Microplastics in Cetaceans Stranded on the Portuguese Coast
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Collection
2.3. Sample Processing
2.4. Quality Assurance and Quality Control (QA/QC) Procedures
2.5. Data Analysis
3. Results
3.1. Microplastic Types
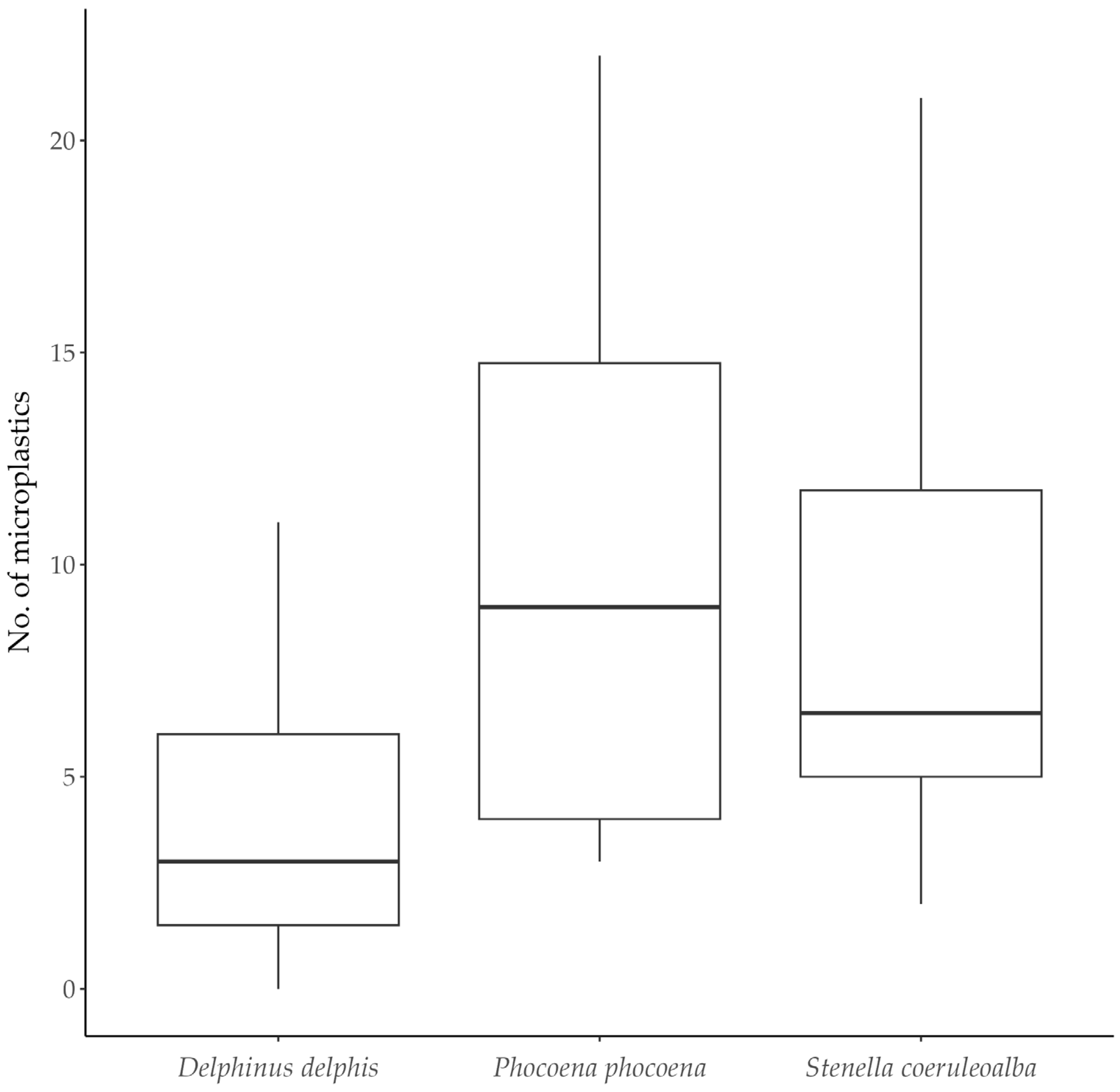
3.2. Microplastics According to Cetacean Species
3.3. Microplastics According to Biological and Health-Related Features
3.4. Microplastic Colour
3.5. Microplastic Sizes and Shape
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gutow, L.; Bergmann, M. Contamination of Our Oceans by Plastics. In Encyclopedia of Ocean Sciences, 3rd ed.; Cochran, J.K., Bokuniewicz, H.J., Yager, P.L., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 6, pp. 264–270. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Worm, B.; Lotze, H.K.; Jubinville, I.; Wilcox, C.; Jambeck, J. Plastic as a persistent marine pollutant. Annu. Rev. Environ. Resour. 2017, 42, 1–26. [Google Scholar] [CrossRef]
- Kühn, S.; van Franeker, J. Quantitative overview of marine debris ingested by marine megafauna. Mar. Pollut. Bull. 2020, 151, 110858. [Google Scholar] [CrossRef]
- CBD—Secretariat of the Convention on Biological Diversity. Marine Debris: Understanding, Preventing and Mitigating the Significant Adverse Impacts on Marine and Coastal Biodiversity; Technical Series No. 83; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2016; pp. 1–78. [Google Scholar]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Thompson, R.C. Microplastics in the marine environment: Sources, consequences and solutions. In Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer: Cham, Switzerland, 2015; pp. 185–200. [Google Scholar] [CrossRef]
- Rellán, A.G.; Ares, D.V.; Brea, C.V.; Lopez, A.F.; Bugallo, P.M.B. Sources, sinks and transformations of plastics in our oceans: Review, management strategies and modelling. Sci. Total Environ. 2022, 854, 158745. [Google Scholar] [CrossRef]
- Klingelhöfer, D.; Braun, M.; Quarcoo, D.; Brüggmann, D.; Groneberg, D.A. Research landscape of a global environmental challenge: Microplastics. Water Res. 2020, 170, 115358. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef]
- Shim, W.J.; Kim, S.K.; Lee, J.; Eo, S.; Kim, J.S.; Sun, C. Toward a long-term monitoring program for seawater plastic pollution in the north Pacific Ocean: Review and global comparison. Environ. Pollut. 2022, 311, 119911. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Zhu, M.; Liang, J.; Zheng, S.; Zhao, Y. Ingestion of microplastics by natural zooplankton groups in the northern South China Sea. Mar. Pollut. Bull. 2017, 115, 217–224. [Google Scholar] [CrossRef]
- Meaza, I.; Toyoda, J.H.; Wise, J.P. Microplastics in Sea Turtles, Marine Mammals and Humans: A One Environmental Health Perspective. Front. Environ. Sci. 2021, 8, 575614. [Google Scholar] [CrossRef]
- Hernandez-Milian, G.; Lusher, A.; MacGabban, S.; Rogan, E. Microplastics in grey seal (Halichoerus grypus) intestines: Are they associated with parasite aggregations? Mar. Pollut. Bull. 2019, 146, 349–354. [Google Scholar] [CrossRef]
- Nelms, S.E.; Galloway, T.S.; Godley, B.J.; Jarvis, D.S.; Lindeque, P.K. Investigating microplastic trophic transfer in marine top predators. Environ. Pollut. 2018, 238, 999–1007. [Google Scholar] [CrossRef]
- Van Franeker, J.A.; Bravo Rebolledo, E.L.; Hesse, E.; IJsseldijk, L.L.; Kühn, S.; Leopold, M.; Mielke, L. Plastic ingestion by harbour porpoises Phocoena phocoena in the Netherlands: Establishing a standardised method. Ambio 2018, 47, 387–397. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hernandez-Milian, G.; Berrow, S.; Rogan, E.; O’Connor, I. Incidence of marine debris in cetaceans stranded and bycaught in Ireland: Recent findings and a review of historical knowledge. Environ. Pollut. 2018, 232, 467–476. [Google Scholar] [CrossRef]
- Van Franeker, J.A.; Law, K.L. Seabirds, gyres and global trends in plastic pollution. Environ. Pollut. 2015, 203, 89–96. [Google Scholar] [CrossRef]
