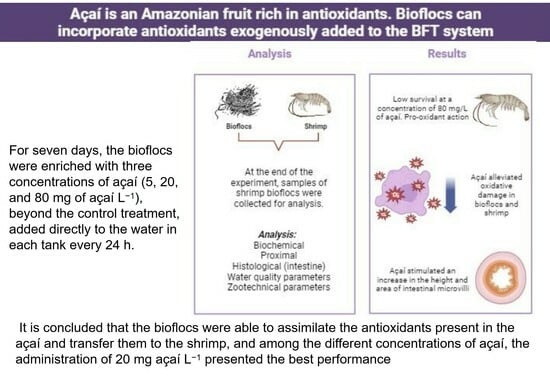
Effects of Lyophilized Açaí (Euterpe oleracea) Supplementation on Oxidative Damage and Intestinal Histology in Juvenile Shrimp Penaeus vannamei Reared in Biofloc Systems
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
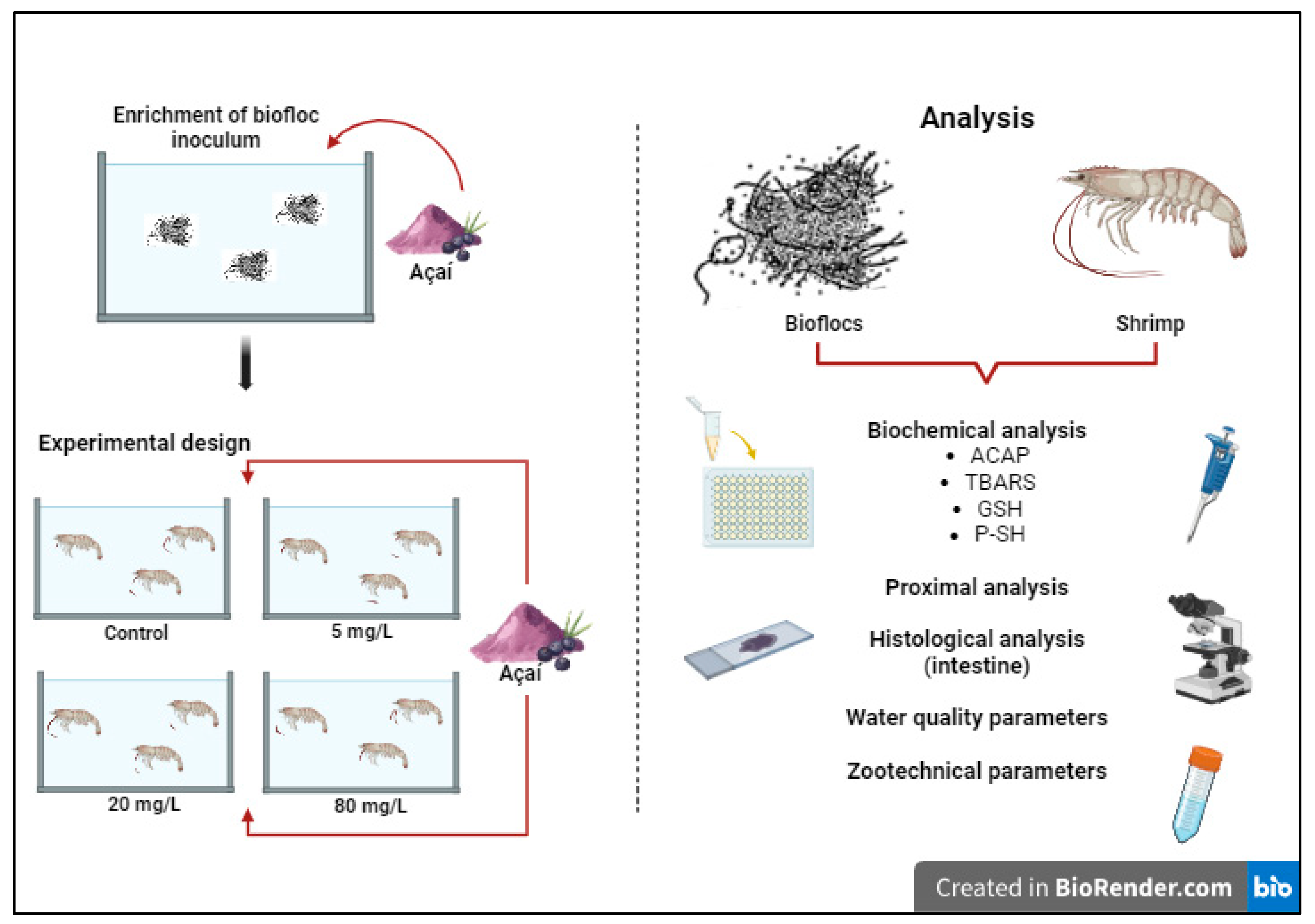
2.1. Shrimp Maintenance and Experimental Design
2.2. Zootechnical Performance and Proximal Analysis of Shrimp Bioflocs and Muscle
- Weight gain (g) = final weight − initial weight.
- Specific growth ratio = [100% × (Ln final weight − Ln initial weight)/trial duration].
- Feed conversion ratio = dry feed intake/weight gain.
- Protein efficiency ratio = weight gain/dry protein intake.
- Survival (%) = (final number of shrimps/initial number of shrimps) × 100.
