Adaptation Response in Sheep: Ewes in Different Cortisol Clusters Reveal Changes in the Expression of Salivary miRNAs
Abstract
:Simple Summary
Abstract
1. Introduction
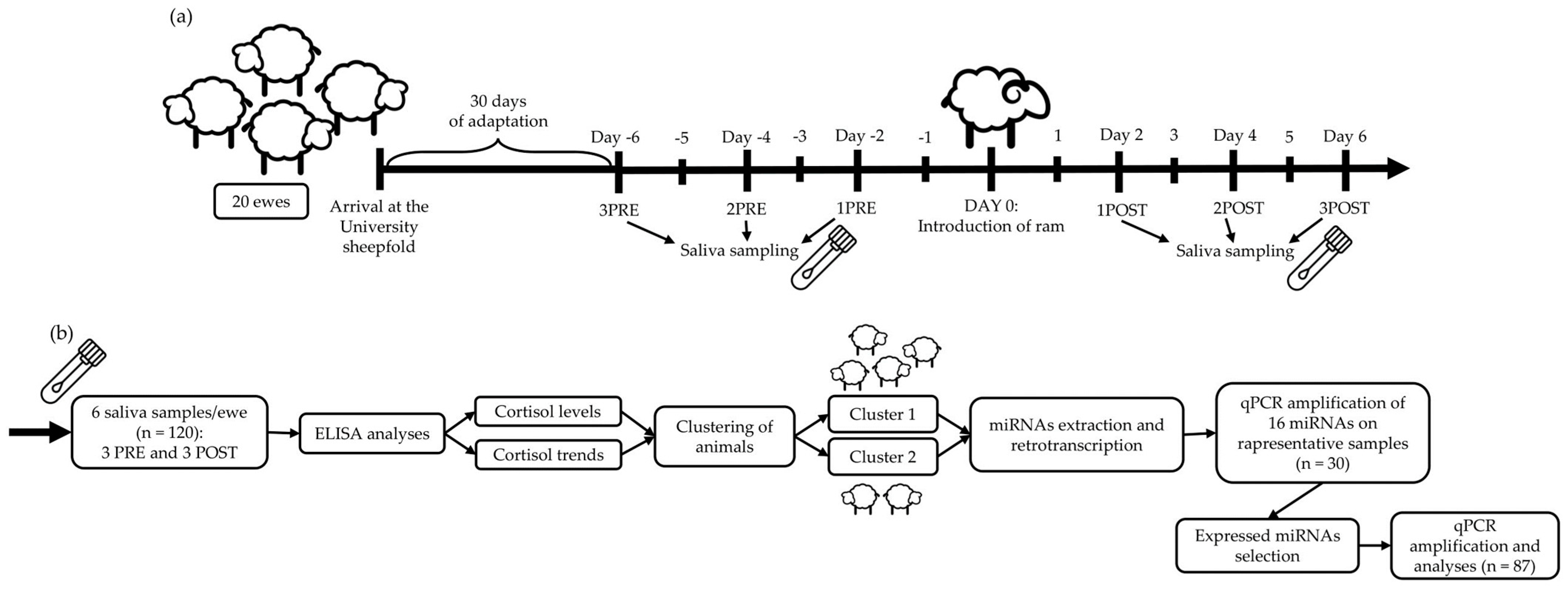
2. Materials and Methods
2.1. Ewes
2.2. Saliva Collection
2.3. Salivary Cortisol Estimation
2.4. Extraction of Salivary miRNAs
2.5. cDNA Synthesis and Quantitative Real-Time PCR
2.6. Statistical Analyses
3. Results
3.1. Salivary Cortisol
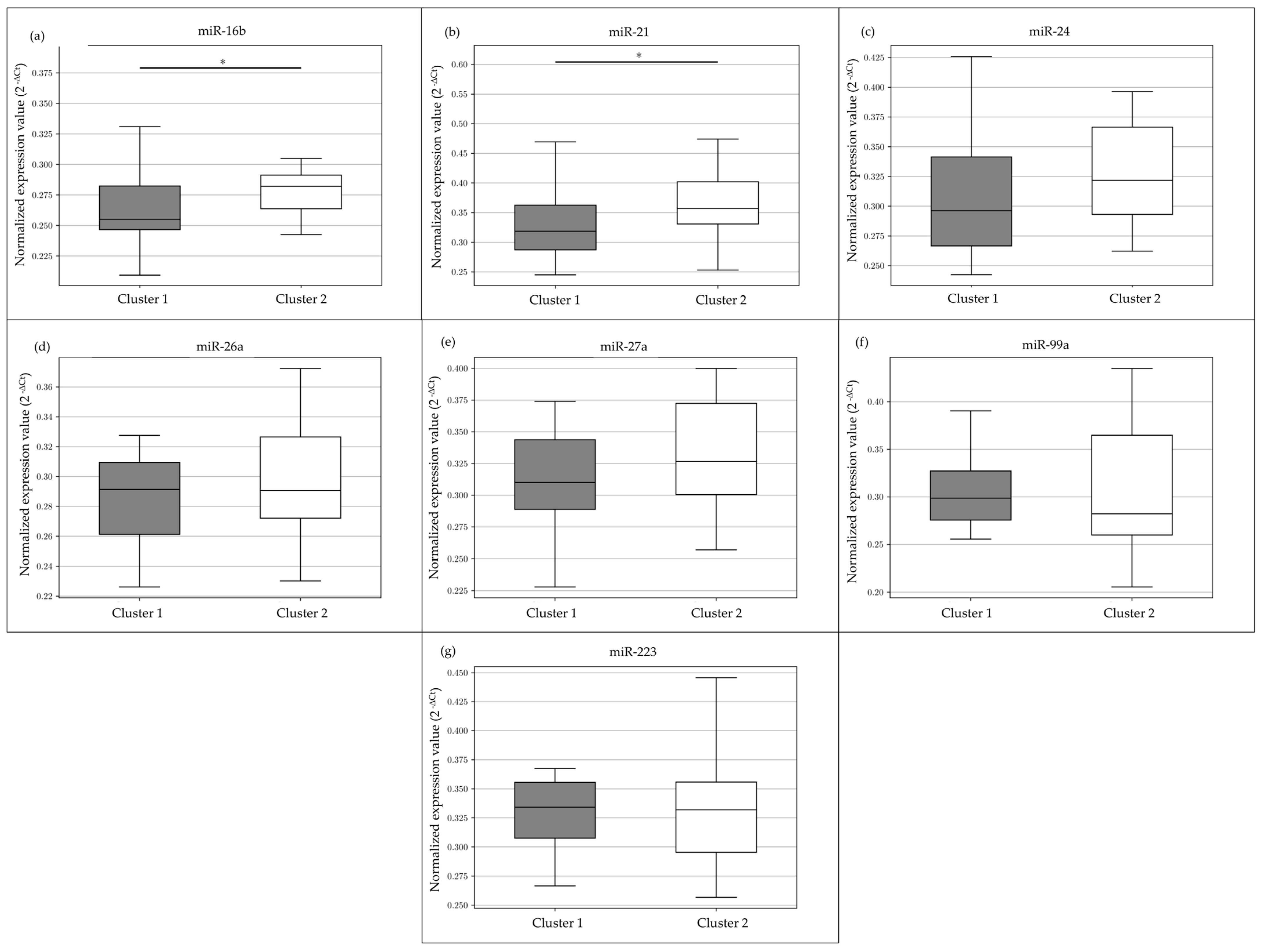
3.2. miRNAs Expression
3.3. miRNAs’ Expression Clustering
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stott, G.H. What Is Animal Stress and How Is It Measured? J. Anim. Sci. 1981, 52, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Etim, N.N.; Williams, M.E.; Evans, E.I.; Offiong, E.E.A. Physiological and Behavioural Responses of Farm Animals to Stress: Implications to Animal Productivity. Am. J. Adv. Agric. Res. 2013, 1, 53–61. [Google Scholar]
- Herman, J.P.; Figueiredo, H.; Mueller, N.K.; Ulrich-Lai, Y.; Ostrander, M.M.; Choi, D.C.; Cullinan, W.E. Central Mechanisms of Stress Integration: Hierarchical Circuitry Controlling Hypothalamo–Pituitary–Adrenocortical Responsiveness. Front. Neuroendocrinol. 2003, 24, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Acharya, R.Y.; Hemsworth, P.H.; Coleman, G.J.; Kinder, J.E. The Animal-Human Interface in Farm Animal Production: Animal Fear, Stress, Reproduction and Welfare. Animals 2022, 12, 487. [Google Scholar] [CrossRef] [PubMed]
- Geß, A.; Viola, I.; Miretti, S.; Macchi, E.; Perona, G.; Battaglini, L.; Baratta, M. A New Approach to LCA Evaluation of Lamb Meat Production in Two Different Breeding Systems in Northern Italy. Front. Vet. Sci. 2020, 7, 651. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.J.; Hynd, P.I.; Ralph, C.R.; Hocking Edwards, J.E.; Burnard, C.L.; Narayan, E.; Tilbrook, A.J. Chronic Elevation of Plasma Cortisol Causes Differential Expression of Predominating Glucocorticoid in Plasma, Saliva, Fecal, and Wool Matrices in Sheep. Domest. Anim. Endocrinol. 2021, 74, 106503. [Google Scholar] [CrossRef] [PubMed]
