Antimicrobial Use in On-Farm Hatching Systems vs. Traditional Hatching Systems: A Case Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Flock Size and Husbandry Conditions
2.2. Study Sample and Data Collection
2.3. AMU Quantification
2.4. Quantitative Analysis
2.5. Qualitative Analysis
3. Results
3.1. AMU at Flock Level
3.2. Indication for AMU
4. Discussion
4.1. Method of Data Collection and Processing
4.2. Husbandry Conditions
4.3. Overall Antimicrobial Use
4.4. Respiratory Disorders and Use of Lincomycin-Spectinomycin
4.5. Impact of On-Farm Hatching on Intestinal Health
4.6. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, K.C.; Gupta, S.; Chander, Y.; Singh, A.K. Antibiotic Use in Agriculture and Its Impact on the Terrestrial Environment. Adv. Agron. 2005, 87, 1–54. [Google Scholar]
- Agyare, C.; Etsiapa Boamah, V.; Ngofi Zumbi, C.; Boateng Osei, F. Antibiotic Use in Poultry Production and Its Effects on Bacterial Resistance. In Antimicrobial Resistance–A Global Threat; Kumar, Y., Ed.; IntechOpen: London, UK, 2019; ISBN 9781789857832. [Google Scholar]
- Windhorst, H.-W. Dynamics and Patterns of Global Poultry-Meat Production. In Poultry Quality Evaluation; Woodhead Publishing: Sawston, UK, 2017; pp. 1–25. ISBN 9780081007631. [Google Scholar]
- Joosten, P.; Sarrazin, S.; Van Gompel, L.; Luiken, R.E.C.; Mevius, D.J.; Wagenaar, J.A.; Heederik, D.J.J.; Dewulf, J.; EFFORT consortium; Graveland, H.; et al. Quantitative and Qualitative Analysis of Antimicrobial Usage at Farm and Flock Level on 181 Broiler Farms in Nine European Countries. J. Antimicrob. Chemoth. 2019, 74, 798–806. [Google Scholar] [CrossRef]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between Veterinary Antimicrobial Use and Antimicrobial Resistance in Food-Producing Animals: A Report on Seven Countries. J. Antimicrob. Chemoth. 2014, 69, 827–834. [Google Scholar] [CrossRef]
- European Commission. Ban on Antibiotics as Growth Promotors in Animal Feed Enters into Effect. 2005. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_05_1687. (accessed on 6 January 2023).
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018. 2015. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2018-trends-2010–2018-tenth-esvac-report_en.pdf. (accessed on 2 January 2023).
- European Commission. A European One Health Action Plan against Antimicrobial Resistance (AMR). 2020. Available online: https://health.ec.europa.eu/system/files/2020-01/amr_2017_action-plan_0.pdf. (accessed on 2 January 2023).
- Shira, E.B.; Sklan, D.; Friedman, A. Impaired Immune Responses in Broiler Hatchling Hindgut Following Delayed Access to Feed. Vet. Immunol. Immunopathol. 2005, 105, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, H.; Debonne, M.; Swennen, Q.; Everaert, N.; Careghi, C.; Han, H.; Bruggeman, V.; Tona, K.; Decuypere, E. Delay in Feed Access and Spread of Hatch: Importance of Early Nutrition. World’s Poultry Sci. J. 2010, 66, 177–188. [Google Scholar] [CrossRef]
- De Jong, I.C.; Van Riel, J.; Bracke, M.B.M.; Van Den Brand, H. A “meta-Analysis” of Effects of Post-Hatch Food and Water Deprivation on Development, Performance and Welfare of Chickens. PLoS ONE 2017, 12, e0189350. [Google Scholar] [CrossRef]
