Influence of Transportation on Stress Response and Cellular Oxidative Stress Markers in Juvenile Meagre (Argyrosomus regius)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Transport Conditions, Animals and Sampling
2.2. Cortisol Measurement
2.3. Immunohistochemistry (IHC)
2.4. Statistical Analysis
3. Results
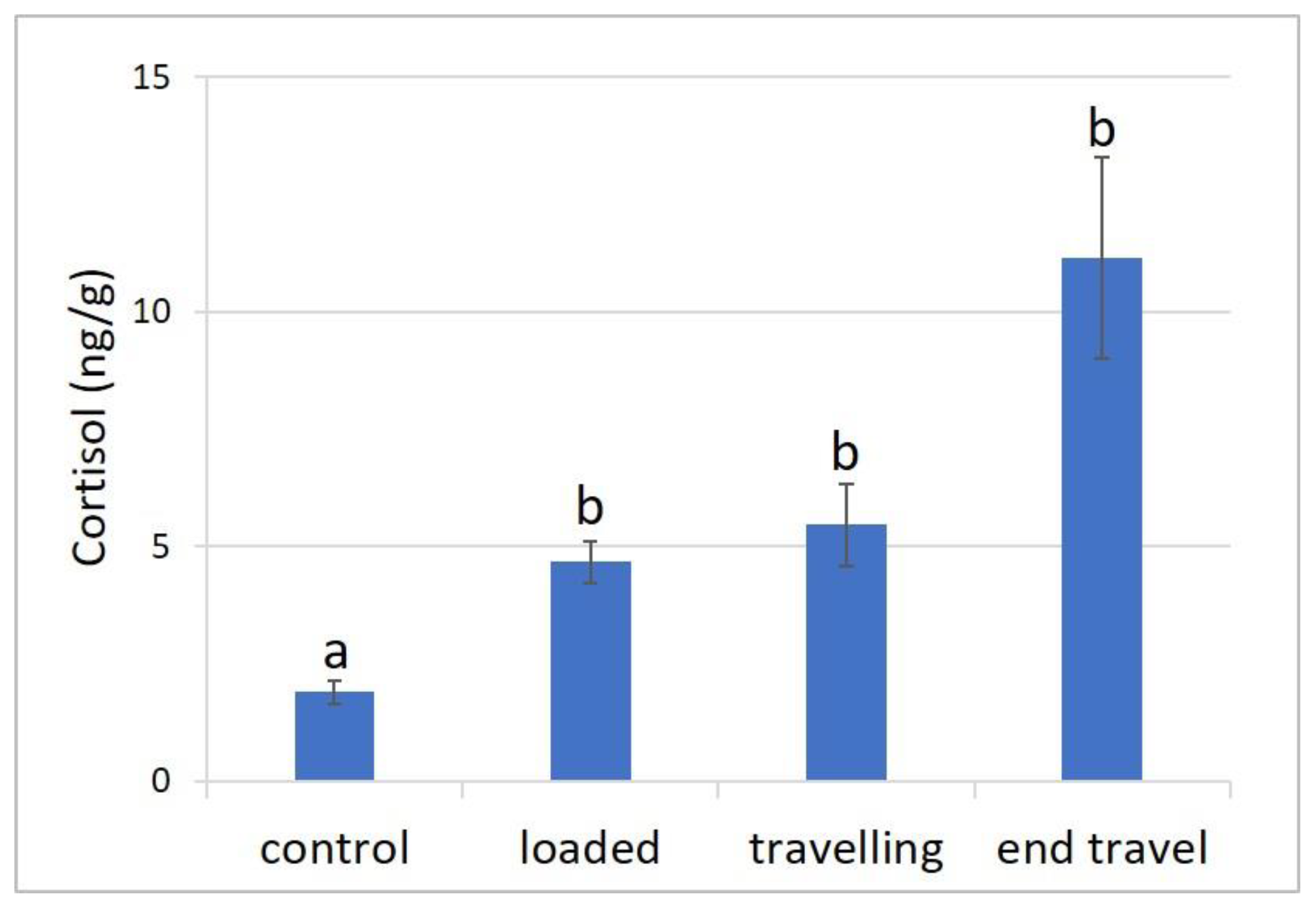
3.1. Stress Response
3.2. HSP70 and Oxidative Stress Markers
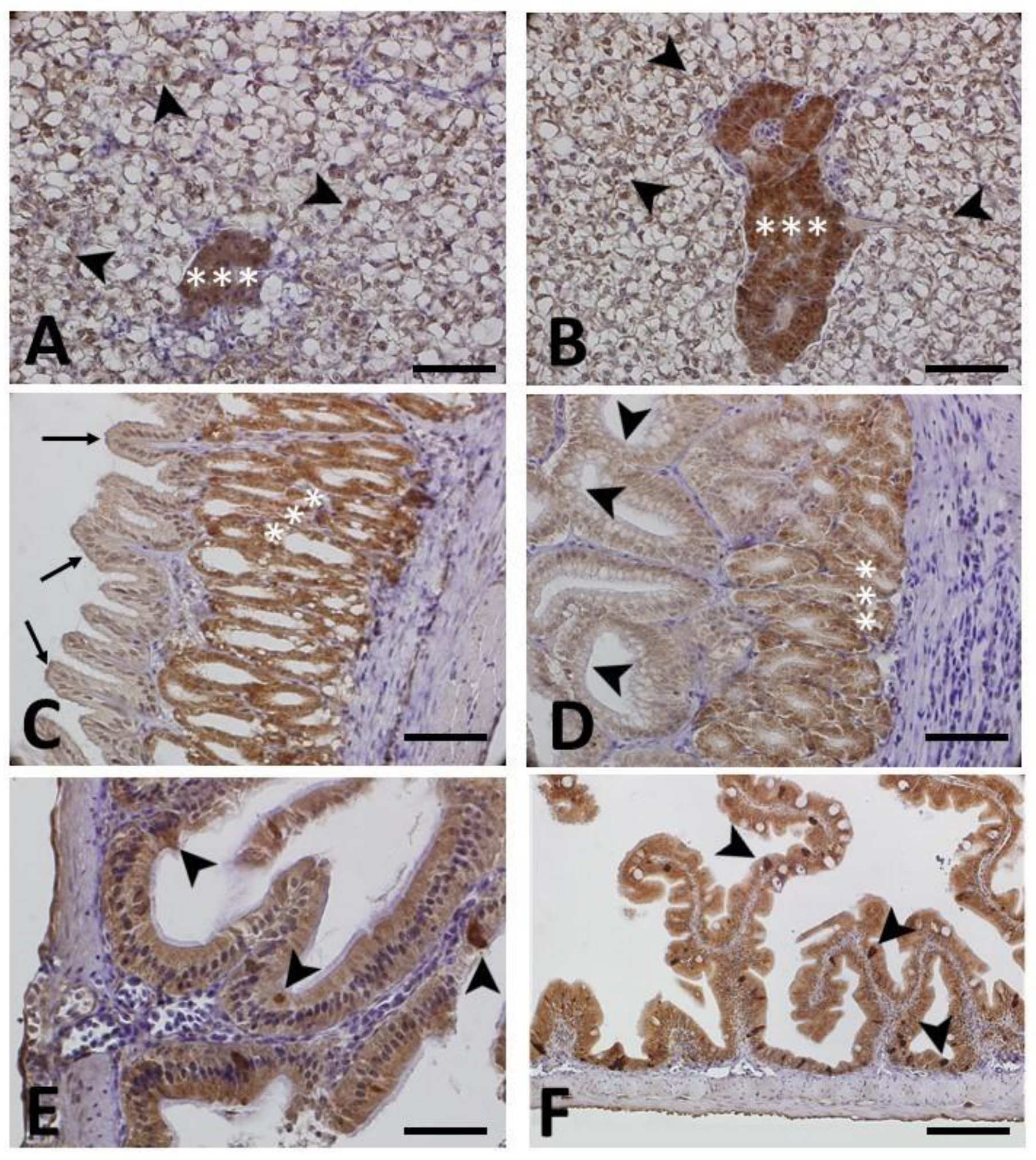
3.2.1. HSP70 Immunohistochemistry
3.2.2. HNE, NT and 8-OHdG Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergqvist, J.; Gunnarsson, S. Finfish Aquaculture: Animal Welfare, the Environment, and Ethical Implications. J. Agric. Environ. Ethics 2013, 26, 75–99. [Google Scholar] [CrossRef]
- Sampaio, F.D.F.; Freire, C.A. An overview of stress physiology of fish transport: Changes in water quality as a function of transport duration. Fish Fish. 2016, 17, 1055–1072. [Google Scholar] [CrossRef]
- King, H.R. Fish transport in the aquaculture sector: An overview of the road transport of Atlantic salmon in Tasmania. J. Vet. Behav. 2009, 4, 163–168. [Google Scholar] [CrossRef]
- Urbinati, E.C.; Abreu, J.S.; Camargo, A.C.; Parra, M.A. Loading and transport stress of juvenile matrinxã (Brycon cephalus, Characidae) at various densities. Aquaculture 2004, 229, 389–400. [Google Scholar] [CrossRef]
- Helmut, S.; Henrik, S.; Kurt, B.; Jessica, D.; Kristina, S.S.; Cédric, M.; Neil, R.; Fredrik, J.; Hilde, T.; Lloyd, V. Health of farmed fish: Its relation to fish welfare and its utility as welfare indicator. Fish Physiol. Biochem. 2011, 38, 85–105. [Google Scholar] [CrossRef]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef]
- Iwama, G.K.; Thomas, P.T.; Forsyth, R.B.; Vijayan, M.M. Heat shock protein expression in fish. Rev. Fish Biol. Fish. 1998, 8, 35–56. [Google Scholar] [CrossRef]
- Yamashita, M.; Yabu, T.; Ojima, N. Stress protein HSP70 in fish. Aqua-Biosci. Monogr. 2010, 3, 111–141. [Google Scholar] [CrossRef]
- Poltronieri, C.; Maccatrozzo, L.; Simontacchi, C.; Bertotto, D.; Funkenstein, B.; Patruno, M.; Radaelli, G. Quantitative RT-PCR analysis and immunohistochemical localization of HSP70 in sea bass Dicentrarchus labrax exposed to transport stress. Eur. J. Histochem. 2007, 51, 125–135. [Google Scholar]