2.3. Biochemical Analysis
2.3.1. Homogenization of the Samples
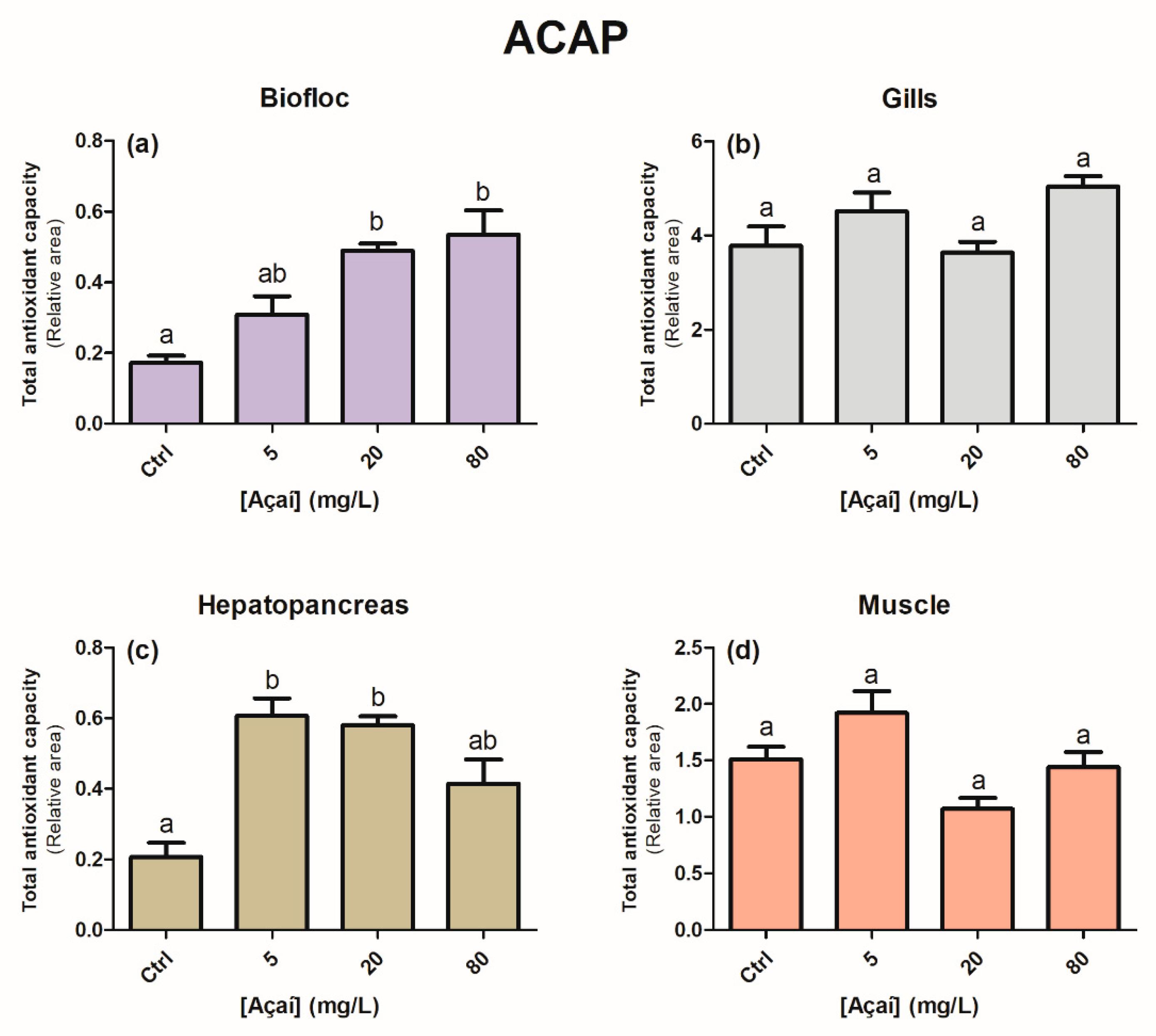
2.3.2. Total Antioxidant Capacity against Peroxyl Radicals (ACAP)
2.3.3. Lipid Peroxidation (TBARS)
2.3.4. Reduced Glutathione (GSH)
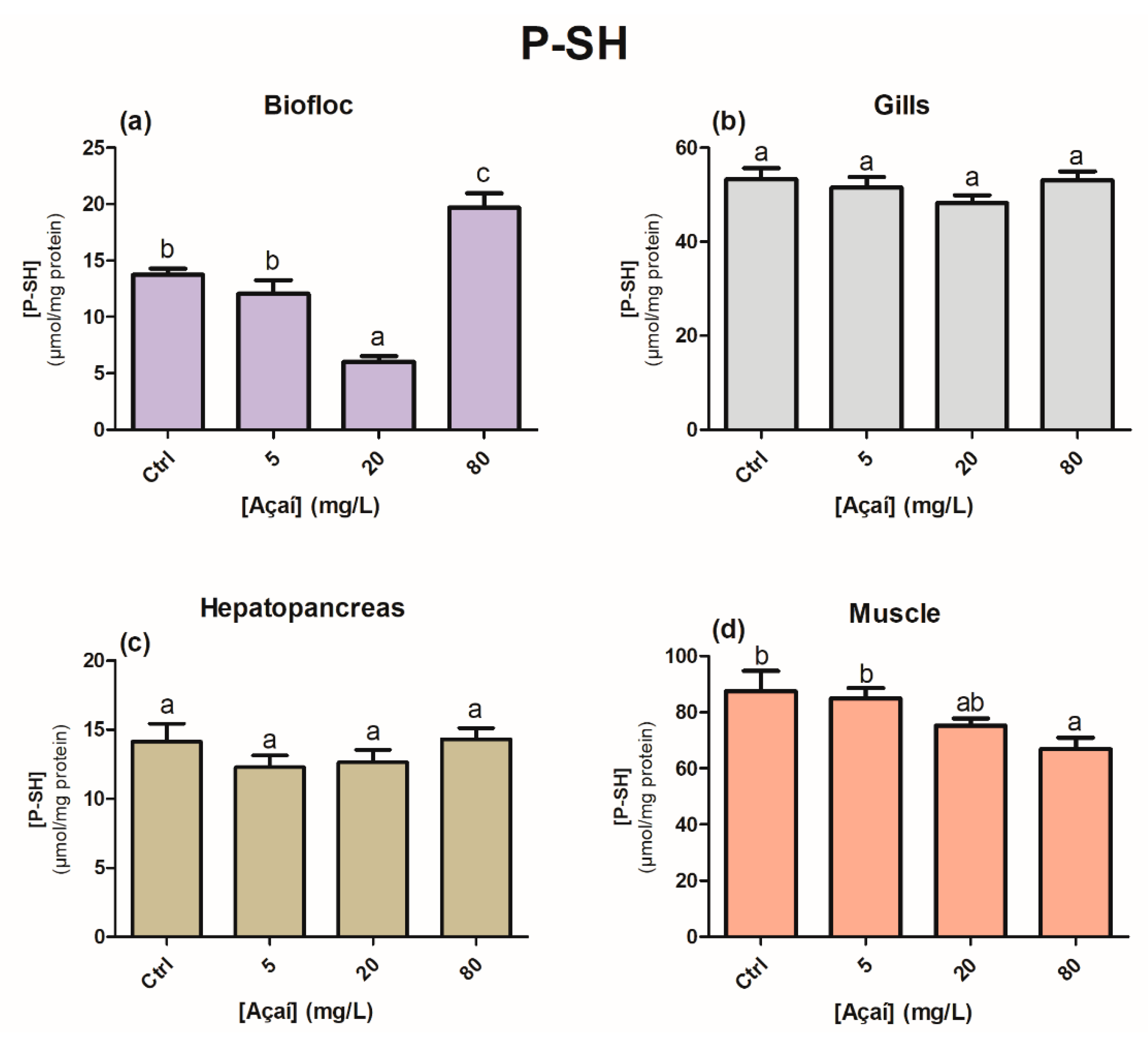
2.3.5. Protein-Associated Sulfhydryl Groups (P-SH)