- Uni, Z.; Ganot, S.; Sklan, D. Posthatch Development of Mucosal Function in the Broiler Small Intestine. Poultry Sci. 1998, 77, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Maiorka, A.; Santin, E.; Dahlke, F.; Boleli, I.C.; Furlan, R.L.; Macari, M. Posthatching Water and Feed Deprivation Affect the Gastrointestinal Tract and Intestinal Mucosa Development of Broiler Chicks. J. Appl. Poultry Res. 2003, 12, 483–492. [Google Scholar] [CrossRef]
- Panda, A.K.; Bhanja, S.K.; Shyam Sunder, G. Early Post Hatch Nutrition on Immune System Development and Function in Broiler Chickens. World’s Poultry Sci. J. 2015, 71, 285–296. [Google Scholar] [CrossRef]
- De Gouw, P.; Van De Ven, L.J.F.; Lourens, S.; Kemp, B.; Van Den Brand, H. Effects of Dust, Formaldehyde and Delayed Feeding on Early Postnatal Development of Broiler Chickens. Res. Vet. Sci. 2017, 112, 201–207. [Google Scholar] [CrossRef]
- Mitchell, M.; Kettlewell, P. Welfare of Poultry during Transport—A Review. In Proceedings of the 8th European Symposium on Poultry Welfare, Cervia, Italy, 18–22 May 2009; pp. 90–100. [Google Scholar]
- De Jong, I.C.; Van Hattum, T.; Van Riel, J.W.; De Baere, K.; Kempen, I.; Cardinaels, S.; Gunnink, H. Effects of On-Farm and Traditional Hatching on Welfare, Health, and Performance of Broiler Chickens. Poultry Sci. 2020, 99, 4662–4671. [Google Scholar] [CrossRef]
- Jessen, C.T.; Foldager, L.; Riber, A.B. Effects of Hatching On-Farm on Performance and Welfare of Organic Broilers. Poultry Sci. 2021, 100, 101292. [Google Scholar] [CrossRef]
- Dibner, J.J.; Knight, C.D.; Kitchell, M.L.; Atwell, C.A.; Downs, A.C.; Ivey, F.J. Early Feeding and Development of the Immune System in Neonatal Poultry. J. Appl. Poultry Res. 1998, 7, 425–436. [Google Scholar] [CrossRef]
- Giersberg, M.F.; Molenaar, R.; De Jong, I.C.; Souza Da Silva, C.; Van Den Brand, H.; Kemp, B.; Rodenburg, T.B. Effects of Hatching System on the Welfare of Broiler Chickens in Early and Later Life. Poultry Sci. 2021, 100, 100946. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare (AHAW); Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar Schmidt, C.; et al. Welfare of Domestic Birds and Rabbits Transported in Containers. EFSA J. 2022, 20, e07441. [Google Scholar] [CrossRef] [PubMed]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare); Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Schmidt, C.G.; et al. Welfare of Broilers on Farm. EFSA J. 2023, 21, e07788. [Google Scholar] [CrossRef]
- NestBorn. The Process. Available online: https://www.nestborn.eu/the-process/ (accessed on 6 February 2023).
- Persoons, D.; Dewulf, J.; Smet, A.; Herman, L.; Heyndrickx, M.; Martel, A.; Catry, B.; Butaye, P.; Haesebrouck, F. Antimicrobial Use in Belgian Broiler Production. Prev. Vet. Med. 2012, 105, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Jensen, V.F.; Jacobsen, E.; Bager, F. Veterinary Antimicrobial-Usage Statistics Based on Standardized Measures of Dosage. Prev. Vet. Med. 2004, 64, 201–215. [Google Scholar] [CrossRef]
- ANIVED. Standard Operating Procedure (SOP) Berekening van de DDDAF voor Antimicrobiële Middelen voor de Pluimveesector. 2017. Available online: https://www.avined.nl/wp-content/uploads/2017-091-e0001-sop_dddaf_pluimveesector_v1.3.pdf (accessed on 6 February 2023).
- AMCRA. Formularium. 2023. Available online: https://formularium.amcra.be/classification.php. (accessed on 10 February 2023).
- Belplume. Periodiek Rapport Pluimvee, Handleiding. Available online: https://www.belplume.be/swfiles/files/Handleiding_ABR-pluimvee_NL_v4_FINAL.pdf. (accessed on 10 February 2023).
