Four Markers Useful for the Distinction of Intrauterine Growth Restriction in Sheep
Abstract
Simple Summary
Abstract
1. Introduction
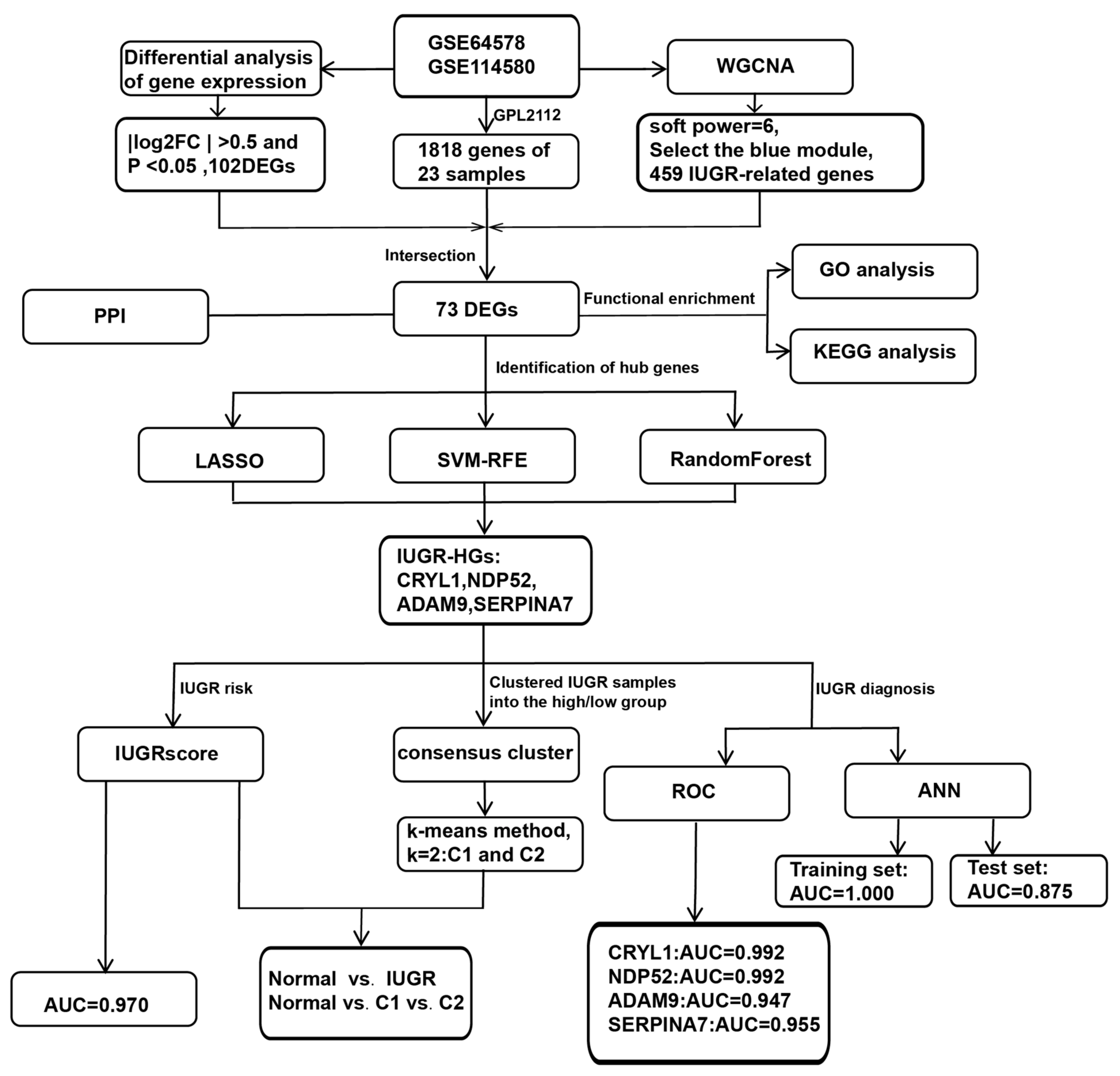
2. Materials and Methods
2.1. Data Acquisition and Preprocessing
2.2. Differential Analysis of Gene Expression
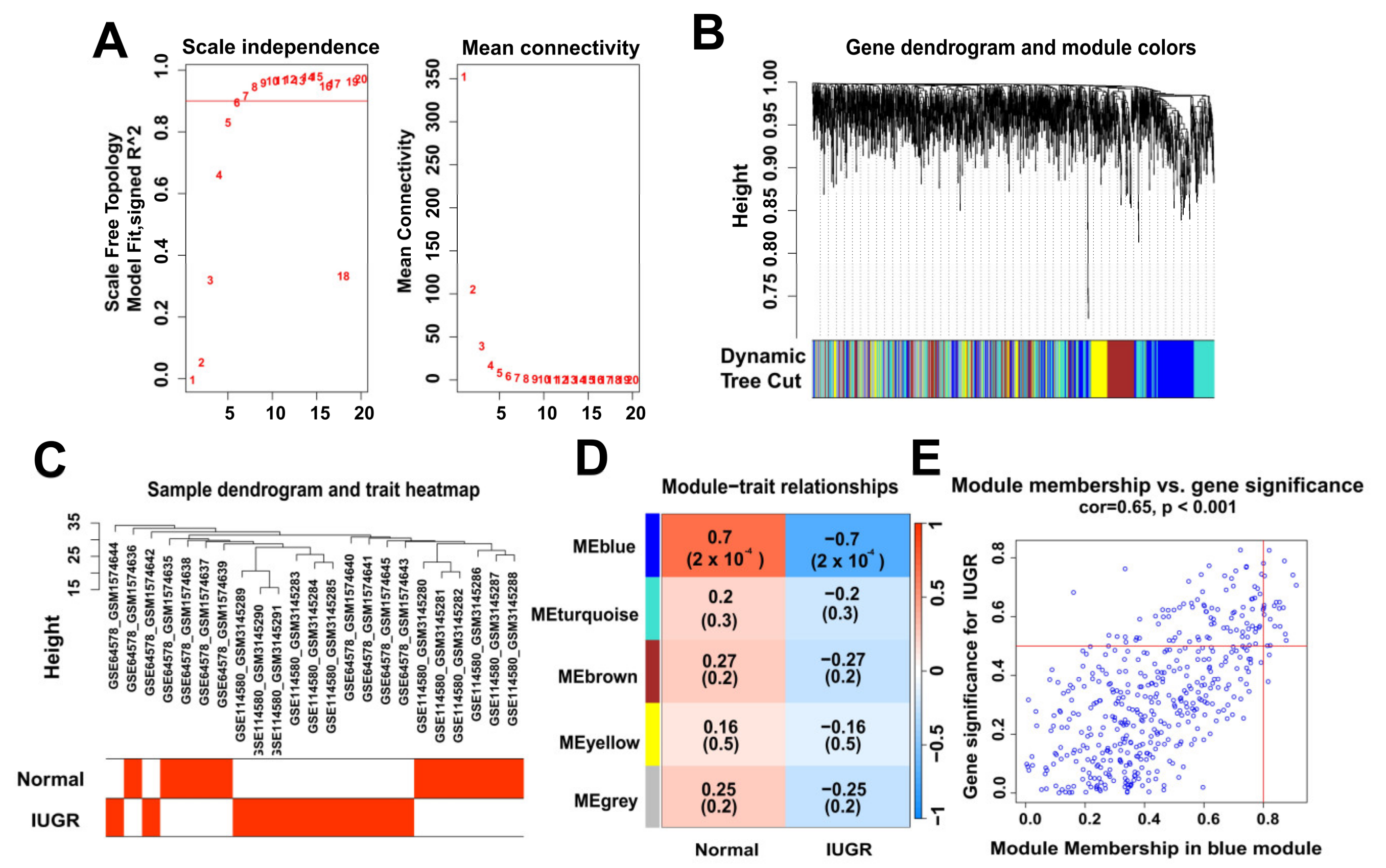
2.3. Weighted Gene Co-Expression Network Analysis