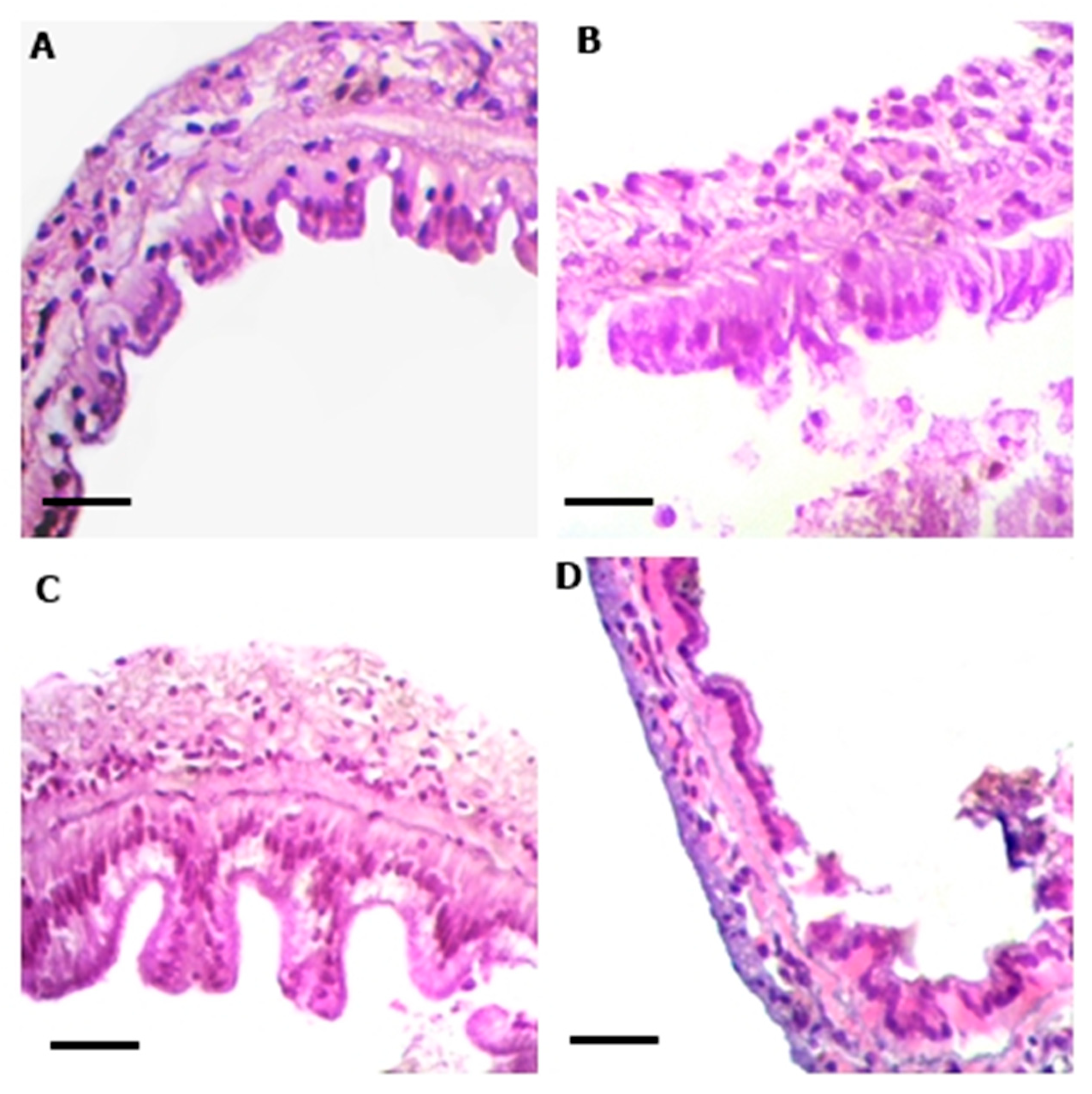
2.4. Histological Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CONAB—Companhia Nacional de Abastecimento. 2020. Available online: https://www.conab.gov.br/info-agro/analises-do-mercado-agropecuario-e-extrativista/analises-do-mercado/historico-mensal-de-acai (accessed on 3 May 2023).