- Maki, J.J.; Bobeck, E.A.; Sylte, M.J.; Looft, T. Eggshell and Environmental Bacteria Contribute to the Intestinal Microbiota of Growing Chickens. J. Anim. Sci. Biotechnol. 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Sert, D.; Aygun, A.; Torlak, E.; Mercan, E. Effect of Ultrasonic Treatment on Reduction of Esherichia Coli ATCC 25922 and Egg Quality Parameters in Experimentally Contaminated Hens’ Shell Eggs. J. Sci. Food Agric. 2013, 93, 2973–2978. [Google Scholar] [CrossRef]
- Kasabova, S.; Hartmann, M.; Freise, F.; Hommerich, K.; Fischer, S.; Wilms-Schulze-Kump, A.; Rohn, K.; Käsbohrer, A.; Kreienbrock, L. Antibiotic Usage Pattern in Broiler Chicken Flocks in Germany. Front. Vet. Sci. 2021, 8, 673809. [Google Scholar] [CrossRef] [PubMed]
- De Briyne, N.; Iatridou, D.; Vanderhaeghen, W.; Ignate, K. Use of Antimicrobials in Practice (Targeted on Cattle, Pigs, Poultry, Horses). Antimicrobials in Livestock 1: Regulation, Science, Practice; Pokludová, L., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 43–79. ISBN 9783030467203. [Google Scholar]
- Ranjitkar, S.; Lawley, B.; Tannock, G.; Engberg, R.M. Bacterial Succession in the Broiler Gastrointestinal Tract. Appl. Environ. Microbiol. 2016, 82, 2399–2410. [Google Scholar] [CrossRef] [PubMed]
