L.; Claudio, A.; Hiroyoshi, A.; Alessando, G.; Eduardo, G.-P.; Dimitri, G.; Jean-Olivier, I.; Matthew, P.; Federico, R. Package “Circular”; CRAN; R Foundation for Statistical Computing: Vienna, Austria, 2022; 142p. [Google Scholar]
- Fisher, N.I. Statistical Analysis of Circular Data; Cambridge University Press: Cambridge, UK, 1995; ISBN 9780521568906. [Google Scholar]
- Pewsey, A.; Neuhauser, M.; Ruxton, G.D. Circular Statistic in R; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Zungu, M.M.; Maseko, M.S.T.; Kalle, R.; Ramesh, T.; Downs, C.T. Factors Affecting the Occupancy of Forest Mammals in an Urban-Forest Mosaic in EThekwini Municipality, Durban, South Africa. Urban For. Urban Green. 2020, 48, 126562. [Google Scholar] [CrossRef]
- Pudyatmoko, S. Free-Ranging Livestock Influence Species Richness, Occupancy, and Daily Behaviour of Wild Mammalian Species in Baluran National Park, Indonesia. Mamm. Biol. 2017, 86, 33–41. [Google Scholar] [CrossRef]
- Challender, D.; Willcox, D.H.A.; Panjang, E.; Lim, N.; Nash, H.; Heinrich, S.; Chong, J. Manis javanica . IUCN Red List. Threat. Species 2019, 8235, 1–26. [Google Scholar]
- Challender, D.W.S.; ’t Sas-Rolfes, M.; Ades, G.W.J.; Chin, J.S.C.; Ching-Min Sun, N.; Chong, J.L.; Connelly, E.; Hywood, L.; Luz, S.; Mohapatra, R.K.; et al. Evaluating the Feasibility of Pangolin Farming and Its Potential Conservation Impact. Glob. Ecol. Conserv. 2019, 20, e00714. [Google Scholar] [CrossRef]
- Van Schaik, C.P.; Griffiths, M. Activity Periods of Indonesian Rain Forest Mammals. Biotropica 1996, 28, 105. [Google Scholar] [CrossRef]
- Withaningsih, S.; Parikesit; Nasrudin, A. Correlation between Landscape Structure and Distribution of Javan Pangolin (Manis javanica) in an Extreme Landscape. Biodiversitas 2021, 22, 920–932. [Google Scholar] [CrossRef]
- Anasari, S.D.; Pusparini, W.; Andayani, N. Predicting the Distribution of Sunda Pangolin (Manis javanica Desmarest, 1822) in Way Canguk Research Station, Bukit Barisan Selatan National Park, Lampung. J. Trop. Biodivers. Biotechnol. 2021, 6, 58612. [Google Scholar] [CrossRef]