2.4. Protein–Protein Interactions between Differentially Expressed IUGR-Associated Genes
2.5. Functional Enrichment Analysis
2.6. Selection of Hub Genes
2.7. Consensus Clustering Analysis
2.8. Construction of the IUGR Scoring System
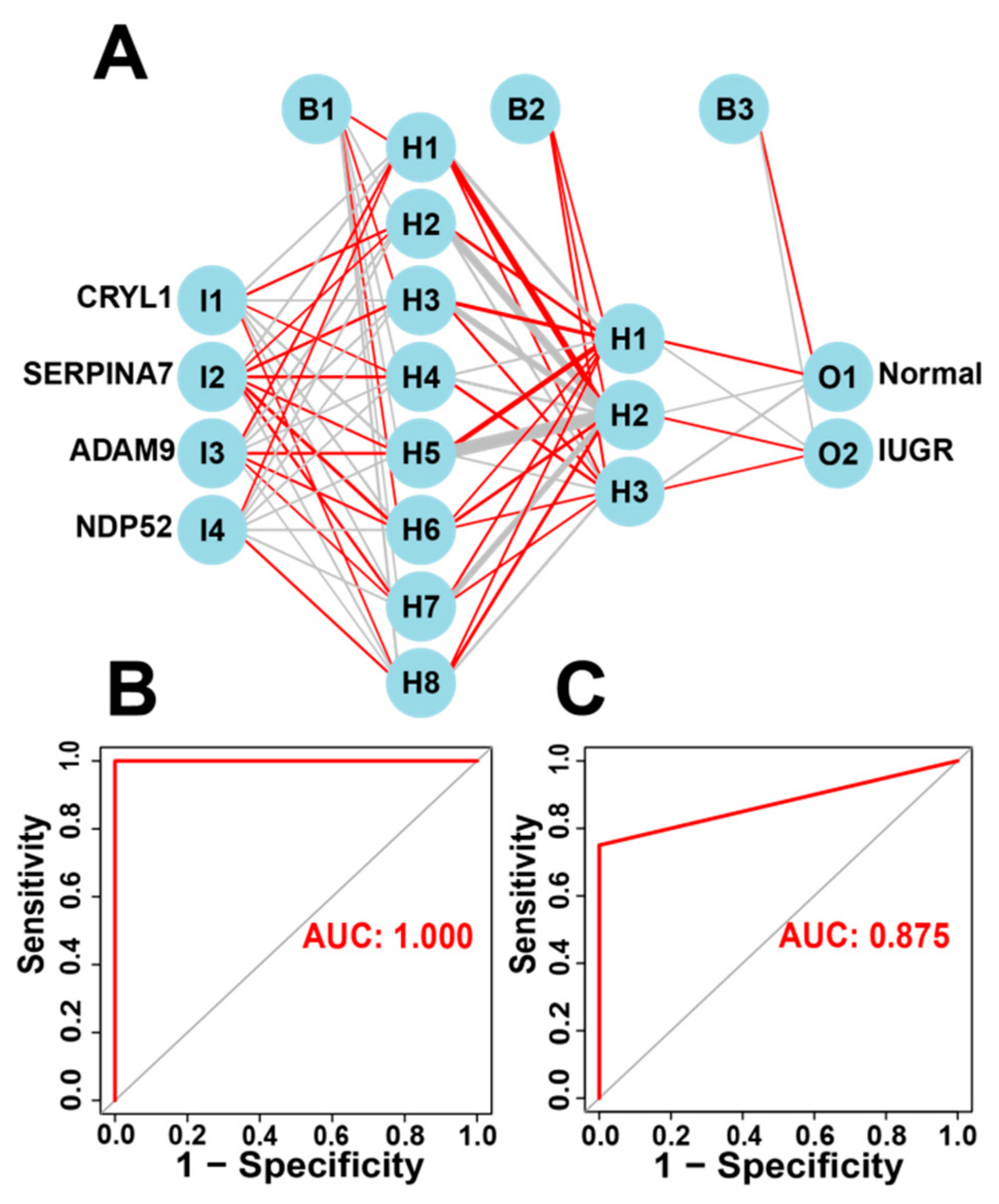
2.9. Construction and Validation of an Artificial Neural Network (ANN) Model
- (1)
- An input layer, which included the gene expression of the four IUGR-HGs;
- (2)
- The first hidden layer, which included the gene expressions and weights of the four IUGR-HGs, and the second hidden layer, which included the weights of all the neurons in hidden layer 1;
- (3)
- The output layer, which indicated whether the sample was “normal” or “IUGR”.