- Dieryck, I.; De Backere, J.; Paeshuyse, J. Effect of Hatching System and Prophylactic Antibiotic Use on Serum Levels of Intestinal Health Biomarker Diamine Oxidase in Broilers at an Early Age. Anim. 2022, 16, 100493. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.D.; Zauszniewski, J.A.; Musil, C.M. How to Determine Whether a Convenience Sample Represents the Population. Appl. Nurs. Res. 2004, 17, 130–133. [Google Scholar] [CrossRef]
- NestBorn. Benefits For Farmers–NestBorn, Turning Animal Welfare into Profit. Available online: https://www.nestborn.eu/for-farmers/ (accessed on 29 July 2022).
- Caekebeke, N.; Ringenier, M.; Jonquiere, F.; Tobias, T.; Postma, M.; Van Den Hoogen, A.; Houben, M.; Velkers, F.; Sleeckx, N.; Stegeman, A.; et al. Coaching Belgian and Dutch Broiler Farmers Aimed at Antimicrobial Stewardship and Disease Prevention. Antibiotics 2021, 10, 590. [Google Scholar] [CrossRef] [PubMed]
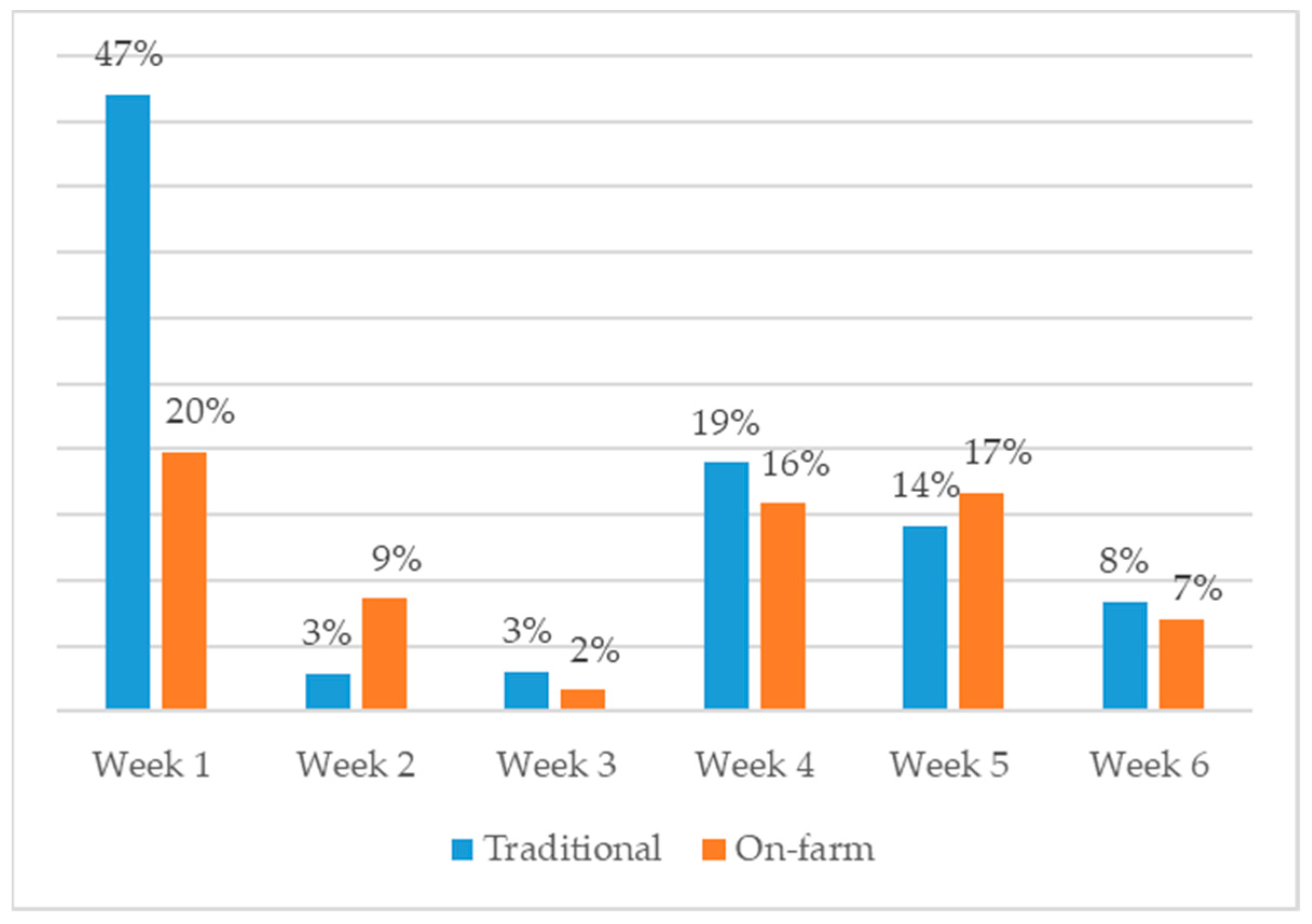
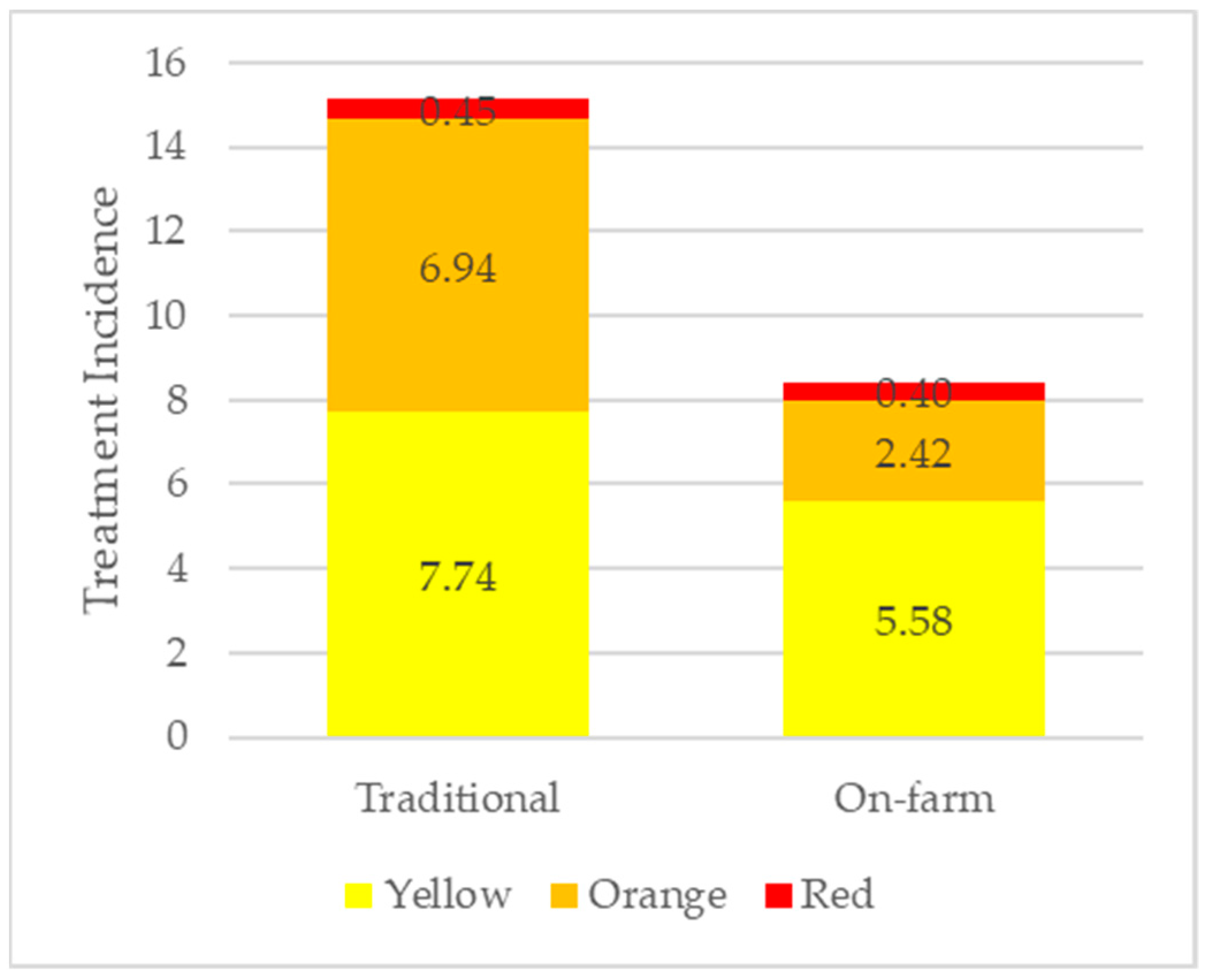
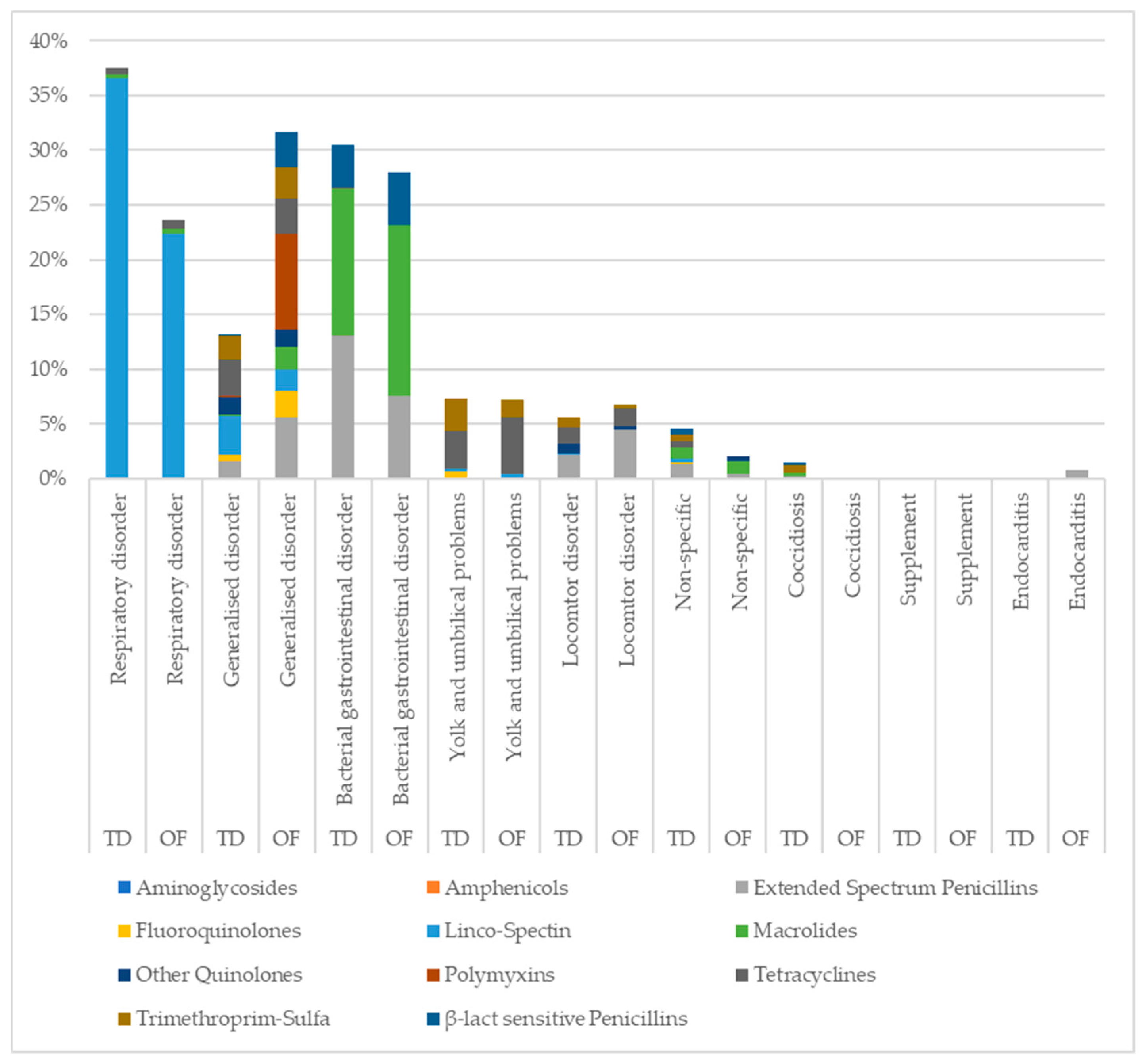
| Traditional | On-Farm | ||||
|---|---|---|---|---|---|
| Indication | Included Diagnoses | Count (n) | Percentage (%) | Count (n) | Percentage (%) |
| Locomotor disorder | Arthritis of the knee joint Arthritis of the hock Arthritis of the hock + knee joint Femoral neck necrosis Kinky back Osteomyelitis | 268 | 5.32 | 17 | 4.74 |
