Dietary Supplementation with Pithecellobium dulce (Roxb) Benth Fruits to Fattening Rabbits
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Proximate Analysis
2.2. Animals and Experimental Design
2.3. Diets
2.4. Productive Performance
2.5. Apparent Digestibility
2.6. Carcass Traits
2.7. Hematological and Biochemical Analysis
2.8. Meat Characteristics
2.9. Analysis of Meatball
2.10. Statistical Analysis
3. Results
3.1. Proximate Analysis
3.2. Productive Performance
3.3. In Vivo Apparent Digestibility
3.4. Biochemical and Blood Analysis
3.5. Carcass Traits
3.6. Meat Characteristics
3.7. Meatball Quality
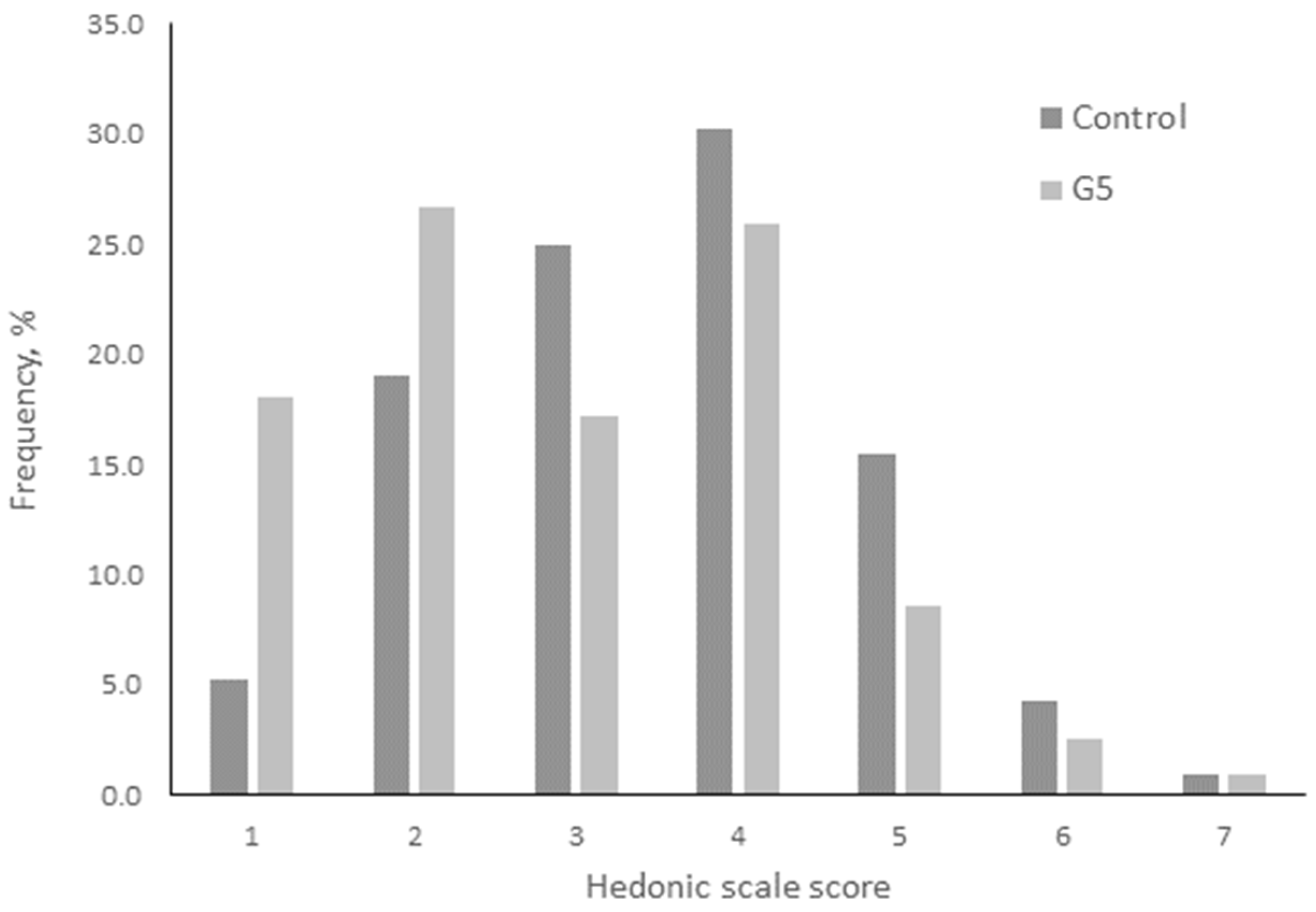
3.8. Sensory Analysis of Meatballs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Blas, C.; Mateos, G.G. Feed Formulation. In Nutrition of the Rabbits, 3rd ed.; de Blas, C., Wiseman, J.W., Eds.; CABI: Oxfordshire, UK, 2020; pp. 243–253. [Google Scholar]
- Dalle Zotte, A.; Szendrő, Z. The role of rabbit meat as functional food. Meat Sci. 2011, 88, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Dalle Zotte, A.; Celia, C.; Szendrő, Z. Herbs and spices inclusion as feedstuff or additive in growing rabbit diets and as additive in rabbit meat: A review. Livest. Sci. 2016, 189, 82–90. [Google Scholar] [CrossRef]
- Rao, G.N. Physico-chemical, mineral, amino acid composition, in vitro antioxidant activity and sorption isotherm of Pithecellobium dulce L. seed protein flour. J. Food Pharm. Sci. 2013, 1, 74–80. [Google Scholar] [CrossRef]
- Murugesan, S.; Lakshmanan, D.K.; Arumugam, V.; Alexander, R.A. Nutritional and therapeutic benefits of medicinal plant Pithecellobium dulce (Fabaceae): A review. J. Appl. Pharm. Sci. 2019, 9, 130–139. [Google Scholar] [CrossRef]
- Dhanisha, S.S.; Drishya, S.; Guruvayoorappan, C. Pithecellobium dulce induces apoptosis and reduce tumor burden in experimental animals via regulating pro-inflammatory cytokines and anti-apoptotic gene expression. Food Chem. Toxicol. 2022, 161, 112816. [Google Scholar] [CrossRef]