- Chojnowska, S.; Ptaszyńska-Sarosiek, I.; Kępka, A.; Knaś, M.; Waszkiewicz, N. Salivary Biomarkers of Stress, Anxiety and Depression. J. Clin. Med. 2021, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.D.; Escribano, D.; Quiles, A.; López-Arjona, M.; Cerón, J.J.; Martínez-Subiela, S.; Hevia, M.L.; Tecles, F. Evaluation of New Biomarkers of Stress in Saliva of Sheep. Animal 2019, 13, 1278–1286. [Google Scholar] [CrossRef]
- Cockrem, J.F. Individual Variation in Glucocorticoid Stress Responses in Animals. Gen. Comp. Endocrinol. 2013, 181, 45–58. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Van Reenen, C.G. ANIMAL BEHAVIOR AND WELL-BEING SYMPOSIUM: Interaction between Coping Style/Personality, Stress, and Welfare: Relevance for Domestic Farm Animals. J. Anim. Sci. 2016, 94, 2284–2296. [Google Scholar] [CrossRef]
- Pant, S.D.; You, Q.; Schenkel, L.C.; Voort, G.; Vander; Schenkel, F.S.; Wilton, J.; Cain, L.; Karrow, N.A. A Genome-Wide Association Study to Identify Chromosomal Regions Influencing Ovine Cortisol Response. Livest. Sci. 2016, 187, 40–47. [Google Scholar] [CrossRef]
- Boluarte-Carbajal, A.; Navarro-Flores, A.; Villarreal-Zegarra, D. Explanatory Model of Perceived Stress in the General Population: A Cross-Sectional Study in Peru During the COVID-19 Context. Front. Psychol. 2021, 12, 673945. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 388354. [Google Scholar] [CrossRef] [PubMed]
- Do, D.N.; Dudemaine, P.L.; Mathur, M.; Suravajhala, P.; Zhao, X.; Ibeagha-awemu, E.M. MiRNA Regulatory Functions in Farm Animal Diseases, and Biomarker Potentials for Effective Therapies. Int. J. Mol. Sci. 2021, 22, 3080. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Schratt, G. miRNA Regulation of Social and Anxiety-Related Behaviour. Cell Mol. Life Sci. 2020, 77, 4347–4364. [Google Scholar] [CrossRef] [PubMed]
- Gururajan, A.; Naughton, M.E.; Scott, K.A.; O’Connor, R.M.; Moloney, G.; Clarke, G.; Dowling, J.; Walsh, A.; Ismail, M.F.; Shorten, G.; et al. MicroRNAs as Biomarkers for Major Depression: A Role for Let-7b and Let-7c. Transl. Psychiatry 2016, 6, e862. [Google Scholar] [CrossRef] [PubMed]