- Menezes, E.M.D.S.; Torres, A.T.; Sabaa Srur, A.U. Valor nutricional da polpa de açaí (Euterpe oleracea Mart) lioflizada. Acta Amaz. 2008, 38, 311–316. [Google Scholar] [CrossRef]
- CONAB—Companhia Nacional de Abastecimento. Boletim da Sociobiodiversidade; CONAB: Brasília, Brazil, 2022; Volume 6. [Google Scholar]
- Lucas, B.F.; Zambiazi, R.C.; Costa, J.A.V. Biocompounds and physical properties of açaí pulp dried by different methods. Lebensm-Wiss Technol. 2018, 98, 335–340. [Google Scholar] [CrossRef]
- Martino, H.S.D.; dos Santos Dias, M.M.; Noratto, G.; Talcott, S.; Mertens-Talcott, S.U. Anti-lipidaemic and anti-inflammatory effect of açai (Euterpe oleracea Martius) polyphenols on 3T3-L1 adipocytes. J. Funct. Foods 2016, 23, 432–443. [Google Scholar] [CrossRef]
- Schauss, A.G. Advances in the study of the health benefts and mechanisms of action of the pulp and seed of the Amazonian palm fruit, Euterpe oleracea Mart., known as “Açai”. In Fruits, Vegetables, and Herbs; Academic Press: Cambridge, MA, USA, 2016; pp. 179–220. [Google Scholar]
- Odendaal, A.Y.; Schauss, A.G. Potent antioxidant and anti-inflammatory flavonoids in the nutrient-rich Amazonian palm fruit, açaí (Euterpe spp.). In Polyphenols in Human Health and Disease; Elsevier Science: Amsterdam, The Netherlands, 2014; Volume 1, pp. 219–239. [Google Scholar] [CrossRef]
- da Costa, C.A.; de Oliveira, P.R.B.; de Bem, G.F.; de Carvalho, L.C.R.M.; Ognibene, D.T.; da Silva, A.F.E.; Valença, S.S.; Pires, K.M.P.; Sousa, P.J.C.; de Moura, R.S.; et al. Euterpe oleracea Mart. derived polyphenols prevent endothelial dysfunction and vascular structural changes in renovascular hypertensive rats: Role of oxidative stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Aranha, L.N.; Silva, M.G.; Uehara, S.K.; Luiz, R.R.; Neto, J.F.N.; Rosa, G.; de Oliveira, G.M.M. Effects of a hypoenergetic diet associated with açaí (Euterpe oleracea Mart.) pulp consumption on antioxidant status, oxidative stress and inflammatory biomarkers in overweight, dyslipidemic individuals. Clin. Nutr. 2019, 39, 1464–1469. [Google Scholar] [CrossRef]
- Colombo, G.M.; dos Santos Simião, C.; Schmitz, M.J.; Pedrosa, V.F.; Romano, L.A.; Tesser, M.B.; Monserrat, J.M. The role of açaí (Euterpe oleracea Mart. 1824) as a chemoprotective agent in the evaluation of antioxidant defence, oxidative damage and histology of juvenile shrimp Litopenaeus vannamei (BOONE, 1931) exposed to ammonia. Aquac. Res. 2020, 51, 1551–1566. [Google Scholar] [CrossRef]
- Monserrat, J.M.; Lima, J.V.; Ferreira, J.L.R.; Acosta, D.; Garcia, M.L.; Ramos, P.B.; Barros Moraes, T.; Cougo dos Santos, L.; Amado, L.L. Modulation of antioxidant and detoxification responses mediated by lipoic acid in the fish Corydoras paleatus (Callychthyidae). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008, 148, 287–292. [Google Scholar] [CrossRef]
- da Silva Martins, A.C.; Artigas Flores, J.; Wasielesky Junior, W.; Zanette, J.; Primel, E.G.; Souza Caldas, S.; Monserrat, J.M. Modulation of antioxidant and detoxification responses induced by lipoic acid in the Pacific white shrimp Litopenaeus vannamei (Boone, 1931) submitted to hypoxia and reoxygenation. Mar. Freshw. Behav. Physiol. 2014, 47, 335–348. [Google Scholar] [CrossRef]
- da Silva, T.V.; Torres, M.F.; Sampaio, L.A.; Hamoy, M.; Monserrat, J.M.; Barbas, L.A.L. Dietary Euterpe oleracea Mart. attenuates seizures and damage to lipids in the brain of Colossoma macropomum. Fish Physiol. Biochem. 2021, 47, 1851–1864. [Google Scholar] [CrossRef]
- Brol, J.; Müller, L.; Prates, E.C.A.; de Farias, B.S.; Pedrosa, V.F.; de Almeida Pinto, L.A.; Cadaval, T.R.S., Jr.; Tesser, M.T.; Wasielesky, W.; Ventura-Lima, J. Dietary chitosan supplementation in Litopenaeus vannamei reared in a biofloc system: Effect on antioxidant status facing saline stress. Aquaculture 2021, 544, 737034. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef]
- de Moura, L.B.; Cavalcante, J.G.; Nascimento, E.T.D.S.; Silva, I.C.; Salaro, A.L.; Barbas, L.A.L.; Veras, G.C.; Campelo, D.A.V. Dietary Euterpe oleracea essential oil, the Amazon Açaí, as feed additive to Amazonian ornamental fish, during post-larvae growing stage: A preliminary study. Fishes 2022, 7, 369. [Google Scholar] [CrossRef]