- Poltronieri, C.; Negrato, E.; Bertotto, D.; Majolini, D.; Simontacchi, C.; Radaelli, G. Immunohistochemical localization of constitutive and inducible heat shock protein 70 in carp (Cyprinus carpio) and trout (Oncorhynchus mykiss) exposed to transport stress. Eur. J. Histochem. 2008, 52, 191–198. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 1999; pp. 1–905. [Google Scholar] [CrossRef]
- Davies, K.J.A. Oxidative stress, the paradox of aerobic life. In Free Radical and Oxidative Stress: Environment, Drugs and Food Additives, 2nd ed.; Rice Evans, C., Halliwell, B., Land, G.G., Eds.; Portland Press: London, UK, 1995; Volume 40, pp. 1–31. [Google Scholar]
- Winston, G.W.; Di Giulio, R.T. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat. Toxicol. 1991, 19, 137–161. [Google Scholar] [CrossRef]
- Aldini, G.; Dalle-donne, I.; Facino, R.M.; Milzani, A.; Carini, M. Intervention Strategies to Inhibit Protein Carbonylation by Reactive Carbonyls. Med. Res. Rev. 2006, 27, 817–868. [Google Scholar] [CrossRef]
- Ischiropoulos, H.; Zhu, L.; Chen, J.; Tsai, M.; Martin, J.C.; Smith, C.D.; Beckman, J.S. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys. 2002, 298, 431–437. [Google Scholar] [CrossRef]
- Ploch, S.A.; Lee, Y.; MacLean, E.; Di Giulio, R.T. Oxidative stress in liver of brown bullhead and channel catfish following exposure to tertbutyl hydroperoxide. Aquat. Toxicol. 1999, 46, 231–240. [Google Scholar] [CrossRef]
- Pilger, A.; Rüdiger, H.W. 8-Hydroxy-2′-deoxyguanosine as a marker of oxidative DNA damage related to occupational and environmental exposure. Int. Arch. Occup. Environ. Health 2006, 80, 1–15. [Google Scholar] [CrossRef]
- Bortoletti, M.; Maccatrozzo, L.; Radaelli, G.; Caberlotto, S.; Bertotto, D. Muscle Cortisol Levels, Expression of Glucocorticoid Receptor and Oxidative Stress Markers in the Teleost Fish Argyrosomus regius Exposed to Transport Stress. Animals 2021, 11, 1160. [Google Scholar] [CrossRef]
- Fiocchi, E.; Civettini, M.; Carbonara, P.; Zupa, W.; Lembo, G.; Manfrin, A. Development of molecular and histological methods to evaluate stress oxidative biomarkers in sea bass (Dicentrarchus labrax). Fish Physiol. Biochem. 2020, 46, 1577–1588. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Bortoletti, M.; Olivotto, I.; Ratti, S.; Poltronieri, C.; Negrato, E.; Caberlotto, S.; Radaelli, G.; Bertotto, D. Salinity, Temperature and Ammonia Acute Stress Response in Seabream (Sparus aurata) Juveniles: A Multidisciplinary Study. Animals 2021, 11, 97. [Google Scholar] [CrossRef]