- Shandilya, U.K.; Sharma, A.; Naylor, D.; Canovas, A.; Mallard, B.; Karrow, N.A. Expression Profile of MiRNA from High, Middle, and Low Stress-Responding Sheep during Bacterial Endotoxin Challenge. Animals 2023, 13, 508. [Google Scholar] [CrossRef] [PubMed]
- Gröschl, M.; Rauh, M. Influence of Commercial Collection Devices for Saliva on the Reliability of Salivary Steroids Analysis. Steroids 2006, 71, 1097–1100. [Google Scholar] [CrossRef]
- Nater, U.M.; La Marca, R.; Florin, L.; Moses, A.; Langhans, W.; Koller, M.M.; Ehlert, U. Stress-Induced Changes in Human Salivary Alpha-Amylase Activity—Associations with Adrenergic Activity. Psychoneuroendocrinology 2006, 31, 49–58. [Google Scholar] [CrossRef]
- Topkas, E.; Keith, P.; Dimeski, G.; Cooper-White, J.; Punyadeera, C. Evaluation of Saliva Collection Devices for the Analysis of Proteins. Clin. Chim. Acta 2012, 413, 1066–1070. [Google Scholar] [CrossRef]
- Guelfi, G.; Iaboni, M.; Sansone, A.; Capaccia, C.; Santoro, M.M.; Diverio, S. Extracellular Circulating MiRNAs as Stress-Related Signature to Search and Rescue Dogs. Sci. Rep. 2022, 12, 3213. [Google Scholar] [CrossRef]
- Patel, R.S.; Jakymiw, A.; Yao, B.; Pauley, B.A.; Carcamo, W.C.; Katz, J.; Cheng, J.Q.; Chan, E.K.L. High Resolution of MicroRNA Signatures in Human Whole Saliva. Arch. Oral. Biol. 2011, 56, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Heusser, P.; Klinger, C.; Cysarz, D.; Büssing, A.; Ostermann, T.; Savelsbergh, A. Stress-Associated Changes in Salivary MicroRNAs Can Be Detected in Response to the Trier Social Stress Test: An Exploratory Study. Sci. Rep. 2018, 8, 7112. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Caroprese, M.; Albenzio, M.; Marzano, A.; Schena, L.; Annicchiarico, G.; Sevi, A. Relationship between Cortisol Response to Stress and Behavior, Immune Profile, and Production Performance of Dairy Ewes. J. Dairy. Sci. 2010, 93, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.; Dowling, S.; Shaw, R.; Hickey, J.; Fraser, D.; Cameron, C.; Sutherland, I. Stress-Induced Immunomodulation in Low and High Reactive Sheep. Animals 2019, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Arfuso, F.; Fazio, F.; Chikhi, L.; Aymond, G.; Piccione, G.; Giannetto, C. Acute Stress Response of Sheep to Shearing Procedures: Dynamic Change of Cortisol Concentration and Protein Electrophoretic Pattern. Animals 2022, 12, 862. [Google Scholar] [CrossRef]