- dos Santos Simião, C.; Colombo, G.M.; Gomes, R.M.M.; Ramos, P.B.; Tesser, M.B.; Wasielesky Junior, W.; Monserrat, J.M. Chemoprotection of amazonian Mauritia flexuosa fruit pulp against ammonia and nitrite toxicity to postlarvae shrimps Litopenaeus vannamei. Bol. Inst. Pesca 2022, 48, e679. [Google Scholar] [CrossRef]
- Ramos, P.B.; Colombo, G.M.; Schmitz, M.J.; Simião, C.S.; dos Santos Machado, K.; Werhli, A.V.; Monserrat, J.M. Chemoprotection mediated by açaí berry (Euterpe oleracea) in white shrimp Litopenaeus vannamei exposed to the cyanotoxin saxitoxin analyzed by in vivo assays and docking modeling. Aquat. Toxicol. 2022, 246, 106148. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J.J. Engineering analysis of the stoichiometry of hotoautotrophic, autotrophic, and heterotrophic control of ammonia-nitrogen in aquaculture production systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef]
- Krummenauer, D.; Samocha, T.; Poersch, L.H.; Lara, G.R.; Wasielesky, W. Effect of water reuse on the culture of Pacifc white shrimp Litopenaeus vannamei in BFT system. J. World Aquac. Soc. 2014, 45, 3–14. [Google Scholar] [CrossRef]
- Wasielesky, W.J.; Atwood, H.I.; Stokes, A.; Browdy, C.L. Effect of natural production in brown water super-intensive culture system for white shrimp Litopenaeus vannamei. Aquaculture. 2006, 258, 396–403. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M. Biofloc technology as a promising tool to improve aquaculture production. Rev. Aquac. 2020, 12, 1836–1850. [Google Scholar] [CrossRef]
- Xu, W.J.; Pan, L.Q. Effects of bioflocs on growth performance, digestive enzyme activity and body composition of juvenile Litopenaeus vannamei in zero-water exchange tanks manipulating C/N ratio in feed. Aquaculture 2012, 356, 147–152. [Google Scholar] [CrossRef]
- da Silva Martins, A.C.; Artigas Flores, J.; Porto, C.; Wasielesky Junior, W.; Monserrat, J.M. Antioxidant and oxidative damage responses in different organs of Pacific white shrimp Litopenaeus vannamei (Boone, 1931) reared in a biofloc technology system. Mar. Freshw. Behav. Physiol. 2015, 48, 279–288. [Google Scholar] [CrossRef]
- Leon, D.C.M.; Wasiliesky Junior, W.; Monserrat, J.M. Quercetin influence in water quality and biochemical responses of shrimp Litopenaeus vannamei reared in biofloc technology system. Aquac. Res. 2018, 49, 3569–3576. [Google Scholar] [CrossRef]
- Silva, S.M.; Ramos, P.B.; Buitrago, J.R.; da Silva, T.V.; Simião, C.S.; Colombo, G.M.; Schmitz, M.; Tesser, M.B.; Prentice, C.; Wasielesky, W., Jr.; et al. Zootechnical performance, biochemical response, and chromaticity in Pacific white shrimp (Litopenaeus vannamei) (Boone, 1931) after the inclusion of lyophilized açaí (Euterpe oleracea) in the diet. Aquac. Int. 2020, 28, 1563–1577. [Google Scholar] [CrossRef]
- Colombo, G.M.; dos Santos Simião, C.; Ramírez, J.R.B.; de Sousa Araujo, A.C.; Gomes, R.M.M.; Buitagro, S.A.M.; Wasielesky, W.; Monserrat, J.M. Bioflocs enriched with lyophilized açaí (Euterpe oleracea) improved the survival and weight gain of Litopenaeus vannamei post-larvae cultivated in the BFT system. Aquaculture 2023, 51, 1551–1566. [Google Scholar] [CrossRef]
- Jory, D.E. Feed management practices for a healthy pond environment. In The New Wave: Proceedings of the Special Session on Sustainable Shrimp Culture; Browdy, C., Jory, D.E., Eds.; World Aquaculture Society: Baton Rouge, LA, USA, 2001; pp. 118–143. [Google Scholar]
- UNESCO. Chemical methods for use in marine environmental monitoring. In Manuals and Guides 12; Intergovernmental Oceanographic Commission: Paris, France, 1983. [Google Scholar]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998; 1193p. [Google Scholar]
- Gaona, C.A.P.; Poersch, L.H.; Krummenauer, D.; Foes, G.K.; Wasielesky, W. The effect of solids removal on water quality, growth and survival of Litopenaeus vannamei in a biofloc technology culture system. Int. J. Recirc. Aquac. 2011, 11, 54–73. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology—A Practical Guide Book; The World Aquaculture: Baton Rouge, LA, USA, 2015. [Google Scholar]