- SAPEA, Science Advice for Policy by European Academies. A Scientific Perspective on Microplastics in Nature and Society; SAPEA: Berlin, Germany, 2019; p. 173. [Google Scholar]
- Burns, E.E.; Boxall, A.B.A. Microplastics in the aquatic environment: Evidence for or against adverse impacts and major knowledge gaps. Environ. Toxicol. Chem. 2018, 37, 2776–2796. [Google Scholar] [CrossRef]
- Tanaka, K.; Takada, H.; Yamashita, R.; Mizukawa, K.; Fukuwaka, M.; Watanuki, Y. Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Mar. Pollut. Bull. 2013, 69, 219–222. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Zhang, S.; Dong, Y.; Pang, Q.; Lynch, I.; Xie, C.; Guo, Z.; Zhang, P. From marine to freshwater environment: A review of the ecotoxicological effects of microplastics. Ecotoxicol. Environ. Saf. 2023, 251, 114564. [Google Scholar] [CrossRef]
- Browne, M.A.; Niven, S.T.; Galloway, T.S.; Rowland, S.J.; Thompson, R.C. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr. Biol. 2013, 23, 2388–2392. [Google Scholar] [CrossRef] [PubMed]
- Mak, C.W.; Ching-Fong Yeung, K.; Chan, K.M. Acute toxic effects of polyethylene microplastic on adult zebrafish. Ecotoxicol. Environ. Saf. 2019, 182, 109442. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Li, B.; Liao, M.; Zhang, Z.; Chen, S.; Xia, B.; Wang, Y. Ingestion, egestion and physiological effects of polystyrene microplastics on the marine jellyfish Rhopilema esculentum. Mar. Pollut. Bull. 2023, 187, 114609. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, C.; León, V.M.; Calles, S.; Gomáriz-Olcina, M.; Vethaak, A.D. The adverse effects of virgin microplastics on the fertilization and larval development of sea urchins. Mar. Environ. Res. 2017, 130, 69–76. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The Impact of Polystyrene Microplastics on Feeding, Function and Fecundity in the Marine Copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef]
- Watts, A.J.; Urbina, M.A.; Corr, S.; Lewis, C.; Galloway, T.S. Ingestion of Plastic Microfibers by the Crab Carcinus maenas and Its Effect on Food Consumption and Energy Balance. Environ. Sci. Technol. 2015, 49, 14597–14604. [Google Scholar] [CrossRef]
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, E.J.; Goïc, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. USA 2016, 113, 2430–2435. [Google Scholar] [CrossRef]
- Lee, K.; Shim, W.J.; Kwon, O.Y.; Kang, J. Size-dependent effects of micro polystyrene particles in the marine copepod Tigriopus japonicus. Environ. Sci. Technol. 2013, 47, 11278–11283. [Google Scholar] [CrossRef]
- Espinosa, C.; Esteban, M.Á.; Cuesta, A. Dietary administration of PVC and PE microplastics produces histological damage, oxidative stress and immunoregulation in European sea bass (Dicentrarchus labrax L.). Fish Shellfish Immunol. 2019, 95, 574–583. [Google Scholar] [CrossRef]
- Junaid, M.; Liu, S.; Chen, G.; Liao, H.; Wang, J. Transgenerational impacts of micro(nano)plastics in the aquatic and terrestrial environment. J. Hazard. Mater. 2023, 443, 130274. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Vieira, L.R.; Branco, V.; Figueiredo, N.; Carvalho, F.; Carvalho, C.; Guilhermino, L.G. Microplastics cause neurotoxicity, oxidative damage and energy related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat. Toxicol. 2018, 195, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Chenet, T.; Mancia, A.; Bono, G.; Falsone, F.; Scannella, D.; Vaccaro, C.; Baldi, A.; Catani, M.; Cavazzini, A.; Pasti, L. Plastic ingestion by atlantic horse mackerel (Trachurus trachurus) from central mediterranean sea: A potential cause for endocrine disruption. Environ. Pollut. 2021, 284, 117449. [Google Scholar] [CrossRef]