- Fabre-Nys, C.; Kendrick, K.M.; Scaramuzzi, R.J. The “ram Effect”: New Insights into Neural Modulation of the Gonadotropic Axis by Male Odors and Socio-Sexual Interactions. Front. Neurosci. 2015, 9, 111. [Google Scholar] [CrossRef]
- McCosh, R.B.; Berry, E.M.; Wehrman, M.E.; Redden, R.R.; Hallford, D.M.; Berardinelli, J.G. Temporal Concentrations of Cortisol and LH in Virgin Ewes Acutely Exposed to Rams during the Transition into the Breeding Season. Anim. Reprod. Sci. 2015, 154, 25–28. [Google Scholar] [CrossRef]
- Rubio, C.P.; Contreras-Aguilar, M.D.; Quiles, A.; López-Arjona, M.; Cerón, J.J.; Martínez-Subiela, S.; Hevia, M.L.; Escribano, D.; Tecles, F. Biomarkers of Oxidative Stress in Saliva of Sheep: Analytical Performance and Changes after an Experimentally Induced Stress. Res. Vet. Sci. 2019, 123, 71–76. [Google Scholar] [CrossRef]
- Andanson, S.; Boissy, A.; Veissier, I. Conditions for Assessing Cortisol in Sheep: The Total Form in Blood v. the Free Form in Saliva. Animal 2020, 14, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Katsuura, S.; Kuwano, Y.; Yamagishi, N.; Kurokawa, K.; Kajita, K.; Akaike, Y.; Nishida, K.; Masuda, K.; Tanahashi, T.; Rokutan, K. MicroRNAs MiR-144/144* and MiR-16 in Peripheral Blood Are Potential Biomarkers for Naturalistic Stress in Healthy Japanese Medical Students. Neurosci. Lett. 2012, 516, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Lecchi, C.; Zamarian, V.; Gini, C.; Avanzini, C.; Polloni, A.; Nodari, S.R.; Ceciliani, F. Salivary MicroRNAs Are Potential Biomarkers for the Accurate and Precise Identification of Inflammatory Response after Tail Docking and Castration in Piglets. J. Anim. Sci. 2020, 98, skaa153. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Yajima, Y.; Fujikura, Y.; Zhuang, H.; Higo-Yamamoto, S.; Toyoda, A.; Oishi, K. Identification of Salivary MicroRNA Profiles in Male Mouse Model of Chronic Sleep Disorder. Stress 2023, 26, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, S.; Israel, S.; Nagy, C.; Turecki, G. The Emerging Role of Exosomes in Mental Disorders. Transl. Psychiatry 2019, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Roser, A.E.; Gomes, L.C.; Schünemann, J.; Maass, F.; Lingor, P. Circulating MiRNAs as Diagnostic Biomarkers for Parkinson’s Disease. Front. Neurosci. 