| Respiratory disorder | Airsacculitis Sinusitis Inflammation of the upper respiratory tract Pneumonia | 1788 | 35.50 | 59 | 16.43 |
| Bacterial gastrointestinal disorder | Enteritis Dysbacteriosis Necrotic enteritis Haemorrhagic enteritis | 1453 | 28.85 | 70 | 19.50 |
| Coccidiosis | Coccidiosis E. Acervulina Coccidiosis E. Tenella Coccidiosis E. Maxima | 71 | 1.41 | 0 | 0.00 |
| Yolk and umbilical disorder | Omphalitis Yolk sac infection Yolk retention | 347 | 6.89 | 18 | 5.01 |
| Generalised disorder | Image of bacterial infection Septicaemia Polyserositis Pericarditis | 621 | 12.33 | 79 | 22.01 |
| Supplement | Supplement | 3 | 0.06 | 0 | 0.00 |
| Endocarditis | Endocarditis | 0 | 0.00 | 2 | 0.56 |
| Non-specific | No diagnosis given | 215 | 4.27 | 5 | 1.39 |
| Grand Total | 4766 | 94.62 | 250 | 69.64 | |
| Traditional | On-Farm | |||
|---|---|---|---|---|
| Antimicrobial Class | Mean | Standard Deviation | Mean | Standard Deviation |
| TI Aminoglycosides | 0.00 a | 0.04 | 0.00 a | 0.00 |
| TI Amphenicols | 0.01 a | 0.04 | 0.00 a | 0.00 |
| TI Extended Spectrum Penicillins | 1.53 a | 3.79 | 1.15 a | 4.03 |
| TI Fluoroquinolones | 0.24 a | 1.74 | 0.22 a | 1.45 |
| TI Lincomycin-spectinomycin | 6.83 a | 7.41 | 2.13 b | 5.65 |
| TI Macrolides | 0.36 a | 1.41 | 0.18 a | 0.44 |
| TI Other Quinolones | 0.22 a | 1.27 | 0.19 a | 1.56 |
| TI Polymyxins | 0.01 a | 0.26 | 0.29 b | 3.12 |