- Vargas-Madriz, Á.F.; Kuri-García, A.; Vargas-Madriz, H.; Chávez-Servín, J.L.; Ferriz-Martínez, R.A.; Hernández-Sandoval, L.G.; Guzmán-Maldonado, S.H. Phenolic profile and antioxidant capacity of Pithecellobium dulce (Roxb) Benth: A review. J. Food Sci. Technol. 2020, 57, 4316–4336. [Google Scholar] [CrossRef]
- Kahindi, R.K.; Abdulrazak, S.A.; Muinga, R.W. Effect of supplementing Napier grass (Pennisetum purpureum) with Madras thorn (Pithecellobium dulce) on intake, digestibility and live weight gains of growing goats. Small Rumin. Res. 2007, 69, 83–87. [Google Scholar] [CrossRef]
- Olivares-Perez, J.; Avilez-Nova, F.; Albarran-Portillo, B.; Castelan-Ortega, O.; Rojas-Hernandez, S. Use of three fodder trees in the feeding of goats in the subhumid tropics in Mexico. Trop. Anim. Health Prod. 2013, 45, 1573–7438. [Google Scholar] [CrossRef]
- Olivares-Pérez, J.; Avilés-Nova, F.; Albarrán-Portillo, B.; Castelán-Ortega, O.A.; Rojas-Hernández, S. Nutritional quality of Pithecellobium dulce and Acacia cochliacantha fruits, and its evaluation in goats. Livest. Sci. 2013, 154, 74–81. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2018. [Google Scholar]
- Van Soest, P.; Robertson, J.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Rabbits, 2nd ed.; National Research Council: Washington, DC, USA, 1977. [Google Scholar] [CrossRef]
- FEDNA. FEDNA/Fundación Española para el Desarrollo de la Nutricion Animal. 2020. Available online: http://www.fundacionfedna.org/ (accessed on 7 July 2022).
- Perez, J.M.; Lebas, F.; Gidenne, T.; Maertens, L.; Xiccato, G.; Parigi-Bini, R.; Dalle Zotte, A.; Cossu, M.E.; Carazzolo, A.; Villamide, M.J.; et al. European reference method for in vivo determination of diet digestibility in rabbits. World Rabbit Sci. 1995, 3, 41–43. [Google Scholar] [CrossRef]
- NOM-033-SAG/ZOO-2014. Métodos para dar Muerte a los Animales Domésticos y Silvestres. Diario Oficial de la Federación, México. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5405210&fecha=26/08/2015#gsc.tab=0 (accessed on 7 July 2022).