- Manshur, A.A.P.K. dan B.M. Karakteristik Habitat Trenggiling Jawa (Manis javanica) Di Taman Nasional Gunung Halimun Salak. Media Konserv. 2015, 20, 77–83. [Google Scholar]
- Farida, W.R. Diversitas Tumbuhan Pakan, Habitat Dan Pemanfaatan Landak (Hystrix Sp.) Di Sumatera Selatan Dan Kalimantan Timur. Pros. Semin. Nas. Masy. Biodiversitas Indones. 2015, 1, 673–681. [Google Scholar] [CrossRef]
- Inayah, N.; Sari, A.P.; Farida, W.R.; Nugroho, H.A.; Handayani, T.H.; Amalia, R.L.R.; Shidiq, F. Diet Enrichment and the Reproductive Season of Captive Sunda Porcupine (Hystrix javanica F. Cuvier 1823). BIO Web Conf. 2020, 19, 00011. [Google Scholar] [CrossRef]
- Gomez, L. The Illegal Hunting and Exploitation of Porcupines for Meat and Medicine in Indonesia. Nat. Conserv. 2021, 43, 109–122. [Google Scholar] [CrossRef]
- Mustikasari, I.A.; Withaningsih, S.; Megantara, E.N.; Husodo, T. Parikesit Population and Distribution of Sunda Porcupine (Hystrix javanica F. Cuvier, 1823) in Designated Area of Cisokan Hydropower, West Java, Indonesia. Biodiversitas 2019, 20, 762–769. [Google Scholar] [CrossRef]
- Jothish, P.S. Diet of the Common Palm Civet Paradoxurus Hermaphroditus in a Rural Habitat in Kerala, India, and Its Possible Role in Seed Dispersal. Small Carniv. Conserv. 2011, 45, 14–17. [Google Scholar]
- Campbell, T.A.; Long, D.B. Activity Patterns of Wild Boars (Sus scrofa) in Southern Texas. Southwest. Nat. 2010, 55, 564–600. [Google Scholar] [CrossRef]
- Caruso, N.; Valenzuela, A.E.J.; Burdett, C.L.; Luengos Vidal, E.M.; Birochio, D.; Casanave, E.B. Summer Habitat Use and Activity Patterns of Wild Boar Sus scrofa in Rangelands of Central Argentina. PLoS ONE 2018, 13, e0206513. [Google Scholar] [CrossRef]
- Ogurtsov, S.S.; Zheltukhin, A.S.; Kotlov, I.P. Daily Activity Patterns of Large and Medium-Sized Mammals Based on Camera Traps Data in the Central Forest Nature Reserve, Valdai Upland, Russia. Nat. Conserv. Res. 2018, 3, 68–88. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, E.J. Diet of the Wild Boar (Sus scrofa): Implications for Management in Forest-Agricultural and Urban Environments in South Korea. PeerJ 2019, 7, e7835. [Google Scholar] [CrossRef] [PubMed]