- Pascoli, F.; Negrato, E.; Di Giancamillo, A.; Bertotto, D.; Domeneghini, C.; Simontacchi, C.; Mutinelli, F.; Radaelli, G. Evaluation of oxidative stress biomarkers in Zosterisessor ophiocephalus from the Venice Lagoon, Italy. Aquat. Toxicol. 2011, 101, 512–520. [Google Scholar] [CrossRef]
- Topal, A.; Gergit, A.; Özkaraca, M. Assessment of oxidative DNA damage, oxidative stress responses and histopathological alterations in gill and liver tissues of Oncorhynchus mykiss treated with linuron. Hum. Exp. Toxicol. 2020, 40, 1112–1121. [Google Scholar] [CrossRef]
- Parisi, G.; Terova, G.; Gasco, L.; Piccolo, G.; Roncarati, A.; Moretti, V.M.; Centoducati, G.; Gatta, P.P.; Pais, A. Current status and future perspectives of Italian finfish aquaculture. Rev. Fish Biol. Fish. 2014, 24, 15–73. [Google Scholar] [CrossRef]
- Bertotto, D.; Poltronieri, C.; Negrato, E.; Majolini, D.; Radaelli, G.; Simontacchi, C. Alternative matrices for cortisol measurement in fish. Aquac. Res. 2010, 41, 1261–1267. [Google Scholar] [CrossRef]
- Gomes, L.C.; Edsandra, C.C.; Richard, P.B.; Rodrigo, R.; Carlos, E.C.; Bernardo, B. Use of salt during transportation of air breathing pirarucu juveniles (Arapaima gigas) in plastic bags. Aquaculture 2006, 256, 521–528. [Google Scholar] [CrossRef]
- Honryo, T.; Oakada, T.; Kawahara, M.; Kurata, M.; Agawa, Y.; Sawada, Y.; Miyashita, S.; Takii, K.; Ishibashi, Y. Estimated time for recovery from transportation stress and starvation in juvenile Pacific bluefin tuna Thunnus orientalis. Aquaculture 2018, 484, 175–183. [Google Scholar] [CrossRef]
- Morteza Yousefi, M.; Hoseini, S.M.; Weber, R.A.; Silva, E.; Rajabiesterabadi, H.; Arghideh, M.; Delavar, F.H. Alleviation of transportation-induced stress in Nile tilapia, Oreochromis niloticus, using brackish water. Aquac. Rep. 2022, 27, 101378. [Google Scholar] [CrossRef]
- Refaey, M.M.; Tian, X.; Tang, R.; Li, D. Changes in physiological responses, muscular composition and flesh quality of channel catfish Ictalurus punctatus suffering from transport stress. Aquaculture 2017, 478, 9–15. [Google Scholar] [CrossRef]