- Rochman, C.M.; Kurobe, T.; Flores, I.; Teh, S.J. Early warning signs of endocrine disruption in adult fish from the ingestion of polyethylene with and without sorbed chemical pollutants from the marine environment. Sci. Total Environ. 2014, 493, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Bossart, G.D. 2011. Marine mammals as sentinel species for oceans and human health. Vet. Pathol. 2011, 48, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Baini, M.; Panti, C.; Baulch, S. Impacts of marine litter on cetaceans: A focus on plastic pollution. In Marine Mammal Ecotoxicology: Impacts of Multiple Stressors on Population Health; Fossi, M.C., Panti, C., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 147–184. [Google Scholar] [CrossRef]
- Fossi, M.C.; Baini, M.; Simmonds, M.P. Cetaceans as Ocean Health Indicators of Marine Litter Impact at Global Scale. Front. Environ. Sci. 2020, 8, 586627. [Google Scholar] [CrossRef]
- De Vere, A.J.; Lilley, M.K.; Frick, E.E. Anthropogenic impacts on the welfare of wild marine mammals. Aquat. Mamm. 2018, 44, 150–180. [Google Scholar] [CrossRef]
- Torres-Pereira, A.; Araújo, H.; Monteiro, S.S.; Ferreira, M.; Bastos-Santos, J.; Sá, S.; Nicolau, L.; Marçalo, A.; Marques, C.; Tavares, A.S.; et al. Assessment of harbour porpoise bycatch along the Portuguese and Galician coast: Insights from strandings over two decades. Animals 2023, 13, 2632. [Google Scholar] [CrossRef]
- Torres-Pereira, A.; Ferreira, M.; Eira, C.; López, A.; Sequeira, M. Phocoena phocoena boto. In Livro Vermelho dos Mamíferos de Portugal Continental; Mathias, M.L., Fonseca, C., Rodrigues, L., Grilo, C., Lopes-Fernandes, M., et al., Eds.; Associação para a Investigação e Desenvolvimento de Ciências and Instituto da Conservação da Natureza e das Florestas: Lisboa, Portugal, 2023; pp. 190–191. [Google Scholar]
- IWC—International Whaling Commission. Report of the IWC Workshop on Mitigation and Management of the Threats Posed by Marine Debris to Cetaceans; IWC/65/CCRep04; IWC: Cambridge, UK, 2014; p. 40. [Google Scholar]
- Baulch, S.; Perry, C. Evaluating the impacts of marine debris on cetaceans. Mar. Pollut. Bull. 2014, 80, 210–221. [Google Scholar] [CrossRef]
- Nelms, S.E.; Barnett, J.; Brownlow, A.; Davison, N.J.; Deaville, R.; Galloway, T.S.; Lindeque, P.K.; Santillo, D.; Godley, B.J. Microplastics in marine mammals stranded around the British coast: Ubiquitous but transitory? Sci. Rep. 2019, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Fossi, M.C.; Panti, C.; Baini, M.; Lavers, J.L. A Review of Plastic-Associated Pressures: Cetaceans of the Mediterranean Sea and Eastern Australian Shearwaters as Case Studies. Front. Mar. Sci. 2018, 5, 173. [Google Scholar] [CrossRef]
- Montoto-Martínez, T.; De la Fuente, J.; Puig-Lozano, R.; Marques, N.; Arbelo, M.; Hernandez-Brito, J.J.; Fernandez, A.; Gelado-Caballero, M.D. Microplastics, bisphenols, phthalates and pesticides in odontocete species in the macaronesian region (eastern North Atlantic). Mar. Pollut. Bull. 2021, 173, 113105. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gonzalez, A.; Saavedra, C.; Gago, J.; Covelo, P.; Santos, M.B.; Pierce, G.J. Microplastics in the stomach contents of common dolphin (Delphinus delphis) stranded on the galician coasts (NW Spain, 2005–2010). Mar. Pollut. Bull. 2018, 137, 526–532. [Google Scholar] [CrossRef]
- Fiúza, A.F. Upwelling patterns off Portugal. In Coastal Upwelling; Suess, E., Thiede, J., Eds.; Plenum Publishers: London, UK, 1983; pp. 85–87. [Google Scholar]
- Leitão, F.; Baptista, V.; Vieira, V.; Laginha Silva, P.; Relvas, P.; Alexandra Teodósio, M. A 60-Year Time Series Analyses of the Upwelling along the Portuguese Coast. Water 2019, 11, 1285. [Google Scholar] [CrossRef]
- Mann, K.; Lazier, J. Dynamics of Marine Ecosystems. In Biological–Physical Interactions in the Oceans, 3rd ed.; Blackwell Publishing: Malden, MA, USA, 2006. [Google Scholar]
- Ballance, L.T.; Pitman, R.L.; Fiedler, P.C. Progress in Oceanography Oceanographic influences on seabirds and cetaceans of the eastern tropical Pacific: A review. Prog. Oceanogr. 2006, 69, 360–390. [Google Scholar] [CrossRef]
- RCM 17/2019. Presidency of the Council of Ministers, Republic Diary No. 16/2019, Series I of 23 January 2019; pp. 474–475. Available online: https://data.dre.pt/eli/resolconsmin/17/2019/01/23/p/dre/pt/html (accessed on 3 August 2023).