- Djuwantoko, D.; Utami, R.N.; Wiyono, W. Aggressive Behavior of Macaques, Macaca Fascicularis (Raffles, 1821) on Tourists at Kaliurang Nature Recreation Forest, Yogyakarta. Biodiversitas J. Biol. Divers. 2008, 9, 301–305. [Google Scholar] [CrossRef]
- Contreras-Abarca, R.; Crespin, S.J.; Moreira-Arce, D.; Simonetti, J.A. Redefining Feral Dogs in Biodiversity Conservation. Biol. Conserv. 2022, 265, 109434. [Google Scholar] [CrossRef]
- Perry, M.C. Studies of Deer-Related Dog Activity in Virginia. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 1970. [Google Scholar]


| Model | No. of Independent Captures | Relative Abundance Index | Naïve Occupancy | Probability of Occupancy ψ ± SE | Elevation Mean (Min–Max) | |
|---|---|---|---|---|---|---|
| Human | ψ(.)p(.) | 57 | 4.19 | 0.26 | 0.27 ± 0.08 | 1145 (980–1575) |
| Domestic dog | ψ(.)p(.) | 27 | 1.99 | 0.29 | 0.30 ± 0.10 | 1381 (986–2155) |
| Barking deer (Muntiacus muntjac) | ψ(.)p(.) | 264 | 19.43 | 0.65 | 0.65 ± 0.09 | 1313 (980–2155) |
| Wild boar (Sus scrofa) | ψ(.)p(.) | 30 | 2.21 | 0.32 | 0.33 ± 0.12 | 1320 (991–1867) |
| Long-tailed macaque (Macaca fascicularis) | ψ(.)p(.) | 471 | 34.66 | 0.94 | 0.94 ± 0.04 | 1262 (848–2155) |
| Leopard cat (Prionailurus bengalensis) | ψ(.)p(.) | 77 | 5.67 | 0.56 | 0.59 ± 0.09 | 1313 (980–2155) |
| Asian palm civet (Paradoxurus hermaphroditus) | ψ(.)p(.) | 142 | 10.45 | 0.79 | 0.80 ± 0.07 | 1253 (848–1867) |
| Small indian civet (Viverricula indica) | ψ(.)p(.) | 54 | 3.97 | 0.35 | 0.36 ± 0.11 | 1242 (959–1575) |
| Javan ferret badger (Melogale orientalis) | ψ(.)p(.) | 54 | 3.97 | 0.47 | 0.48 ± 0.13 | 1321 (848–1867) |
| Sunda porcupine (Hystrix javanica) | ψ(.)p(.) | 156 | 11.48 | 0.41 | 0.44 ± 0.09 | 1215 (848–1648) |
| Malayan pangolin (Manis javanica) | ψ(.)p(.) | 16 | 1.18 | 0.21 | 0.22 ± 0.18 | 1319 (1016–1687) |
| Model | ψA | ψBA | ψBa | Φ (SIF) | φ (95% CI) | |
|---|---|---|---|---|---|---|
| Human–domestic dog | ψ(.)p(ASP.DFG.DFM.DFS.HDF.HIG.SLO) | 0.60 | 0.76 | 0.08 | 1.55 | 0.56–2.53 |
| SE | 0.12 | 0.02 | 0.01 | 0.20 | ||
| Human–wild boar | ψ(.)p(ASP.DFD.DFM.DFS) | 0.38 | 0.23 | 0.15 | 0.33 | 0.03–0.63 |
| SE | 0.11 | 0.01 | 0.02 | 0.02 | ||
| Human–barking deer | ψ(.)p(ASP.DFM.DFS.HDF.LDF) | 0.42 | 0.85 | 0.61 | 1.19 | 0.90–1.48 |