- Refaey, M.M.; Li, D. Transport stress changes blood biochemistry, antioxidant defense system, and hepatic HSPs mRNA expressions of channel catfish Ictalurus punctatus. Front. Physiol. 2018, 9, 1628. [Google Scholar] [CrossRef]
- Tacchi, L.; Lowrey, L.; Musharrafieh, R.; Crossey, K.; Larragoite, E.T.; Salinas, I. Effects of transportation stress and addition of salt to transport water on the skin mucosal homeostasis of rainbow trout (Oncorhynchus mykiss). Aquaculture 2015, 435, 120–127. [Google Scholar] [CrossRef]
- Schreck, C.; Solazzi, M.; Johnson, S.; Nickelson, T. Transportation stress affects performance of coho salmon, Oncorhynchus kisutch. Aquaculture 1989, 82, 15–20. [Google Scholar] [CrossRef]
- Weirich, C.; Tomasso, J. Confinement- and Transport-Induced Stress on Red Drum Juveniles: Effect of Salinity. Progress. Fish-Cult. 1991, 53, 146–149. [Google Scholar] [CrossRef]
- Erikson, U.; Sigholt, T.; Rustad, T.; Einarsdóttir, I.E.; Jørgensen, L. Contribution of Bleeding to Total Handling Stress During Slaughter of Atlantic Salmon. Aquac. Int. 1999, 7, 101–115. [Google Scholar] [CrossRef]
- Dobšíková, R.; Svobodová, Z.; Blahova, J.; Modra, H.; Velisek, J. The effect of transport on biochemical and haematological indices of common carp (Cyprinus carpio L.). Czech J. Anim. Sci. 2009, 54, 510–518. [Google Scholar] [CrossRef]
- Skjervold, P.O.; Fjæra, S.O.; Østby, P.B.; Einen, O. Live-chilling and crowding stress before slaughter of Atlantic salmon (Salmo salar). Aquaculture 2001, 192, 265–280. [Google Scholar] [CrossRef]
- Ren, Y.; Men, X.; Yu, Y.; Li, B.; Zhou, Y.; Zhao, C. Effects of transportation stress on antioxidation, immunity capacity and hypoxia tolerance of rainbow trout (Oncorhynchus mykiss). Aquac. Rep. 2022, 22, 100940. [Google Scholar] [CrossRef]
- Liu, X.L.; Xi, Q.Y.; Yang, L.; Li, H.Y.; Jiang, Q.Y.; Shu, G.; Wang, S.B.; Gao, P.; Zhu, X.T.; Zhang, Y.L. The effect of dietary Panax ginseng polysaccharide extract on the immune responses in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2011, 30, 495–500. [Google Scholar] [CrossRef] [PubMed]

| Target Protein | Antibody | Species | Dilution | Characterization | Immunizing Antigen/Source |
|---|---|---|---|---|---|
| Heat Shock Protein 70 (HSP70) | Anti-HSP70 | mouse monoclonal | 1:600 (IHC) | Immunohistochemistry | Recombinant fragment from human Hsp70 (Abcam, Cambridge, UK) |