- Geraci, R.; Lounsbury, V.J. Marine Mammals Ashore: A Field Guide for Strandings, 2nd ed.; National Aquarium in Baltimore: Baltimore, MD, USA, 2005; p. 371. [Google Scholar]
- Kuiken, T.; Garcia-Hartmann, M. Cetacean pathology: Dissection techniques and tissue sampling. In Proceedings of the European Cetacean Society Workshop, ECS, Leiden, The Netherlands, 13–14 September 1991. [Google Scholar]
- Camarão, B.C. Estudo da Reprodução de Pequenos Cetáceos Através da Morfologia do Ovário. Master’s Thesis, University of Aveiro, Aveiro, Portugal, 2017. [Google Scholar]
- Read, F. Understanding Cetacean and Fisheries Interactions in the North-West Iberian Peninsula. Ph.D. Thesis, University of Vigo, Vigo, Spain, 16 September 2015. [Google Scholar]
- Pugliares, K.R.; Bogomolni, A.; Touhey, K.M.; Herzig, S.M.; Harry, C.T.; Moore, M.J. Marine Mammal Necropsy: An Introductory Guide for Stranding Responders and Field Biologists; Woods Hole Oceanographic Institution Technical Report (WHOI-2007-06); Woods Hole Oceanographic Institution: Woods Hole, MA, USA, 2007; p. 133. [Google Scholar]
- Kuiken, T. (Ed.) A review of the criteria for the diagnosis of by-catch in cetaceans. In Diagnosis of Bycatch in Cetaceans, Proceedings of the Second European Cetacean Society Workshop on Cetacean Pathology, Montpelier, France, 2 March 1994; European Cetacean Society: Saskatoon, SK, Canada, 1994. [Google Scholar]
- Moore, M.J.; van der Hoop, J.; Barco, S.G.; Costidis, A.M.; Gulland, F.M.; Jepson, P.D.; Moore, K.T.; Raverty, S.; McLellan, W.A. Criteria and case definitions for serious innjury and death of pinnipeds and cetaceans caused by anthropogenic trauma. Dis. Aquat. Org. 2013, 103, 229–264. [Google Scholar] [CrossRef]
- Prata, J.C.; Costa, J.P.; Girão, A.V.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Identifying a quick and efficient method of removing organic matter without damaging microplastic samples. Sci. Total Environ. 2019, 686, 131–139. [Google Scholar] [CrossRef]
- Lavers, J.L.; Oppel, S.; Bond, A.L. Factors influencing the detection of beach plastic debris. Mar. Environ. Res. 2016, 119, 245–251. [Google Scholar] [CrossRef]
- Bessa, F.; Ratcliffe, N.; Otero, V.; Sobral, P.; Marques, J.C.; Waluda, C.M.; Trathan, P.N.; Xavier, J.C. Microplastics in gentoo penguins from the Antarctic region. Sci. Rep. 2019, 9, 14191. [Google Scholar] [CrossRef]
- Fragão, J.; Bessa, F.; Otero, V.; Barbosa, A.; Sobral, P.; Waluda, C.M.; Guímaro, H.R.; Xavier, J.C. Microplastics and other anthropogenic particles in Antarctica: Using penguins as biological samplers. Sci. Total Environ. 2021, 788, 147698. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Hernandez-Milian, G.; O’Brien, J.; Berrow, S.; O’Connor, I.; Officer, R. Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: The True’s beaked whale Mesoplodon mirus. Environ. Pollut. 2015, 199, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Dehaut, A.; Cassone, A.L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Munno, K.; Helm, P.A.; Jackson, D.A.; Rochman, C.; Sims, A. Impacts of temperature and selected chemical digestion methods on microplastic particles. Environ. Toxicol. Chem. 2018, 37, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Norén, F. Small Plastic Particles in Coastal Swedish Waters; KIMO Report; KIMO Sweden: Gothenburg, Sweden, 2007; p. 12. [Google Scholar]
- Abiñon, B.S.F.; Camporedondo, B.S.; Mercadal, E.M.B.; Olegario, K.M.R.; Palapar, E.M.H.; Ypil, C.W.R.; Tambuli, A.E.; Lomboy, C.A.; Garces, J.J.C. Abundance and characteristics of microplastics in commercially sold fishes from Cebu Island, Philippines. Int. J. Aquat. Biol 2020, 8, 424–433. [Google Scholar] [CrossRef]
- Devriese, L.I.; Van der Meulen, M.D.; Maes, T.; Bekaert, K.; Paul-Pont, I.; Frère, L.; Robbens, J.; Vethaak, A.D. Microplastic contamination in brown shrimp (Crangon crangon, Linnaeus 1758) from coastal waters of the southern North Sea and channel area. Mar. Pollut. Bull. 2015, 98, 179–187. [Google Scholar] [CrossRef]
- De Witte, B.; Devriese, L.; Bekaert, K.; Hoffman, S.; Vandermeersch, G.; Cooreman, K.; Robbens, J. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull. 2014, 85, 146–155. [Google Scholar] [CrossRef]
- Hanke, G.; Galgani, F.; Werner, S.; Oosterbaan, L.; Nilsson, P.; Fleet, D.; Kinsey, S.; Thompson, R.; Palatinus, A.; Van Franeker, J.; et al. MSFD GES Technical Subgroup on Marine Litter. Guidance on Monitoring of Marine Litter in European Seas; Joint Research Centre–Institute for Environment and Sustainability, Publications Office of the European Union: Luxembourg, 2013; p. 128. Available online: https://mcc.jrc.ec.europa.eu/documents/201702074014.pdf (accessed on 22 August 2023).
- Bessa, F.; Frias, J.; Kögel, T.; Lusher, A.; Andrade, J.M.; Antunes, J.; Sobral, P.; Pagter, E.; Nash, R.; O’Connor, I.; et al. Harmonized Protocol for Monitoring Microplastics in Biota; JPI-Oceans BASEMAN Project; JPI-Oceans BASEMAN Project: Brussels, Belgium, 2019; p. 30. [Google Scholar]
- Mann, H.B.; Whitney, D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Dunnett, C.W.; Tamhane, A.C. Step-up multiple testing of parameters with unequally correlated estimates. Biometrics 1995, 51, 217–227. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 22 August 2023).