- Furtado, P.S.; Poersch, L.H.; Wasielesky, W.J. Effect of calcium hydroxide, carbonate and sodium bicarbonate on water quality and zootechnical performance of shrimp Litopenaeus vannamei reared in bio-flocs technology (BFT) systems. Aquaculture 2011, 321, 130–135. [Google Scholar] [CrossRef]
- AOAC International. Offcial Methods of Analysis of the Association of Official Analytical Chemists, 16th ed.; AOAC International: Washington, DC, USA, 1999; p. 114. [Google Scholar]
- AOAC (Association of the Official Analytical Chemists). Official Methods of Analysis, 16th ed.; AOAC: Arlington, VA, USA, 1995. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purifcation. Can. J. Biochem. Physiol. 1959, 27, 911–917. [Google Scholar] [CrossRef]
- Amado, L.L.; Garcia, M.L.; Ramos, P.B.; Freitas, R.F.; Zafalon, B.; Ferreira, J.L.R.; Monserrat, J.M. A method to measure total antioxidant capacity against peroxyl radicals in aquatic organisms: Application to evaluate microcystins toxicity. Sci. Total Environ. 2009, 407, 2115–2123. [Google Scholar] [CrossRef]
- Oakes, K.D.; Van Der Kraak, G.J. Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat. Toxicol. 2003, 63, 447–463. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Luna, L.G. Manual of histologic staining methods of the Armed Forces Institute of Pathology. In Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology; McGraw-Hill: New York, NY, USA, 1968; p. xii-258. [Google Scholar]
- Romano, L.A.; Pedrosa, V.F. Re-claiming H&E: Back to the future. Postgrad. Med. J. 2020, 96, 58. [Google Scholar]
- Bullerwell, C.N.; Collins, S.A.; Lall, S.P.; Anderson, D.M. Growth performance, proximate and histological analysis of rainbow trout fed diets containing Camelina sativa seeds, meal (high-oil and solvent-extracted) and oil. Aquaculture 2016, 452, 342–350. [Google Scholar] [CrossRef]
- Searle, S.R.; Casella, G.; McCulloch, C.E. Variance Components; John Wiley and Sons: Hoboken, NJ, USA, 2006; p. 528. [Google Scholar]
- Schwalfenberg, G.K. The alkaline diet: Is there evidence that an alkaline pH diet benefits health? J. Environ. Public Health 2012, 2012, 727630. [Google Scholar] [CrossRef] [PubMed]
- Fardet, A.; Richonnet, C. Nutrient density and bioaccessibility, and the antioxidant, satiety, glycemic, and alkalinizing potentials of fruit-based foods according to the degree of processing: A narrative review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3233–3258. [Google Scholar] [CrossRef] [PubMed]
- Rosas, C.; Sanchez, A.; Diaz, E.; Soto, L.A.; Gaxiola, G.; Brito, R. Effect of dietary protein level on apparent heat increment and post-prandial nitrogen excretion of Penaeus setiferus, P. schmitti, P. duorarum, and P. notialis postlarvae. J. World Aquac. Soc. 1996, 27, 92–102. [Google Scholar] [CrossRef]
- Zhao, M.; Yao, D.; Li, S.; Zhang, Y.; Aweya, J.J. Effects of ammonia on shrimp physiology and immunity: A review. Rev. Aquac. 2020, 12, 2194–2211. [Google Scholar] [CrossRef]
- Sui, Z.; Wei, C.; Wang, X.; Zhou, H.; Liu, C.; Mai, K.; He, G. Nutrient sensing signaling and metabolic responses in shrimp Litopenaeus vannamei under acute ammonia stress. Ecotoxicol. Environ. Saf. 2023, 253, 114672. [Google Scholar] [CrossRef]
- Erickson, A.J.; Ramsewak, R.S.; Smucker, A.J.; Nair, M.G. Nitrifcation inhibitors from the roots of Leucaena leucocephala. J. Agric. Food Chem. 2000, 48, 6174–6177. [Google Scholar] [CrossRef]
- Cesco, S.; Mimmo, T.; Tonon, G.; Tomasi, N.; Pinton, R.; Terzano, R.; Neumann, G.; Weisskopf, L.; Renella, G.; Landi, L.; et al. Plant-borne flavonoids released into the rhizosphere: Impact on soil bio-activities related to plant nutrition. A review. Biol. Fertil. Soils 2012, 48, 123–149. [Google Scholar] [CrossRef]
- Tang, S.; Ma, Q.; Luo, J.; Xie, Y.; Pan, W.; Zheng, N.; Liu, M.; Wu, L. The inhibition effect of tea polyphenols on soil nitrification is greater than denitrification in tea garden soil. Sci. Total Environ. 2021, 778, 146328. [Google Scholar] [CrossRef]
- Fernandes Da Silva, C.; Ballester, E.; Monserrat, J.; Geracitano, L.; Wasielesky, W., Jr.; Abreu, P.C. Contribution of microorganisms to the biofilm nutritional quality: Protein and lipid contents. Aquac. Nutr. 2008, 14, 507–514. [Google Scholar] [CrossRef]