- Blasco, A.; Ouhayoun, J.; Masoero, G. Harmonization of criteria and terminology in rabbit meat research. World Rabbit Sci. 1993, 1, 3–10. [Google Scholar] [CrossRef]
- AMSA. Meat Color Measurement Guidelines; American Meat Science Association: Champaign, IL, USA, 2012; Available online: https://meatscience.org/publications-resources/printed-publications/amsa-meat-color-measurement-guidelines (accessed on 7 July 2022).
- Honikel, K.O. How to measure the water-holding capacity of meat? Recommendation of standardized methods. In Evaluation and Control of Meat Quality in Pigs; Current Topics in Veterinary Medicine and Animal, Science; Tarrant, P.V., Eikelenboom, G., Monin, G., Eds.; Springer: Dordrecht, Germany, 1987; p. 38. [Google Scholar]
- NOM-210-SSA1-2014. Productos y Servicios. Métodos de Prueba Microbiológicos. Determinación de Microorganismos Indicadores. Determinación de Microorganismos Patógenos. Available online: http://dof.gob.mx/nota_detalle.php?codigo=5398468&fecha=26/06/2015 (accessed on 7 July 2022).
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Drake, M.A. Invited review: Sensory analysis of dairy foods. J. Dairy Sci. 2007, 90, 4925–4937. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.N.; Nagender, A.; Satyanarayana, A.; Rao, D.G. Preparation, chemical composition and storage studies of quamachil (Pithecellobium dulce L.) aril powder. J. Food Sci. Technol. 2011, 4, 90–95. [Google Scholar] [CrossRef]
- Dhanisha, S.S.; Drishya, S.; Guruvayoorappan, C. Traditional knowledge to clinical trials: A review on nutritional and therapeutic potential of Pithecellobium dulce. J. Basic Clin. Physiol. Pharmacol. 2021, 33, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.; Simonetti, A.; Grassi, G.; Gambacorta, E. Effect of a cauliflower (Brassica oleraceae var. Botrytis) leaf powder-enriched diet on performance, carcass and meat characteristics of growing rabbit. Meat Sci. 2019, 149, 134–140. [Google Scholar] [CrossRef]
- Mancini, S.; Moruzzo, R.; Minieri, S.; Turchi, B.; Cerri, D.; Gatta, D.; Sagona, S.; Felicioli, A.; Paci, G. Dietary supplementation of quebracho and chestnut tannins mix in rabbit: Effects on live performances, digestibility, carcase traits, antioxidant status, faecal microbial load and economic value. Ital. J. Anim. Sci. 2019, 18, 621–629. [Google Scholar] [CrossRef]
- Kovitvadhi, A.; Gasco, L.; Ferrocino, I.; Rotolo, L.; Dabbou, S.; Malfatto, V.; Gai, F.; Peirette, P.G.; Falzone, M.; Vignolini, C.; et al. Effect of purple loosestrife (Lythrum salicaria) diet supplementation in rabbit nutrition on performance, digestibility, health and meat quality. Animal 2016, 10, 10–18. [Google Scholar] [CrossRef]
- Khalid, A.R.; Yasoob, T.B.; Zhang, Z.; Yu, D.; Feng, J.; Zhu, X.; Hang, S. Supplementation of Moringa oleifera leaf powder orally improved productive performance by enhancing the intestinal health in rabbits under chronic heat stress. J. Therm. Biol. 2020, 93, 102680. [Google Scholar] [CrossRef]