- Moore, R.C.; Loseto, L.; Noel, M.; Etemadifar, A.; Brewster, J.D.; MacPhee, S.; Bendell, L.; Ross, P.S. Microplastics in beluga whales (Delphinapterus leucas) from the eastern Beaufort Sea. Mar. Pollut. Bull. 2020, 150, 110723. [Google Scholar] [CrossRef]
- Novillo, O.; Raga, J.T.; Tomas, J. Evaluating the presence of microplastics in striped dolphins (Stenella coeruleoalba) stranded in the Western Mediterranean Sea. Mar. Pollut. Bull. 2020, 160, 111557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yu, X.; Zhang, Q.; Li, Y.; Tan, S.; Li, D.; Yang, Z.; Wang, J. Cetaceans and microplastics: First report of microplastic ingestion by a coastal delphinid, Sousa chinensis. Sci. Total Environ. 2018, 659, 649–654. [Google Scholar] [CrossRef]
- Villanova-Solano, C.; Díaz-Peña, F.J.; Hernández-Sánchez, C.; González-Sálamo, J.; González-Pleiter, M.; Vega-Moreno, D.; Fernández-Piñas, F.; Fraile-Nuez, E.; Machín, F.; Hernández-Borges, J. Microplastic pollution in sublittoral coastal sediments of a North Atlantic island: The case of La Palma (Canary Islands, Spain). Chemosphere 2022, 288, 12. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Burke, A.; O’Connor, I.; Officer, R. Microplastic pollution in the Northeast Atlantic Ocean: Validated and opportunistic sampling. Mar. Pollut. Bull. 2014, 88, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Chouchene, K.; Prata, J.C.; da Costa, J.; Duarte, A.C.; Rocha-Santos, T.; Ksibi, M. Microplastics on Barra beach sediments in Aveiro, Portugal. Mar. Pollut. Bull. 2021, 167, 112264. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, P.M.; Serra-Gonçalves, C.; Ferreira, J.L.; Catry, T.; Granadeiro, J.P. Plastic and other microfibers in sediments, macroinvertebrates and shorebirds from three intertidal wetlands of southern Europe and west Africa. Environ. Pollut. 2017, 231, 123–133. [Google Scholar] [CrossRef]
- Frias, J.P.; Gago, J.; Otero, V.; Sobral, P. Microplastics in coastal sediments from Southern Portuguese shelf waters. Mar. Environ. Res. 2016, 114, 24–30. [Google Scholar] [CrossRef]
- Duncan, E.M.; Broderick, A.C.; Fuller, W.J.; Galloway, T.S.; Godfrey, M.H.; Hamann, M.; Limpus, C.J.; Lindeque, P.K.; Mayes, A.G.; Omeyer, L.C. Microplastic ingestion ubiquitous in marine turtles. Glob. Chang. Biol. 2019, 25, 744–752. [Google Scholar] [CrossRef]
- Vingada, J.; Eira, C. Conservation of Cetaceans and Seabirds in Continental Portugal. In The LIFE + MarPro Project; Rainho & Neves, Lda.: Aveiro, Portugal, 2018; p. 257. [Google Scholar]
- Maximenko, N.; Hafner, J.; Niiler, P. Pathways of marine debris derived from trajectories of Lagrangian drifters. Mar. Pollut. Bull. 2012, 65, 51–62. [Google Scholar] [CrossRef]
- Law, K.L.; Moret-Ferguson, S.; Maximenko, N.A.; Proskurowski, G.; Peacock, E.E.; Hafner, J.; Reddy, C.M. Plastic accumulation in the North Atlantic Subtropical Gyre. Science 2010, 329, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- IPRC-International Pacific Research Center. Tracking ocean debris. IPRC Climate 2008, 8, 14. Available online: http://iprc.soest.hawaii.edu/newsletters/iprc_climate_vol8_no2.pdf (accessed on 22 August 2023).
- Alimi, O.S.; Claveau-Mallet, D.; Lapointe, M.; Biu, T.; Liu, L.; Hernandez, L.M.; Bayen, S.; Tufenkji, N. Effects of weathering on the properties and fate of secondary microplastics from a polystyrene single-use cup. J. Hazard. Mater. 2023, 459, 131855. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhardwaj, A.; Thakur, M.; Saini, A. Understanding microplastic pollution of marine ecosystem: A review. Environ Sci. Pollut. Res. 2023, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.M.; Almeida, C.M.R.; Ramos, S. Microplastics contamination along the coastal waters of NW Portugal. Case Stud. Chem. Environ. Eng. 2020, 2, 100056. [Google Scholar] [CrossRef]
- Hocking, D.P.; Marx, F.G.; Park, T.; Fitzgerald, E.M.G.; Evans, A.R. A behavioural framework for the evolution of feeding in predatory aquatic mammals. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162750. [Google Scholar] [CrossRef]
- Werth, A.J. Mandibular and dental variation and the evolution of suction feeding in Odontoceti. J. Mammal. 2006, 87, 579–588. [Google Scholar] [CrossRef]
- Lopes, C.; Ambrosino, A.C.; Figueiredo, C.; Caetano, M.; Santos, M.M.; Garrido, S.; Raimundo, J. Microplastic distribution in different tissues of small pelagic fish of the Northeast Atlantic Ocean. Sci. Total Environ. 2023, 901, 166050. [Google Scholar] [CrossRef]
- da Silva, J.M.; Alves, L.M.F.; Laranjeiro, M.I.; Bessa, F.; Silva, A.V.; Norte, A.C.; Lemos, M.F.L.; Ramos, J.A.; Novais, S.C.; Ceia, F.R. Accumulation of chemical elements and occurrence of microplastics in small pelagic fish from a neritic environment. Environ. Pollut. 2022, 292, 118451. [Google Scholar] [CrossRef]
- Pequeno, J.; Antunes, J.; Dhimmer, V.; Bessa, F.; Sobral, P. Microplastics in marine and estuarine species from the coast of Portugal. Front. Environ. Sci. 2021, 9, 579127. [Google Scholar] [CrossRef]
- Lopes, C.; Raimundo, J.; Caetano, M.; Garrido, S. Microplastic ingestion and diet composition of planktivorous fish. Limnol. Oceanogr. 2020, 5, 103–112. [Google Scholar] [CrossRef]
- Neves, D.; Sobral, P.; Ferreira, J.L.; Pereira, T. Ingestion of microplastics by commercial fish off the Portuguese coast. Mar. Pollut. Bull. 2015, 101, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Guilhermino, L.; Martins, A.; Lopes, C.; Raimundo, J.; Vieira, L.R.; Barboza, L.G.A.; Costa, J.; Antunes, C.; Caetano, M.; Vale, C. Microplastics in fishes from an estuary (minho river) ending into the NE atlantic ocean. Mar. Pollut. Bull. 2021, 173, 113008. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Suspected microplastics in Atlantic horse mackerel fish (Trachurus trachurus) captured in Portugal. Mar. Pollut. Bull. 2022, 174, 113249. [Google Scholar] [CrossRef]
- Marçalo, A.; Nicolau, L.; Giménez, J.; Ferreira, M.; Santos, J.; Araújo, H.; Silva, A.; Vingada, J.; Pierce, G.J. Feeding ecology of the common dolphin (Delphinus delphis) in Western Iberian waters: Has the decline in sardine (Sardina pilchardus) afected dolphin diet? Mar. Biol. 2018, 165, 44. [Google Scholar] [CrossRef]
- Pinheiro, G.A.J. Contribuição para o estudo da dieta de pequenos cetáceos em Portugal Continental. Master’s Thesis, University of Aveiro, Aveiro, Portugal, 18 December 2017. Available online: http://hdl.handle.net/10773/21949 (accessed on 22 August 2023).