- Reis, W.G.; Wasielesky, W., Jr.; Abreu, P.C.; Brandão, H.; Krummenauer, D. Rearing of the Pacifc white shrimp Litopenaeus vannamei (Boone, 1931) in BFT system with different photoperiods: Effects on the microbial community, water quality and zootechnical performance. Aquaculture 2019, 508, 19–29. [Google Scholar] [CrossRef]
- Suita, S.M.; Cardozo, A.P.; Romano, L.A.; Abreu, P.C.; Wasielesky, W., Jr. Development of the hepatopancreas and quality analysis of post-larvae Pacifc white shrimp Litopenaeus vannamei produced in a BFT system. Aquac. Int. 2014, 23, 449–463. [Google Scholar] [CrossRef]
- Yun, H.; Shahkar, E.; Katya, K.; Jang, I.K.; Kim, S.K.; Bai, S.C. Effects of bioflocs on dietary protein requirement in juvenile whiteleg shrimp, Litopenaeus vannamei. Aquac. Res. 2016, 47, 3203–3214. [Google Scholar] [CrossRef]
- Habotta, O.A.; Dawood, M.A.; Kari, Z.A.; Tapingkae, W.; Van Doan, H. Antioxidative and immunostimulant potential of fruit derived biomolecules in aquaculture. Fish Shellfish Immunol. 2022, 130, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Naiel, M.A.; El-Kholy, A.I.; Negm, S.S.; Ghazanfar, S.; Shukry, M.; Zhang, Z.; Ahmadifar, E.; Abdel-Latif, H.M. A mini-review on plant-derived phenolic compounds with particular emphasis on their possible applications and beneficial uses in aquaculture. Ann. Anim. Sci. 2023; ahead of print. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015; p. 851. [Google Scholar]
- Pinn, E.H.; Nickell, L.A.; Rogerson, A.; Atkinson, R.J. Comparison of gut morphology and gut microflora of seven species of mud shrimp (Crustacea: Decapoda: Thalassinidea). Mar. Biol. 1999, 133, 103–114. [Google Scholar] [CrossRef]
- Garibay-Valdez, E.; Cicala, F.; Martinez-Porchas, M.; Gómez-Reyes, R.; Vargas-Albores, F.; Gollas-Galván, T.; Martínez-Córdova, L.F.; Calderón, K. Longitudinal variations in the gastrointestinal microbiome of the white shrimp, Litopenaeus vannamei. PeerJ 2021, 9, e11827. [Google Scholar] [CrossRef]
- Talbot, P.; Clark, W.H.; Lawrence, A.L. Fine structure of the midgut epithelium in the developing brown shrimp Penaeus aztecus. J. Morphol. 1972, 138, 467–485. [Google Scholar] [CrossRef]
- Duan, Y.; Xiong, D.; Wang, Y.; Dong, H.; Huang, J.; Zhang, J. Effects of Microcystis aeruginosa and microcystin-LR on intestinal histology, immune response, and microbial community in Litopenaeus vannamei. Environ. Pollut. 2020, 265, 114774. [Google Scholar] [CrossRef]
- Klahan, R.; Deevong, P.; Wiboonsirikul, J.; Yuangsoi, B. Growth Performance, feed utilisation, endogenous digestive enzymes, intestinal morphology, and antimicrobial effect of Pacific White Shrimp (Litopenaeus vannamei) fed with feed supplemented with pineapple waste crude extract as a functional feed additive. Aquac. Nutr. 2023, 2023, 1160015. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, G.B.; Smagghe, G.; Grootaert, C.; Zotti, M.; Raes, K.; Camp, J.V. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure–activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015, 47, 175–190. [Google Scholar] [CrossRef]
- Kang, J.; Li, Z.; Wu, T.; Jensen, G.S.; Schauss, A.G.; Wu, X. Anti-oxidant capacities of flavonoid compounds isolated from acai pulp (Euterpe oleracea Mart.). Food Chem. 2010, 122, 610–617. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, Q.; Ding, Z.; Zheng, J.; Zhou, D.; Wei, S.; Han, X.; Chang, X.; Li, X.; Xue, Y. Dietary α-lipoic acid requirement and its effects on antioxidant status, carbohydrate metabolism, and intestinal microflora in oriental river prawn Macrobrachium nipponense (De Haan). Aquaculture 2022, 547, 737531. [Google Scholar] [CrossRef]



| Parameters | Treatments | |||
|---|---|---|---|---|
| Control | 5 mg L−1 | 20 mg L−1 | 80 mg L−1 | |
| Dissolved oxygen (mg L−1) | 6.13 ± 0.03 a | 6.25 ± 0.04 a | 6.19 ± 0.04 a | 6.16 ± 0.03 a |
| Temperature | 29.85 ± 0.06 a | 29.67 ± 0.07 a | 29.84 ± 0.07 a | 29.84 ± 0.05 a |
| pH | 7.93 ± 0.009 a | 7.92 ± 0.008 a | 7.97 ± 0.008 b | 8.01 ± 0.005 c |
| Ammonia (mg TAN L−1) | 0.31 ± 0.05 a | 0.30 ± 0.04 a | 0.32 ± 0.04 a | 0.71 ± 0.06 b |
| Nitrite—N (mg L−1) | 0.16 ± 0.02 a | 0.16 ± 0.02 a | 0.21 ± 0.04 a | 0.27 ± 0.05 a |
| Nitrate—N (mg L−1) | 44.95 ± 4.61 a | 40.78 ± 3.68 a | 32.51 ± 2.72 a | 17.63 ± 1.48 b |
| Phosphorus—P (mg L−1) | 2.06 ± 0.35 a | 2.16 ± 0.37 a | 1.86 ± 0.28 a | 1.70 ± 0.28 a |