| 4-Hydroxy-2-nonenal (HNE) | Anti-HNE | mouse monoclonal | 1:50 (IHC) | Immunohistochemistry | 4-Hydroxy-2-nonenal modified KLH (Abcam, UK) |
| 3-Nitrotyrosine (NT) | Anti-NT | mouse monoclonal | 1:1000 (IHC) | Immunohistochemistry | 3-(4-Hydroxy-3-nitrophenylacetamido) propionic acid conjugated to bovine serum albumin (BSA) (GeneTex, Inc., Irvine, CA, USA) |
| 8-Hydroxy-2′-guanosine (8-OHdG) | Anti-8-OHdG | mouse monoclonal | 1:3000 (IHC) | Immunohistochemistry | 8-Hydroxy-2’-deoxyguanosine conjugated Keyhole Limpet Hemocyanin (Abcam, UK) |
| Tissue | Control | Transported | ||||||
|---|---|---|---|---|---|---|---|---|
| HSP70 | NT | HNE | 8-OHdG | HSP70 | NT | HNE | 8-OHdG | |
| Skin | + | - | - | + | + | - | - | + |
| Stomach | ++ | + | ++ | ++ | ++ | + | ++ | ++ |
| Intestine | ++ | + | + | ++ | ++ | + | ++ | ++ |
| Hepatopancreas | ++ | +/- | ++ | + | ++ | +/- | ++ | + |
| Gills | ++ | +/- | ++ | ++ | ++ | +/- | ++ | ++ |
| Muscle | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortoletti, M.; Fonsatti, E.; Leva, F.; Maccatrozzo, L.; Ballarin, C.; Radaelli, G.; Caberlotto, S.; Bertotto, D. Influence of Transportation on Stress Response and Cellular Oxidative Stress Markers in Juvenile Meagre (Argyrosomus regius). Animals 2023, 13, 3288. https://doi.org/10.3390/ani13203288
Bortoletti M, Fonsatti E, Leva F, Maccatrozzo L, Ballarin C, Radaelli G, Caberlotto S, Bertotto D. Influence of Transportation on Stress Response and Cellular Oxidative Stress Markers in Juvenile Meagre (Argyrosomus regius). Animals. 2023; 13(20):3288. https://doi.org/10.3390/ani13203288
Chicago/Turabian StyleBortoletti, Martina, Elisa Fonsatti, Federico Leva, Lisa Maccatrozzo, Cristina Ballarin, Giuseppe Radaelli, Stefano Caberlotto, and Daniela Bertotto. 2023. "Influence of Transportation on Stress Response and Cellular Oxidative Stress Markers in Juvenile Meagre (Argyrosomus regius)" Animals 13, no. 20: 3288. https://doi.org/10.3390/ani13203288
APA StyleBortoletti, M., Fonsatti, E., Leva, F., Maccatrozzo, L., Ballarin, C., Radaelli, G., Caberlotto, S., & Bertotto, D. (2023). Influence of Transportation on Stress Response and Cellular Oxidative Stress Markers in Juvenile Meagre (Argyrosomus regius). Animals, 13(20), 3288. https://doi.org/10.3390/ani13203288