- Margarido, I. Contribuição para a avaliação da dieta do golfinho-comum (Delphinus delphis) na costa continental portuguesa. Master’s Thesis, University of Aveiro, Aveiro, Portugal, 2015. Available online: http://hdl.handle.net/10773/15926 (accessed on 22 August 2023).
- Aguiar, Z.V.P. Ecologia alimentar do bôto (Phocoena phocoena) ao longo da costa continental Portuguesa. Master’s Thesis, University of Porto, Porto, Portugal, 11 December 2013. Available online: https://repositorio-aberto.up.pt/handle/10216/70768 (accessed on 22 August 2023).
- Marçalo, A.; Giménez, J.; Nicolau, L.; Frois, J.; Ferreira, M.; Sequeira, M.; Eira, C.; Pierce, G.J.; Vingada, J. Stranding patterns and feeding ecology of striped dolphins, Stenella coeruleoalba, in Western Iberia (1981–2014). J. Sea Res. 2021, 169, 101996. [Google Scholar] [CrossRef]
- Kastelein, R.A.; Staal, C.; Terlouw, A.; Muller, M. Pressure changes in the mouth of a feeding harbour porpoise (Phocoena phocoena). In The Biology of the Harbor Porpoise; Read, A.J., Wiepkma, P.R., Nachtigall, P.E., Eds.; DeSpil Publishers: Woerden, The Netherlands, 1997; pp. 279–291. [Google Scholar]
- Smith, G.J.D. The stomach of the harbor porpoise Phocoena phocoena (L.). Can. J. Zool. 2011, 50, 1611–1616. [Google Scholar] [CrossRef]
- Bessa, F.; Barría, P.; Neto, J.M.; Frias, J.P.G.L.; Otero, V.; Sobral, P.; Marques, J.C. Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 2018, 128, 575–584. [Google Scholar] [CrossRef]
- Bellas, J.; Martínez-Armental, J.; Martínez-Cámara, A.; Besada, V.; Martínez-Gómez, C. Ingestion of microplastics by demersal fish from the spanish Atlantic and mediterranean coasts. Mar. Pollut. Bull. 2016, 109, 55–60. [Google Scholar] [CrossRef]
- Barry, J.; Rindorf, A.; Gago, J.; Silburn, B.; McGoran, A.; Russell, J. Top 10 marine litter items on the seafloor in European seas from 2012 to 2020. Sci. Total Environ. 2023, 902, 165997. [Google Scholar] [CrossRef]
- Galgani, F.; Leaute, J.P.; Moguedet, P.; Souplet, A.; Verin, Y.; Carpentier, A.; Goraguer, H.; Latrouite, D.; Andral, B.; Cadiou, Y.; et al. Litter on the sea floor along European coasts. Mar. Pollut. Bull. 2000, 40, 516–527. [Google Scholar] [CrossRef]
- Anastasopoulou, A.; Virsek, M.K.; Varezic, D.B.; Digka, N.; Fortibuoni, T.; Koren, S.; Mandic, M.; Mytilineou, C.; Pesic, A.; Ronchi, F.; et al. Assessment on marine litter ingested by fish in the Adriatic and NE Ionian Sea macro-region (Mediterranean). Mar. Pollut. Bull. 2018, 133, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Rummel, C.D.; Loder, M.G.J.; Fricke, N.F.; Lang, T.; Griebeler, E.M.; Janke, M.; Gerdts, G. Plastic ingestion by pelagic and demersal fish from the North Sea and Baltic Sea. Mar. Pollut. Bull. 2016, 102, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the english channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef]
- Hernandez-Milian, G.; Tsangaris, C.; Anestis, A.; Fossi, M.C.; Baini, M.; Caliani, I.; Panti, C.; Bundone, L.; Panou, A. Monk seal faeces as a non-invasive technique to monitor the incidence of ingested microplastics and potential presence of plastic additives. Mar. Pollut. Bull. 2023, 193, 115227. [Google Scholar] [CrossRef]
- Merrill, G.B.; Hermabessiere, L.; Rochman, C.M.; Nowacek, D.P. Microplastics in marine mammal blubber, melon, & other tissues: Evidence of translocation. Environ. Pollut. 2023, 335, 122252. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and release of chemicals from plastics to the environment and to wildlife. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef]
- Galli, M.; Garcia, T.O.; Baini, M.; Urbán, J.; Ramírez-Macías, D.; Viloria-Gómora, L.; Panti, C.; Martellini, T.; Cincinelli, A.; Fossi, M.C. Microplastic occurrence and phthalate ester levels in neuston samples and skin biopsies of filter-feeding megafauna from La Paz Bay (Mexico). Mar. Pollut. Bull. 2023, 192, 115086. [Google Scholar] [CrossRef]
- Routti, H.; Harju, M.; Lühmann, K.; Aars, J.; Ask, A.; Goksøyr, A.; Kovacs, K.M.; Lydersen, C. Concentrations and endocrine disruptive potential of phthalates in marine mammals from the Norwegian Arctic. Environ. Int. 2021, 152, 106458. [Google Scholar] [CrossRef]
- Fossi, M.C.; Panti, C.; Guerranti, C.; Coppola, D.; Giannetti, M.; Marsili, L.; Minutoli, R. Are baleen whales exposed to the threat of microplastics? a case study of the Mediterranean fin whale (Balaenoptera physalus). Mar. Pollut. Bull. 2012, 64, 2374–2379. [Google Scholar] [CrossRef]