2018, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Savelsbergh, A.; Heusser, P. MicroRNAs in Psychological Stress Reactions and Their Use as Stress-Associated Biomarkers, Especially in Human Saliva. Biomed. Hub. 2017, 2, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gerrard, B.; Singh, V.; Babenko, O.; Gauthier, I.; Wee Yong, V.; Kovalchuk, I.; Luczak, A.; Metz, G.A.S. Chronic Mild Stress Exacerbates Severity of Experimental Autoimmune Encephalomyelitis in Association with Altered Non-Coding RNA and Metabolic Biomarkers. Neuroscience 2017, 359, 299–307. [Google Scholar] [CrossRef]
- Gidron, Y.; De Zwaan, M.; Quint, K.; Ocker, M. Influence of Stress and Health-Behaviour on MiRNA Expression. Mol. Med. Rep. 2010, 3, 455–457. [Google Scholar] [CrossRef]
- Launay, J.M.; Mouillet-Richard, S.; Baudry, A.; Pietri, M.; Kellermann, O. Raphe-Mediated Signals Control the Hippocampal Response to SRI Antidepressants via MiR-16. Transl. Psychiatry 2011, 1, e56. [Google Scholar] [CrossRef]
- Baudry, A.; Mouillet-Richard, S.; Schneider, B.; Launay, J.M.; Kellermann, O. MiR-16 Targets the Serotonin Transporter: A New Facet for Adaptive Responses to Antidepressants. Science 2010, 329, 1537–1541. [Google Scholar] [CrossRef]
- Zurawek, D.; Kusmider, M.; Faron-Gorecka, A.; Gruca, P.; Pabian, P.; Solich, J.; Kolasa, M.; Papp, M.; Dziedzicka-Wasylewska, M. Reciprocal MicroRNA Expression in Mesocortical Circuit and Its Interplay with Serotonin Transporter Define Resilient Rats in the Chronic Mild Stress. Mol. Neurobiol. 2017, 54, 5741. [Google Scholar] [CrossRef]
- Honda, M.; Kuwano, Y.; Katsuura-Kamano, S.; Kamezaki, Y.; Fujita, K.; Akaike, Y.; Kano, S.; Nishida, K.; Masuda, K.; Rokutan, K. Chronic Academic Stress Increases a Group of MicroRNAs in Peripheral Blood. PLoS ONE 2013, 8, 75960. [Google Scholar] [CrossRef]
- MiRBase. Available online: https://mirbase.org/ (accessed on 13 July 2023).