- Elwan, H.A.; Dawood, D.H.; Abd El-Aziz El-Shafei, S.M.; Abd El-Mohsen Abd El-Rahman, A.; Abdel-Latif, S.A.; Mohany, M.; Alqahtani, F.; Alqahtani, S.; Al-Rejaie, S.S. The potential role of citrus limon powder as a natural feed supplement to boost the productive performance, antioxidant status, and blood biochemistry of growing rabbits. Animals 2019, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Dabbou, S.; Ferrocino, I.; Kovitvadhi, A.; Bergagna, S.; Dezzuto, D.; Schiavone, A.; Cocolin, L.; Gai, F.; Santoro, V.; Gasco, L. Bilberry pomace in rabbit nutrition: Effects on growth performance, apparent digestibility, caecal traits, bacterial community and antioxidant status. Animal 2019, 13, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Roselin, C.; Parameshwari, S. A systematic review on the materialistic use of Pithecellobium dulce in food formulations. Mater Today Proc. 2022, 66, 996–1001. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Cullere, M.; Tasoniero, G.; Gerencsér, Z.; Szendrő, Z.; Novelli, E.; Matics, Z. Supplementing growing rabbit diets with chestnut hydrolyzable tannins: Effect on meat quality and oxidative status, nutrient digestibilities, and content of tannin metabolites. Meat Sci. 2018, 146, 101–108. [Google Scholar] [CrossRef]
- Brandão, J. Basic approach to veterinary care of rabbits. In Ferrets, Rabbits and Rodents Clinical Medicine and Surgery; Quesenberry, K.E., Orcutt, C.J., Mans, C., Carpenter, J.W., Eds.; Elsevier: St. Louis, MO, USA, 2021. [Google Scholar]
- Aljohani, N.E.; Abduljawad, S.H. Efficacy of Moringa oleifera leaf supplementation for enhanced growth performance, haematology and serum biochemistry of rabbits. Food Nutr. Sci. 2018, 9, 1285–1298. [Google Scholar] [CrossRef]
- Imbabi, T.; Sabeq, I.; Osman, A.; Mahmoud, K.; Amer, S.A.; Hassan, A.M.; Kostomakhin, N.; Habashy, W.; Easa, A.A. Impact of fennel essential oil as an antibiotic alternative in rabbit diet on antioxidant enzymes levels, growth performance, and meat quality. Antioxidants 2021, 10, 1797. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.; Cappai, M.G. Response of fattening rabbits with acorns (Quercus pubescens willd.) Combined in the diet: First acquaintances on growth performance, carcass traits and perirenal fatty acid profile. Animals 2020, 10, 1394. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, C.; Wang, Y.; Zhou, L.; Hu, H.; Bai, L.; Wang, J. Weighted gene co-expression network analysis reveals potential candidate genes affecting drip loss in pork. Anim. Genet. 2020, 51, 855–865. [Google Scholar] [CrossRef]
- Ramirez, J. Effect of selection for growth rate on biochemical, quality and texture characteristics of meat from rabbits. Meat Sci. 2004, 67, 617–624. [Google Scholar] [CrossRef]
- Sembiring, I.C.B.; Wardhita, A.A.G.; Adi, A.A.M. Muntingia calabura’s leaves extract ointment increased collagen density and enhanced healing of skin incision wound in hyperglycemic mice. Indones. Med. Veterinus 2021, 10, 189–199. [Google Scholar] [CrossRef]