| TI Tetracyclines | 3.82 a | 12.80 | 3.08 a | 11.83 |
| TI Trimethroprim-Sulfa | 1.66 a | 6.52 | 0.66 b | 3.33 |
| TI β-lact sensitive Penicillins | 0.45 a | 2.80 | 0.50 a | 2.40 |
| Sum of TI | 15.11 a | 18.01 | 8.40 b | 15.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerab, J.G.; Chantziaras, I.; Van Limbergen, T.; Van Erum, J.; Boel, F.; Hoeven, E.; Dewulf, J. Antimicrobial Use in On-Farm Hatching Systems vs. Traditional Hatching Systems: A Case Study. Animals 2023, 13, 3270. https://doi.org/10.3390/ani13203270
Jerab JG, Chantziaras I, Van Limbergen T, Van Erum J, Boel F, Hoeven E, Dewulf J. Antimicrobial Use in On-Farm Hatching Systems vs. Traditional Hatching Systems: A Case Study. Animals. 2023; 13(20):3270. https://doi.org/10.3390/ani13203270
Chicago/Turabian StyleJerab, Julia G., Ilias Chantziaras, Tommy Van Limbergen, Johan Van Erum, Filip Boel, Erik Hoeven, and Jeroen Dewulf. 2023. "Antimicrobial Use in On-Farm Hatching Systems vs. Traditional Hatching Systems: A Case Study" Animals 13, no. 20: 3270. https://doi.org/10.3390/ani13203270
APA StyleJerab, J. G., Chantziaras, I., Van Limbergen, T., Van Erum, J., Boel, F., Hoeven, E., & Dewulf, J. (2023). Antimicrobial Use in On-Farm Hatching Systems vs. Traditional Hatching Systems: A Case Study. Animals, 13(20), 3270. https://doi.org/10.3390/ani13203270