| SE | 0.09 | 0.10 | 0.01 | 0.01 | ||
| Human–long-tailed macaque | ψ(.)p(DFM.DFS.HIG.LDF) | 0.66 | 1.00 | 0.83 | 1.06 | 0.97–1.15 |
| SE | 0.09 | 0.00 | 0.11 | 0.05 | ||
| Human–leopard cat | ψ(.)p(ASP.DFM.DFS) | 0.64 | 0.94 | 0.49 | 1.21 | 0.93–1.49 |
| SE | 0.09 | 0.07 | 0.02 | 0.01 | ||
| Human–Asian palm civet | ψ(.)p(ASP.DFM.DFS.HDF) | 0.49 | 0.88 | 1.00 | 0.94 | 0.84–1.03 |
| SE | 0.09 | 0.08 | 0.00 | 0.05 | ||
| Human–small Indian civet | ψ(.)p(ASP.DFD.DFP.DFS) | 0.51 | 0.54 | 0.33 | 1.23 | 0.66–1.81 |
| SE | 0.09 | 0.01 | 0.02 | 0.03 | ||
| Human–Javan ferret badger | ψ(.)p(ASP.DFD.DFP.LDF.SLO) | 0.60 | 0.61 | 0.43 | 0.80 | 0.53–1.06 |
| SE | 0.02 | 0.01 | 0.03 | 0.01 | ||
| Human–Sunda porcupine | ψ(.)p(ASP.DFG.DFM.DFP.HDF.HIG.LDF) | 0.67 | 0.34 | 0.28 | 0.60 | 0.23–0.98 |
| SE | 0.01 | 0.02 | 0.01 | 0.02 | ||
| Human–Malayan pangolin | ψ(.)p(ASP.DFG.DFM.HIG) | 0.31 | 0.10 | 0.56 | 0.24 | 0.19–0.45 |
| SE | 0.09 | 0.09 | 0.18 | 0.03 | ||
| Species | English | No. of Stations | No. of Images | No. of Events | Period | Activity Category | ||
|---|---|---|---|---|---|---|---|---|
| Night | Day | Night | Day | |||||
| Human | 10 | 57 | 3 | 54 | 0.05 | 0.95 | - | |
| Domestic dog | 9 | 27 | 7 | 20 | 0.26 | 0.74 | - | |
| Macaca fascicularis | Long-tailed macaque | 32 | 471 | 11 | 460 | 0.02 | 0.98 | D |
| Herpestes javanicus | Javan mongoose | 9 | 11 | 0 | 11 | 0.00 | 1.00 | D |
| Sus scrofa | Wild boar | 11 | 30 | 1 | 29 | 0.03 | 0.97 | D |
| Muntiacus muntjac | Barking deer | 22 | 264 | 53 | 211 | 0.20 | 0.80 | D |
| Prionailurus bengalensis | Leopard cat | 19 | 77 | 47 | 30 | 0.61 | 0.39 | C |
| Viverricula indica | Small Indian civet | 11 | 54 | 50 | 4 | 0.93 | 0.07 | N |
| Melogale orientalis | Javan ferret badger | 16 | 54 | 53 | 1 | 0.98 | 0.02 | N |
| Paradoxurus hermaphroditus | Asian palm civet | 26 | 142 | 141 | 1 | 0.99 | 0.01 | N |
| Manis javanica | Malayan pangolin | 7 | 16 | 16 | 0 | 1.00 | 0.00 | N |
| Hystrix javanica | Sunda porcupine | 14 | 156 | 156 | 0 | 1.00 | 0.00 | N |
| Trachypithecus auratus | Javan langur | 6 | 0 | 6 | 0.00 | 1.00 | - | |
| Petaurista petaurista | Red giant flying squirrel | 1 | 5 | 5 | 0 | 1.00 | 0.00 | - |