| Alkalinity (mg CaCO3 L−1) | 113.13 ± 5.09 a | 135.63 ± 4.77 a | 159.69 ± 6.02 b | 197.50 ± 5.31 c |
| Salinity | 26.48 ± 0.31 a | 26.65 ± 0.27 a | 27.05 ± 0.36 a | 26.55 ± 0.34 a |
| TSS (mg L−1) | 213.89 ± 16.98 a | 266.85 ± 24.88 b | 323.52 ± 30.05 c | 341.11 ± 25.38 c |
| Parameters | Treatments | |||
|---|---|---|---|---|
| Control | 5 mg L−1 | 20 mg L−1 | 80 mg L−1 | |
| (A) Biofloc | ||||
| Dry matter (%) | 14.64 ± 0.82 a | 13.16 ± 0.86 a | 12.09 ± 0.19 a | 13.91 ± 0.21 a |
| Protein (%) | 30.02 ± 0.57 a | 29.56 ± 0.42 a | 27.73 ± 0.36 a | 32.27 ± 1.58 a |
| Ether extract (%) | 2.62 ± 0.65 a | 2.70 ± 0.49 a | 4.01 ± 0.95 a | 4.27 ± 0.33 a |
| Ash (%) | 45.61 ± 0.26 c | 44.92 ± 0.30 c | 40.47 ± 0.69 b | 27.74 ± 0.88 a |
| (B) Muscle | ||||
| Dry matter (%) | 23.59 ± 0.27 a | 23.47 ± 0.24 a | 24.04 ± 0.33 a | 25.13 ± 0.59 a |
| Protein (%) | 76.23 ± 1.12 ab | 77.75 ± 0.44 b | 74.44 ± 1.35 a | 74.80 ± 0.92 a |
| Ether extract (%) | 2.58 ± 0.34 a | 1.95 ± 0.39 a | 3.68 ± 0.85 a | 3.33 ± 0.21 a |
| Ash (%) | 6.26 ± 0.09 a | 6.42 ± 0.07 a | 6.37 ± 0.08 a | 5.59 ± 0.34 a |
| Parameters | Treatments | |||
|---|---|---|---|---|
| Control | 5 mg L−1 | 20 mg L−1 | 80 mg L−1 | |
| Initial weight (g) | 1.09 ± 0.04 a | 1.10 ± 0.04 a | 1.06 ± 0.05 a | 0.96 ± 0.04 a |
| Final weight (g) | 3.95 ± 0.16 a | 4.14 ± 0.17 a | 3.80 ± 0.16 a | 3.56 ± 0.30 a |
| Weight gain (g) | 2.83 ± 0.15 a | 2.97 ± 0.17 a | 2.78 ± 0.16 a | 2.65 ± 0.30 a |
| SGR (%/day) | 4.05 ± 0.13 a | 4.06 ± 0.15 a | 4.03 ± 0.13 a | 3.98 ± 0.32 a |
| FCR | 2.00 ± 0.10 a | 2.27 ± 0.15 a | 2.27 ± 0.13 a | 1.90 ± 0.26 a |
| PER | 1.52 ± 0.08 a | 1.47 ± 0.08 a | 1.47 ± 0.09 a | 1.91 ± 0.21 a |
| Survival (%) | 79.57 ± 18.84 b | 92.47 ± 7.53 b | 88.45 ± 3.61 b | 38.71 ± 8.53 a |
| Treatments | Middle Intestine | Posterior Intestine | ||||
|---|---|---|---|---|---|---|
| Width (µm) | Height (µm) | Area (µm2) | Width (µm) | Height (µm) | Area (µm2) | |
| Control | 32.70 ± 2.15 a | 24.10 ± 2.91 a | 674.34 ± 101.13 a | 34.86 ± 3.51 a | 29.49 ± 1.73 a | 903.32 ± 127.90 c |
| 5 mg L−1 | 36.50 ± 3.45 a | 36.99 ± 4.31 b | 1311.42 ± 223.48 b | 35.92 ± 3.91 a | 27.26 ± 2.88 a | 819.45 ± 111.33 bc |
| 20 mg L−1 | 40.89 ± 3.05 a | 50.64 ± 3.70 c | 1981.28 ± 212.50 c | 26.77 ± 2.76 a | 19.59 ± 1.84 b | 532.58 ± 98.49 ab |
| 80 mg L−1 | 31.64 ± 2.06 a | 28.31 ± 2.70 ab | 921.03 ± 149.19 ab | 28.66 ± 1.65 a | 16.18 ± 0.58 b | 443.39 ± 33.26 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, G.M.; Marreiro Gomes, R.M.; Muñoz Buitrago, S.A.; Buitrago Ramírez, J.R.; de Sousa Araujo, A.C.; Silva Oliveira, F.P.; Pedrosa, V.F.; Romano, L.A.; Tesser, M.; Wasielesky, W.; et al. Effects of Lyophilized Açaí (Euterpe oleracea) Supplementation on Oxidative Damage and Intestinal Histology in Juvenile Shrimp Penaeus vannamei Reared in Biofloc Systems. Animals 2023, 13, 3282. https://doi.org/10.3390/ani13203282
Colombo GM, Marreiro Gomes RM, Muñoz Buitrago SA, Buitrago Ramírez JR, de Sousa Araujo AC, Silva Oliveira FP, Pedrosa VF, Romano LA, Tesser M, Wasielesky W, et al. Effects of Lyophilized Açaí (Euterpe oleracea) Supplementation on Oxidative Damage and Intestinal Histology in Juvenile Shrimp Penaeus vannamei Reared in Biofloc Systems. Animals. 2023; 13(20):3282. https://doi.org/10.3390/ani13203282
Chicago/Turabian StyleColombo, Grecica Mariana, Robson Matheus Marreiro Gomes, Sonia Astrid Muñoz Buitrago, Juan Rafael Buitrago Ramírez, Alan Carvalho de Sousa Araujo, Fernando Pablo Silva Oliveira, Virgínia Fonseca Pedrosa, Luís Alberto Romano, Marcelo Tesser, Wilson Wasielesky, and et al. 2023. "Effects of Lyophilized Açaí (Euterpe oleracea) Supplementation on Oxidative Damage and Intestinal Histology in Juvenile Shrimp Penaeus vannamei Reared in Biofloc Systems" Animals 13, no. 20: 3282. https://doi.org/10.3390/ani13203282
APA StyleColombo, G. M., Marreiro Gomes, R. M., Muñoz Buitrago, S. A., Buitrago Ramírez, J. R., de Sousa Araujo, A. C., Silva Oliveira, F. P., Pedrosa, V. F., Romano, L. A., Tesser, M., Wasielesky, W., & Monserrat, J. M. (2023). Effects of Lyophilized Açaí (Euterpe oleracea) Supplementation on Oxidative Damage and Intestinal Histology in Juvenile Shrimp Penaeus vannamei Reared in Biofloc Systems. Animals, 13(20), 3282. https://doi.org/10.3390/ani13203282