- Pałka, S.E.; Otwinowska-Mindur, A.; Migdał, Ł.; Kmiecik, M.; Wojtysiak, D. Effect of a diet supplemented with nettle (Urtica dioica L.) or fenugreek (Trigonella foenumgraecum L.) on the post-slaughter traits and meat quality parameters of Termond white rabbits. Animals 2021, 11, 1566. [Google Scholar] [CrossRef]
- García-Vázquez, L.M.; Zepeda-Bastida, A.; Ayala-Martinez, M.; Soto-Simental, S. Infusion of Chenopodium ambrosioides consumed by rabbits: Effects on carcass, meat and burger quality. Food Sci. Technol. 2020, 40, 451–457. [Google Scholar] [CrossRef]
- Koné, A.P.; Desjardins, Y.; Gosselin, A.; Cinq-Mars, D.; Guay, F.; Saucier, L. Plant extracts and essential oil product as feed additives to control rabbit meat microbial quality. Meat Sci. 2019, 150, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.; Preziuso, G.; Dal Bosco, A.; Roscini, V.; Szendrő, Z.; Fratini, F.; Paci, G. Effect of turmeric powder (Curcuma longa L.) and ascorbic acid on physical characteristics and oxidative status of fresh and stored rabbit burgers. Meat Sci. 2015, 110, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Dabbou, S.; Gai, F.; Renna, M.; Rotolo, L.; Dabbou, S.; Lussiana, C.; Kovitvadhi, A.; Brugiapaglia, A.; Helal, M.N.; Zoccarato, I.; et al. Inclusion of bilberry pomace in rabbit diets: Effects on carcass characteristics and meat quality. Meat Sci. 2017, 124, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Castrica, M.; Menchetti, L.; Balzaretti, C.M.; Branciari, R.; Ranucci, D.; Cotozzolo, E.; Vigo, D.; Curone, G.; Brechia, G.; Miraglia, D. Impact of dietary supplementation with goji berries (Lycium barbarum) on microbiological quality, physico-chemical, and sensory characteristics of rabbit meat. Foods 2020, 9, 1480. [Google Scholar] [CrossRef]
- Mancini, S.; Preziuso, G.; Fratini, F.; Torracca, B.; Nuvoloni, R.; Dal Bosco, A.; Paci, G. Qualitative improvement of rabbit burgers using Zingiber officinale Roscoe powder. World Rabbit Sci. 2017, 25, 367–375. [Google Scholar] [CrossRef]
| Ingredients (g·kg−1) | Treatments 1 | |
|---|---|---|
| C | G5 | |
| Barley straw | 13.5 | 11.0 |
| Corn ground | 21.5 | 22.0 |
| Sorghum ground | 12.4 | 12.4 |
| Dry Distilled Grains | 6.5 | 6.0 |
| Canola meal | 4.0 | 3.0 |
| Wheat bran | 8.1 | 8.1 |
| Canola oil | 1.0 | 1.0 |
| Molasses | 2.5 | 2.5 |
| Soybean meal | 15.0 | 14.0 |
| Soybean hulls | 12.5 | 12.0 |
| Minerals and vitamins premix | 3.0 | 3.0 |
| Pithecellobium dulce | 0 | 5.0 |
| Chemical composition, % | ||
| Dry matter | 89.5 | 88.0 |
| Ash | 5.7 | 5.6 |
| Ether extract | 6.2 | 5.4 |
| Protein | 15.7 | 15.7 |
| Acid detergent fiber | 45 | 43 |
| Neutral detergent fiber | 60.1 | 58.3 |
| Digestible energy, Mcal kg MS−1 | 2.6 | 2.6 |
| Productive Parameter | Treatments 1 | SEM 2 | p Value | |
|---|---|---|---|---|
| C (n = 32) | G5 (n = 32) | |||
| Initial weight at 35 d, g | 598 | 576 | 49 | 0.99 |
| Final weight at 63 d, g | 1596 | 1583 | 75 | 0.42 |
| Average daily weight gain, g | 35.64 | 35.96 | 2.88 | 0.28 |
| Average daily feed intake, g | 83.25 | 81.82 | 5.82 | 0.26 |
| Feed conversion ratio | 2.89 a | 2.49 b | 0.32 | 0.19 |
| Total weight gain, g | 998 | 1007 | 67 | 0.28 |