| Species | N | Rayleigh Test | |
|---|---|---|---|
| R | p | ||
| Human | 57 | 0.72 | <0.0001 |
| Domestic dog | 27 | 0.74 | <0.0001 |
| Wild boar | 30 | 0.89 | <0.0001 |
| Barking deer | 264 | 0.58 | <0.0001 |
| Long-tailed macaque | 471 | 0.74 | <0.0001 |
| Leopard cat | 77 | 0.54 | <0.0001 |
| Asian palm civet | 142 | 0.65 | <0.0001 |
| Small Indian civet | 54 | 0.50 | <0.0001 |
| Javan ferret badger | 54 | 0.53 | <0.0001 |
| Sunda porcupine | 156 | 0.72 | <0.0001 |
| Malayan pangolin | 16 | 0.84 | <0.0001 |
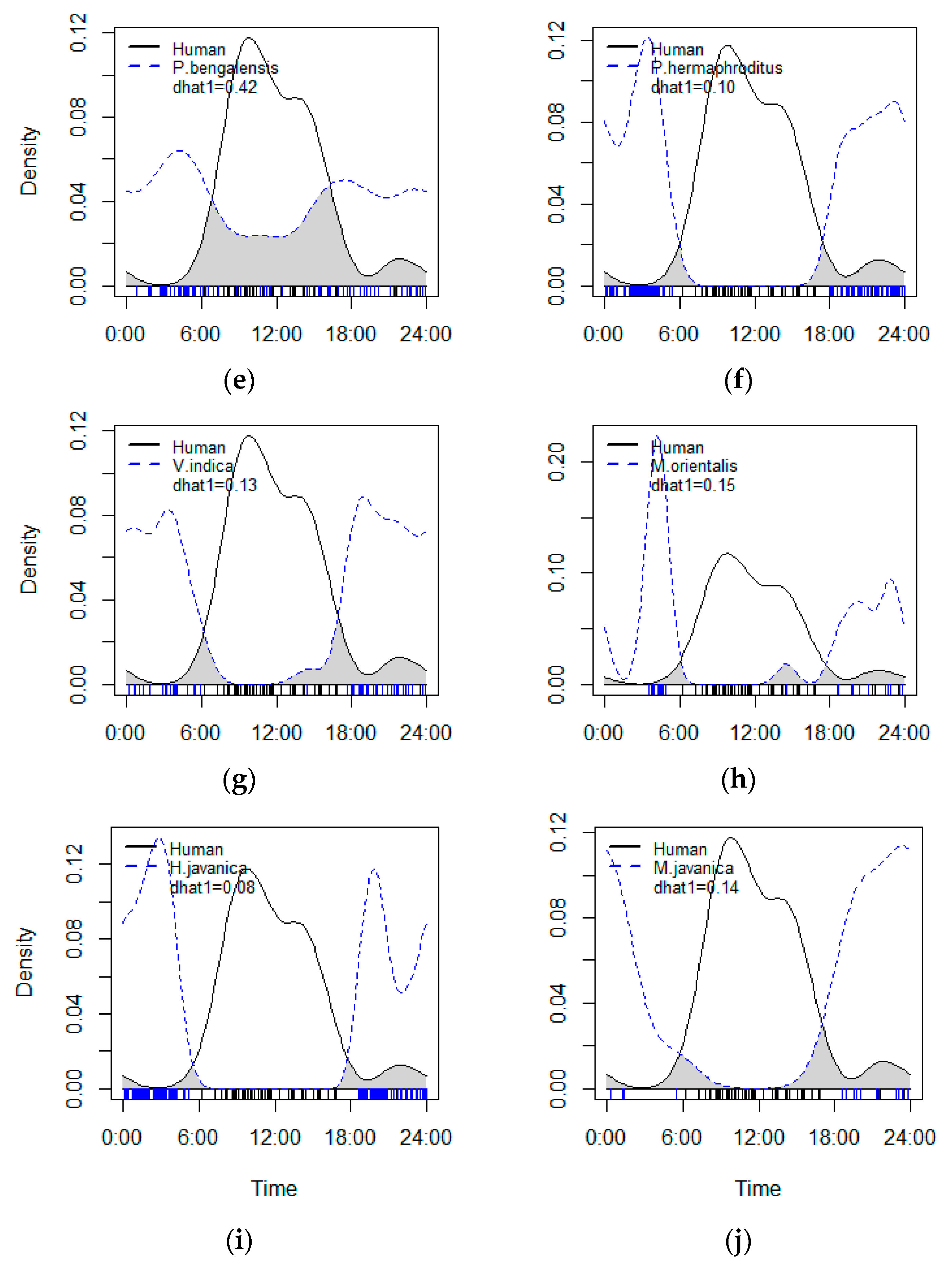
| Δ1 (95% CI) | MWW | p Value | |
|---|---|---|---|
| Human–domestic dog | 0.51 (0.41–0.68) | 30.27 | p < 0.001 |
| Human–wild boar | 0.78 (0.59–0.91) | 3.9788 | p < 0.001 |
| Human–barking deer | 0.59 (0.48–0.68) | 47.679 | p < 0.001 |
| Human–long-tailed macaque | 0.91 (0.83–0.98) | 53.423 | p < 0.001 |
| Human–leopard cat | 0.42 (0.28–0.52) | 56.468 | p < 0.001 |
| Human–Asian palm civet | 0.10 (0.06–0.21) | 101.98 | p < 0.001 |
| Human–small Indian civet | 0.13 (0.06–0.27) | 68.437 | p < 0.001 |
| Human–Javan ferret badger | 0.15 (0.10–0.29) | 43.072 | p < 0.001 |
| Human–Sunda porcupine | 0.08 (0.04–0.17) | 110.47 | p < 0.001 |
| Human–Malayan pangolin | 0.14 (0.06–0.31) | 33.49 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulaksono, N.; Pudyatmoko, S.; Sumardi, S.; Wardhana, W.; Budiman, A. The Effects of Anthropogenic Disturbances on the Spatiotemporal Patterns of Medium–Large Mammals in Tropical Volcanic Landscapes. Animals 2023, 13, 3217. https://doi.org/10.3390/ani13203217
Sulaksono N, Pudyatmoko S, Sumardi S, Wardhana W, Budiman A. The Effects of Anthropogenic Disturbances on the Spatiotemporal Patterns of Medium–Large Mammals in Tropical Volcanic Landscapes. Animals. 2023; 13(20):3217. https://doi.org/10.3390/ani13203217
Chicago/Turabian StyleSulaksono, Nurpana, Satyawan Pudyatmoko, Sumardi Sumardi, Wahyu Wardhana, and Arief Budiman. 2023. "The Effects of Anthropogenic Disturbances on the Spatiotemporal Patterns of Medium–Large Mammals in Tropical Volcanic Landscapes" Animals 13, no. 20: 3217. https://doi.org/10.3390/ani13203217