- Hart, L.B.; Beckingham, B.; Wells, R.S.; Flagg, M.A.; Wischusen, K.; Moors, A.; Kucklick, J.; Pisarski, E.; Wirth, E. Urinary phthalate metabolites in common bottlenose dolphins (Tursiops truncatus) from sarasota Bay, FL, USA. GeoHealth 2018, 2, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Rian, M.B.; Vike-Jonas, K.; Gonzalez, S.V.; Ciesielski, T.M.; Venkatraman, V.; Lindstrøm, U.; Jenssen, B.M.; Asimakopoulos, A.G. Phthalate metabolites in harbor porpoises (Phocoena phocoena) from Norwegian coastal waters. Environ. Int. 2020, 137, 105525. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.S.; Pinto da Costa, J. Methods for the extraction of microplastics in complex solid, water and biota samples. Trends Environ. Anal. Chem. 2022, 33, e00151. [Google Scholar] [CrossRef]

| ID | Species | Year | TBL (cm) | Sex | Age | Maturity | Body Condition | Parasites | Cause of Death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Delphinus delphis | 2016 | −130 * | F | nd | Immature | Moderate | No | Bycatch |
| 2 | Delphinus delphis | 2016 | 184 | M | Juvenile | Immature | Good | Yes | Bycatch |
| 3 | Delphinus delphis | 2017 | 210 | M | Adult | Mature | Moderate | Yes | Bycatch |
| 4 | Delphinus delphis | 2017 | 192 | F | Adult | Mature | Thin | No | Disease |
| 5 | Delphinus delphis | 2017 | 174.5 | M | Juvenile | Immature | Good | Yes | Bycatch |
| 6 | Delphinus delphis | 2017 | 125 | M | Calf | Immature | Moderate | Yes | Bycatch |
| 7 | Delphinus delphis | 2017 | 132 | M | Calf | Immature | Good | No | Bycatch |
| 8 | Delphinus delphis | 2017 | 130 | M | Calf | Immature | Good | Yes | Bycatch |
| 9 | Delphinus delphis | 2017 | 150 | M | Juvenile | Immature | Good | No | Bycatch |
| 10 | Delphinus delphis | 2017 | 145 | M | Juvenile | Immature | Good | Yes | Bycatch |
| 11 | Delphinus delphis | 2017 | 173 | F | Juvenile | Immature | Thin | Yes | Bycatch |
| 12 | Delphinus delphis | 2017 | 175.5 | F | Juvenile | Immature | Moderate | Yes | Disease |
| 13 | Delphinus delphis | 2017 | 192 | F | Adult | Mature | Thin | Yes | Disease |
| 14 | Delphinus delphis | 2017 | 196 | F | Adult | Mature | Thin | Yes | Disease |
| 15 | Delphinus delphis | 2018 | −125 * | F | nd | Immature | Good | Yes | Bycatch |
| 16 | Delphinus delphis | 2018 | 141 | F | Juvenile | Immature | Good | No | Trauma |
| 17 | Delphinus delphis | 2018 | 135 | F | Calf | Immature | Good | Yes | Bycatch |
| 18 | Delphinus delphis | 2018 | 119 | M | Calf | Immature | Moderate | No | Bycatch |
| 19 | Delphinus delphis | 2019 | 138 | M | Calf | Immature | Moderate | Yes | Bycatch |
| 20 | Delphinus delphis | 2019 | 167 | M | Juvenile | Immature | Moderate | Yes | Bycatch |
| 21 | Delphinus delphis | 2019 | 181 | F | Juvenile | Immature | Moderate | Yes | Bycatch |
| 22 | Delphinus delphis | 2019 | 125 | F | Calf | Immature | Good | No | Bycatch |
| 23 | Delphinus delphis | 2019 | 158 | M | Juvenile | Immature | Skeletal | Yes | Disease |
| 24 | Delphinus delphis | 2019 | 196 | F | Adult | Mature | nd | nd | Bycatch |
| 25 | Phocoena phocoena | 2017 | 146 | F | Juvenile | Immature | Good | Yes | Bycatch |
| 26 | Phocoena phocoena | 2017 | 144 | F | Juvenile | Immature | Moderate | Yes | Bycatch |
| 27 | Phocoena phocoena | 2017 | 154 | M | Juvenile | Immature | Thin | Yes | Bycatch |
| 28 | Phocoena phocoena | 2017 | 174 | M | Adult | Mature | Thin | Yes | Bycatch |
| 29 | Phocoena phocoena | 2017 | 147 | M | Juvenile | Immature | Thin | Yes | Bycatch |
| 30 | Phocoena phocoena | 2017 | 150 | F | Juvenile | Immature | Moderate | Yes | Bycatch |
| 31 | Phocoena phocoena | 2017 | 156.5 | F | Juvenile | Immature | Good | Yes | Trauma |
| 32 | Phocoena phocoena | 2018 | 136 | F | Juvenile | Immature | Good | Yes | Bycatch |
| 33 | Stenella coeruleoalba | 2017 | 175 | F | nd | Immature | Thin | Yes | Disease |
| 34 | Stenella coeruleoalba | 2018 | 178 | F | nd | Immature | nd | nd | Disease |
| 35 | Stenella coeruleoalba | 2018 | 176 | M | nd | Immature | nd | Yes | Disease |
| 36 | Stenella coeruleoalba | 2018 | 159 | M | nd | Immature | Thin | Yes | Disease |
| 37 | Stenella coeruleoalba | 2018 | 136 | F | nd | Immature | Thin | Yes | Disease |
| 38 | Stenella coeruleoalba | 2019 | 136 | M | nd | Immature | Moderate | No | nd |
| Species | Type | N (%N) | F.O.% | Mean (±SD) | Median (IQR) | Range |
|---|---|---|---|---|---|---|
| D. delphis | Fibres | 100 (84.75) | 79.17 | 4.17 ± 5.75 | 2.5 (1.0–5.5) | 0–27 |