| Total feed intake, g | 2331 | 2291 | 109 | 0.80 |
| Digestibility Coefficient | Treatments 1 | SEM 2 | p Value | |
|---|---|---|---|---|
| C (n = 8) | G5 (n = 8) | |||
| Dry matter | 0.63 b | 0.65 a | 0.09 | 0.05 |
| Organic matter | 0.67 b | 0.69 a | 0.08 | 0.03 |
| Neutral detergent fiber | 0.37 | 0.36 | 0.09 | 0.20 |
| Acid detergent fiber | 0.59 | 0.59 | 0.09 | 0.79 |
| Carcass Trait | Treatments 1 | SEM 2 | p Value | |
|---|---|---|---|---|
| C (n = 9) | G5 (n = 9) | |||
| Blood analysis | ||||
| Hematocrit, L·L−1 | 0.42 | 0.42 | 0.01 | 0.97 |
| Hemoglobin, g·L−1 | 129.57 | 131.11 | 3.19 | 0.73 |
| Red blood cells, ×1012 | 5.68 | 5.78 | 0.19 | 0.72 |
| Mean corpuscular volume, FI | 71.42 | 70.42 | 68.54 | 0.53 |
| Mean corpuscular hemoglobin concentration, g·L−1 | 318.57 | 319.78 | 2.45 | 0.73 |
| Platelets, ×109·L−1 | 283.85 | 365.89 | 56.29 | 0.32 |
| Total proteins, g·L−1 | 54.71 | 55.22 | 1.43 | 0.80 |
| White blood cells, ×109·L−1 | 5.20 | 6.78 | 1.02 | 0.29 |
| Neutrophiles, ×109·L−1 | 2.84 | 4.17 | 0.75 | 0.23 |
| Lymphocytes, ×109·L−1 | 2.17 | 2.43 | 0.28 | 0.53 |
| Monocytes, ×109·L−1 | 0.14 | 0.17 | 0.04 | 0.54 |
| Biochemical analysis | ||||
| Glucose, mmol·L−1 | 7.69 | 7.90 | 0.30 | 0.63 |
| Creatinine, µmol·L−1 | 83.90 | 86.31 | 4.15 | 0.68 |
| Cholesterol, mmol·L−1 | 1.69 | 2.11 | 0.15 | 0.07 |
| Bilirubin, µmol·L−1 | 4.64 | 8.32 | 1.69 | 0.14 |
| Alanine transferase, UI·L−1 | 32.57 b | 49.77 a | 4.86 | 0.02 |
| Aspartate transferase, UI·L−1 | 38.14 b | 48.55 a | 3.30 | 0.04 |
| Alkaline phosphatase, UI·L−1 | 216.85 | 211.77 | 20.20 | 0.86 |
| Total protein, gL·L−1 | 52.57 | 51.88 | 1.80 | 0.79 |
| Albumin, gL·L−1 | 30.71 | 30.22 | 3.02 | 0.91 |
| Globulin, gL·L−1 | 21.85 | 18.44 | 1.38 | 0.10 |
| Calcium, mmol·L−1 | 3.19 | 3.16 | 0.09 | 0.83 |
| Phosphorus, mmol·L−1 | 2.52 b | 2.85 a | 0.11 | 0.04 |
| Lactate dehydrogenase, UI·L−1 | 702.00 | 903.11 | 121.95 | 0.26 |
| Carcass Trait | Treatments 1 | SEM 2 | p Value | |
|---|---|---|---|---|
| C (n = 32) | G5 (n = 32) | |||
| Live weight, g | 1562.19 | 1566.05 | 80.55 | 0.97 |
| Dressing out percentage, % | 51.30 | 50.26 | 0.94 | 0.44 |
| Body length, cm | 30.00 | 28.55 | 0.73 | 0.16 |
| Body lumbar circumference, cm | 20.54 | 20.16 | 0.65 | 0.67 |
| Skin, g·kg−1 LW 3 | 148.00 | 146.86 | 2.61 | 0.75 |
| Feet, g·kg−1 LW | 27.90 a | 25.01 b | 0.81 | 0.01 |
| Carcass length, cm | 28.94 | 28.03 | 0.68 | 0.34 |
| Carcass lumbar circumference, cm | 14.75 | 14.89 | 0.52 | 0.84 |
| Viscera 4, g·kg−1 LW | 252.98 | 259.47 | 8.58 | 0.59 |
| Hot carcass weight, g | 809.69 | 790.05 | 47.24 | 0.77 |
| Cold carcass weight, g | 790.94 | 759.21 | 46.59 | 0.63 |
| Drip loss, % | 2.35b | 3.47 a | 0.31 | 0.01 |
| Kidney fat, g·kg−1 HCW 5 | 11.36 | 12.05 | 1.80 | 0.78 |
| Scapular fat, g·kg−1 HCW | 3.93 | 3.35 | 0.54 | 0.45 |
| Head, g·kg−1 HCW | 122.33 | 114.18 | 8.84 | 0.51 |
| Forepart weight, g·kg−1 HCW | 254.12 | 232.97 | 20.09 | 0.46 |
| Intermedia part weight, g·kg−1 HCW | 107.28 | 95.47 | 9.07 | 0.36 |
| Hind part weight, g·kg−1 HCW | 182.08 | 163.12 | 17.52 | 0.45 |