- Chanvallon, A.; Blache, D.; Chadwick, A.; Esmaili, T.; Hawken, P.A.R.; Martin, G.B.; Viñoles, C.; Fabre-Nys, C. Sexual Experience and Temperament Affect the Response of Merino Ewes to the Ram Effect during the Anoestrous Season. Anim. Reprod. Sci. 2010, 119, 205–211. [Google Scholar] [CrossRef]

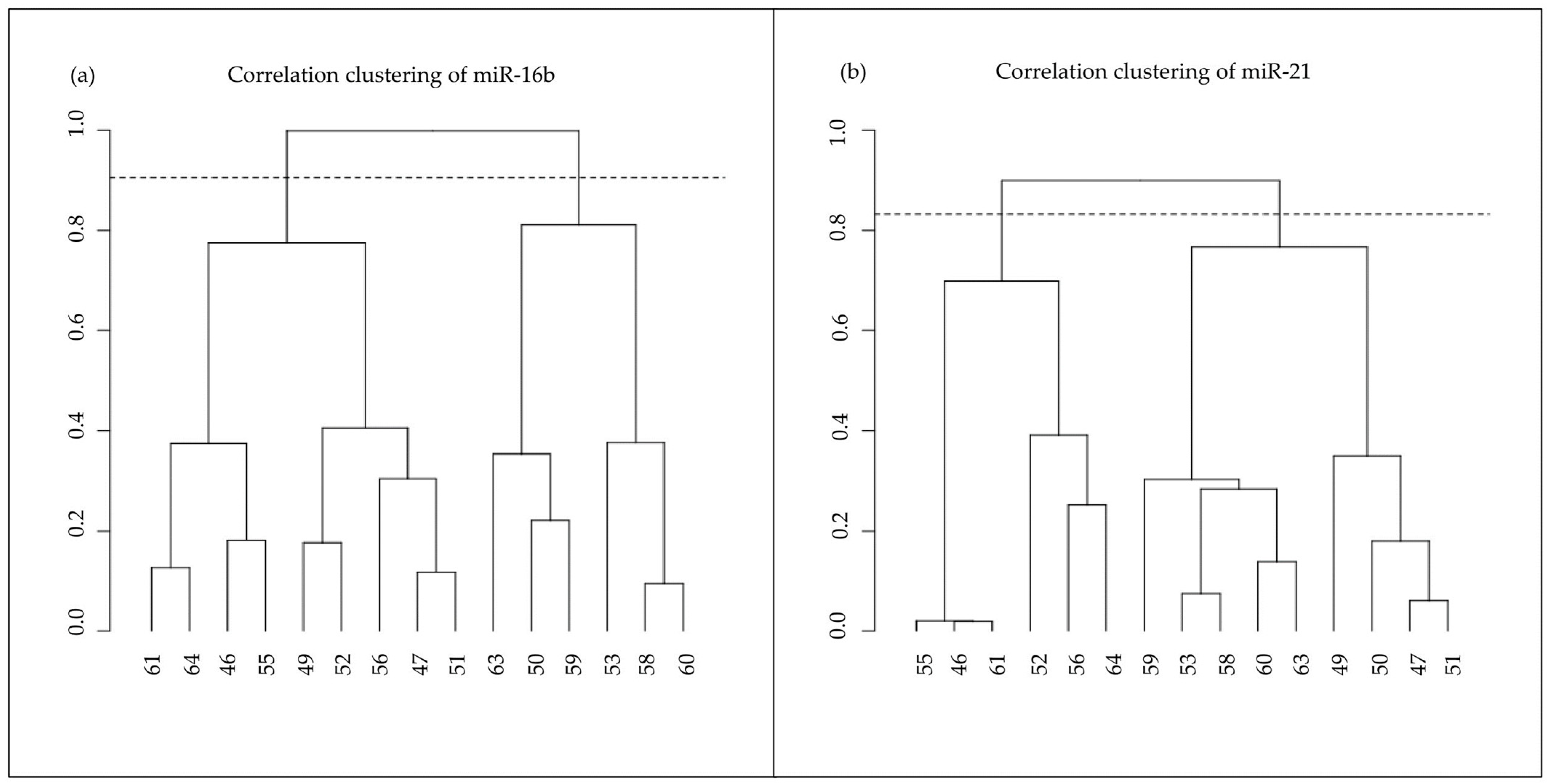
| Cluster 1 | 47 49 50 53 56 59 60 61 63 64 |
| Cluster 2 | 46 51 52 55 58 |
| miRNA Name | Primer ID | Qiagen GeneGlobe ID |
|---|---|---|
| miR-223 | hsa-miR-223-3p | YP00205986 |
| miR-26a | hsa-miR-26a-5p | YP00206023 |
| miR-24 | hsa-miR-24-3p | YP00204260 |
| miR-16b | oar-miR-16b | YP02115941 |
| miR-191 | cfa-miR-191 | YP00205972 |
| miR-27a | hsa-miR-27a-3p | YP00206038 |
| miR-106a | oar-miR-106a | YP02110125 |
| miR-17 | oar-miR-17-5p | YP02110961 |
| miR-29a | oar-miR-29a | YP02105177 |
| miR-19b | hsa-miR-19b-3p | YP00204450 |
| miR-30c | oar-miR-30c | YP02114922 |
| miR-26b | hsa-miR-26b-5p | YP00204172 |
| miR-23a | bta-miR-23a | YP02115608 |
| miR-21 | oar-miR-21 | YP02110192 |