| Fragments | 10 (8.47) | 20.83 | 0.42 ± 1.02 | 0 (0) | 0–4 | |
| Films | 7 (5.93) | 20.83 | 0.29 ± 0.62 | 0 (0) | 0–2 | |
| Fibre clusters | 1 (0.85) | 4.17 | - | - | 0–1 | |
| Total | 118 (100) | 87.50 | 4.92 ± 5.76 | 3 (1.75–6.25) | 0–27 | |
| P. phocoena | Fibres | 66 (80.49) | 100 | 8.25 ± 6.27 | 5 (3.75–14.50) | 2–17 |
| Fragments | 11 (13.41) | 25.00 | 1.38 ± 2.88 | 0 (0–0.75) | 0–8 | |
| Films | 5 (6.10) | 25.00 | 0.63 ± 1.19 | 0 (0–0.50) | 0–3 | |
| Fibre clusters | 0 | 0 | - | - | - | |
| Total | 82 (100) | 100 | 10.25 ± 7.21 | 9 (4.0–14.75) | 3–22 | |
| S. coeruleoalba | Fibres | 29 (53.70) | 100 | 4.83 ± 4.49 | 3 (2.25–6.00) | 1–13 |
| Fragments | 23 (42.59) | 83.33 | 3.83 ± 6.97 | 1 (1–1.75) | 0–18 | |
| Films | 2 (3.70) | 16.67 | 0.33 ± 0.82 | 0 (0) | 0–2 | |
| Fibre clusters | 0 | 0 | - | - | - | |
| Total | 54 (100) | 100 | 9.00 ± 6.96 | 6.5 (5.0–11.75) | 2–21 | |
| All individuals | Fibres | 195 (76.77) | 86.84 | 5.13 ± 5.79 | 3 (1.0–7.0) | 0–27 |
| Fragments | 44 (17.32) | 31.58 | 1.16 ± 3.21 | 0 (0–1) | 0–18 | |
| Films | 14 (5.51) | 21.05 | 0.37 ± 0.79 | 0 (0) | 0–3 | |
| Fibre clusters | 1 (0.39) | 2.63 | - | - | - | |
| Total | 254 (100) | 92.11 | 6.68 ± 6.53 | 4.5 (2.25–9.50) | 0–27 |
| Study area | Species | Plastic Particles | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number | % F.O. | Mean Number ± SD (Range) | Colour | Size Class (mm) | Mean Size ± SD mm | Digestive Compartment | |||
| NE Atlantic (Portugal) | |||||||||
| common dolphin (24) harbour porpoise (8) striped dolphin (6) | 254 micro | 92.11 | 7.05 ± 6.45 micro/mesoplastics (0–27) | blue, black | 0.5–1.0 | micro/mesoplastics, 2.01 ± 1.73 microfibres, 1.88 ± 1.12 micro/meso, 2.14 ± 1.68 | intestine | Present study | |
| NE Atlantic, Macaronesia (Canary and Madeira archipelagos) | |||||||||
| striped dolphins (5) bottlenose dolphin (2) Risso’s dolphins (2) Short-finned pilot whale (1) Pygmy sperm whale (1) Fraser’s dolphin (1) | 722 * micro/meso | 100 | 59.08 ± 40.52 fibres 3.00 ± 1.15 fragments | green, red | na | micro/meso, 2.66 ± 2.51 | oesophagus, stomach, duodenal ampulla and intestine | [50] | |
| W Mediterranean (Valencia, Spain) | |||||||||
| striped dolphin (43) | 672 micro | 90.5 | 14.9 ± 22.3 (0–82) | black, red | na | microfibres, no size | digestive tract | [82] | |
| Arctic (Beaufort Sea) | |||||||||
| belugas (7) | 81 micro | 100 | 11.6 ± 6.6 | na | na | fragments, no size | stomach intestine | [81] | |
| NE Atlantic (UK) | |||||||||
| cetaceans (43) pinnipeds (7) | 261 micro | 100 | 5.5 ± 2.7 (1–12) | blue, black | 0.5–1.0 | micro/meso, 2.00 ± 2.30 | stomachs and intestines | [48] | |
| W Pacific Ocean, South China Sea (Guangxi Beibu Gulf, China) | |||||||||
| Indo-Pacific humpback dolphin (3) | 77 micro | 100 | na | white, blue | na | microfibres, 2.20 ± 0.40 | intestine | [83] | |
| NE Atlantic (Galicia, Spain) | |||||||||
| common dolphins (35) | 411 micro/meso | 100 | 12.0 ± 8.0 (3–41) | blue, black | na | microfibres, 2.11 ± 1.26 | stomachs | [51] | |
| NE Atlantic (Ireland) | |||||||||
| delphinids (19) beaked whales (2) | 598 micro/meso | 100 | (1–88) | blue, grey | 1.0–2.0 | microfibres, no size | oesophagus, stomachs, intestine | [19] | |
| NE Atlantic (Ireland) | |||||||||
| True’s beaked whale (1) | 88 micro/meso | - | na | na | na | micro/mesofibres, 2.16 ± 1.39 | stomach, intestine | [68] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sá, S.; Torres-Pereira, A.; Ferreira, M.; Monteiro, S.S.; Fradoca, R.; Sequeira, M.; Vingada, J.; Eira, C. Microplastics in Cetaceans Stranded on the Portuguese Coast. Animals 2023, 13, 3263. https://doi.org/10.3390/ani13203263
Sá S, Torres-Pereira A, Ferreira M, Monteiro SS, Fradoca R, Sequeira M, Vingada J, Eira C. Microplastics in Cetaceans Stranded on the Portuguese Coast. Animals. 2023; 13(20):3263. https://doi.org/10.3390/ani13203263
Chicago/Turabian StyleSá, Sara, Andreia Torres-Pereira, Marisa Ferreira, Sílvia S. Monteiro, Raquel Fradoca, Marina Sequeira, José Vingada, and Catarina Eira. 2023. "Microplastics in Cetaceans Stranded on the Portuguese Coast" Animals 13, no. 20: 3263. https://doi.org/10.3390/ani13203263
APA StyleSá, S., Torres-Pereira, A., Ferreira, M., Monteiro, S. S., Fradoca, R., Sequeira, M., Vingada, J., & Eira, C. (2023). Microplastics in Cetaceans Stranded on the Portuguese Coast. Animals, 13(20), 3263. https://doi.org/10.3390/ani13203263