| Legs, g·kg−1 HCW | 363.35 | 330.77 | 29.36 | 0.43 |
| Meat, g·kg−1 Legs | 561.66 | 550.69 | 36.80 | 0.83 |
| Bone, g·kg−1 Legs | 234.12 | 225.11 | 10.99 | 0.56 |
| Dissectible fat, g·kg−1 Legs | 4.28 | 5.23 | 1.01 | 0.51 |
| Carcass Trait | Treatments 1 | SEM 2 | p Value | |
|---|---|---|---|---|
| C (n = 32) | G5 (n = 32) | |||
| L* | 47.28 | 47.63 | 0.41 | 0.54 |
| a* | 1.27 a | 0.28 b | 0.14 | 0.00 |
| b* | 7.52 | 7.29 | 0.18 | 0.37 |
| C* | 7.72 | 7.42 | 0.18 | 0.25 |
| h* | 1.51 | 1.52 | 0.01 | 0.44 |
| Water holding capacity | 19.91 | 19.26 | 0.87 | 0.60 |
| pH | 5.96 a | 5.97 b | 0.02 | 0.79 |
| Cooking loss, % | 8.28 | 8.86 | 0.74 | 0.58 |
| Hardness, N | 24.66 a | 19.31 b | 0.92 | 0.00 |
| Adhesiveness, N (×10) | 0.43 | 0.31 | 0.05 | 0.08 |
| Resilience | 0.17 | 0.18 | 0.01 | 0.16 |
| Cohesiveness | 0.49 | 0.49 | 0.01 | 0.74 |
| Springiness | 0.57 | 0.56 | 0.01 | 0.53 |
| Chewiness, N | 7.28 | 6.61 | 0.40 | 0.24 |
| Parameter | Treatment 1 | Time (d) | SEM 2 | p Value | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | T | t | T × t | |||
| pH | C | 5.823 b | 5.890 b | 6.703 aA | 0.020 | 0.002 | 0.001 | 0.001 |
| G5 | 5.907 | 5.830 | 6.203 B | 0.020 | ||||
| Aw 3 | C | 0.980 a | 0.957 b | 0.967 a | 0.004 | 0.210 | 0.101 | 0.024 |
| G5 | 0.963 | 0.950 | 0.967 | 0.004 | ||||
| TCV 4, Log UFC·g−1 | C | 3.085 c | 6.756 b | 9.619 a | 0.093 | 0.938 | 0.001 | 0.270 |
| G5 | 3.213 c | 6.660 b | 9.553 a | 0.152 | ||||
| Staphylococcus, Log UFC·g−1 | C | 2.971 c | 7.650 b | 10.826 a | 0.077 | 0.001 | 0.001 | 0.705 |
| G5 | 2.553 c | 6.460 b | 10.320 a | 0.125 | ||||
| Enterobacteriaceae, Log UFC·g−1 | C | 2.649 c | 7.835 b | 12.661 a | 0.060 | 0.615 | 0.001 | 0.521 |
| G5 | 2.400 c | 8.043 b | 12.593 a | 0.148 | ||||
| Antioxidant activity (mg·mL−1) | C | 11.301 a | 4.469 bB | 3.825 bB | 0.463 | 0.344 | 0.001 | 0.001 |
| G5 | 10.066 a | 5.908 bA | 5.229 bA | 0.463 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apáez-Barrios, J.; Ocampo-López, J.; Soto-Simental, S.; Aguilar-Raymundo, V.G.; Ayala-Martínez, M. Dietary Supplementation with Pithecellobium dulce (Roxb) Benth Fruits to Fattening Rabbits. Animals 2023, 13, 3249. https://doi.org/10.3390/ani13203249
Apáez-Barrios J, Ocampo-López J, Soto-Simental S, Aguilar-Raymundo VG, Ayala-Martínez M. Dietary Supplementation with Pithecellobium dulce (Roxb) Benth Fruits to Fattening Rabbits. Animals. 2023; 13(20):3249. https://doi.org/10.3390/ani13203249
Chicago/Turabian StyleApáez-Barrios, Jairo, Juan Ocampo-López, Sergio Soto-Simental, Victoria Guadalupe Aguilar-Raymundo, and Maricela Ayala-Martínez. 2023. "Dietary Supplementation with Pithecellobium dulce (Roxb) Benth Fruits to Fattening Rabbits" Animals 13, no. 20: 3249. https://doi.org/10.3390/ani13203249
APA StyleApáez-Barrios, J., Ocampo-López, J., Soto-Simental, S., Aguilar-Raymundo, V. G., & Ayala-Martínez, M. (2023). Dietary Supplementation with Pithecellobium dulce (Roxb) Benth Fruits to Fattening Rabbits. Animals, 13(20), 3249. https://doi.org/10.3390/ani13203249