| miR-221 | bta-miR-221 | YP02111922 |
| miR-99a | bta- miR-99a-5p | YP00205945 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manenti, I.; Viola, I.; Ala, U.; Cornale, P.; Macchi, E.; Toschi, P.; Martignani, E.; Baratta, M.; Miretti, S. Adaptation Response in Sheep: Ewes in Different Cortisol Clusters Reveal Changes in the Expression of Salivary miRNAs. Animals 2023, 13, 3273. https://doi.org/10.3390/ani13203273
Manenti I, Viola I, Ala U, Cornale P, Macchi E, Toschi P, Martignani E, Baratta M, Miretti S. Adaptation Response in Sheep: Ewes in Different Cortisol Clusters Reveal Changes in the Expression of Salivary miRNAs. Animals. 2023; 13(20):3273. https://doi.org/10.3390/ani13203273
Chicago/Turabian StyleManenti, Isabella, Irene Viola, Ugo Ala, Paolo Cornale, Elisabetta Macchi, Paola Toschi, Eugenio Martignani, Mario Baratta, and Silvia Miretti. 2023. "Adaptation Response in Sheep: Ewes in Different Cortisol Clusters Reveal Changes in the Expression of Salivary miRNAs" Animals 13, no. 20: 3273. https://doi.org/10.3390/ani13203273