2.10. Evaluation of the Diagnostic Value of the Selected Hub Genes in IUGR
2.11. Statistical Analysis
3. Results
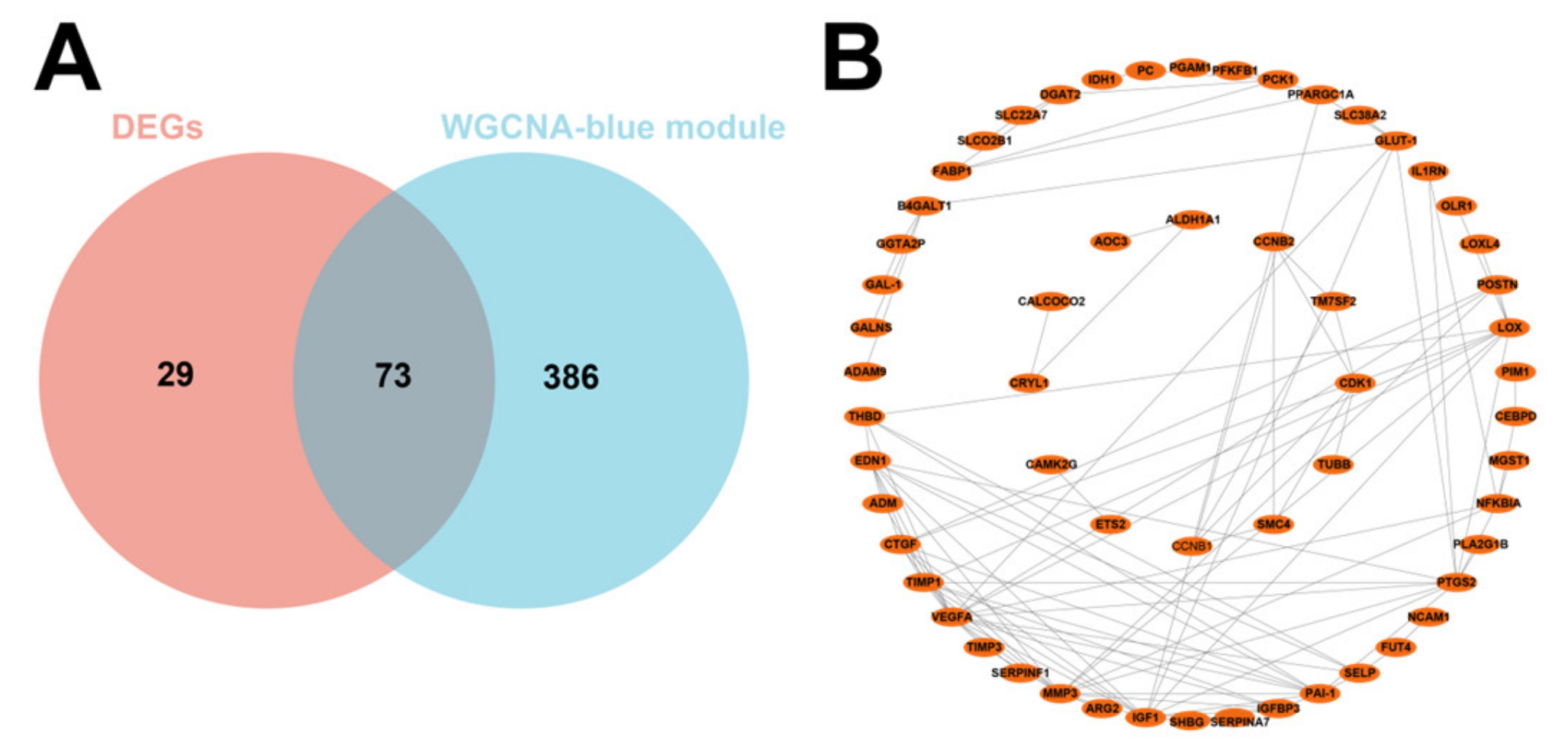
3.1. Screening for DEGs by Comparing IUGR and Normal Samples
3.2. Identification of Modular Genes Associated with IUGR by WGCNA
3.3. GO and KEGG Pathway Analysis of 73 DEGs
3.4. Identification of Hub Genes via Machine Learning
3.5. Identification of Molecular Subtypes Based on IUGR-HGs and Verification of Molecular Subtypes Using the IUGR Score
3.6. Construction and Validation of Artificial Neural Network (ANN) Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hinchliffe, S.A.; Lynch, M.R.J.; Sargent, P.H.; Howard, C.V.; Van, V.D. The effect of intrauterine growth retardation on the development of renal nephrons. Br. J. Obstet. Gynaecol. 1992, 99, 296–301. [Google Scholar] [CrossRef]
- Sultana, Z.; Maiti, K.; Aitken, J.; Morris, J.; Dedman, L.; Smith, R. Oxidative stress, placental ageing-related pathologies and adverse pregnancy outcomes. Am. J. Reprod. Immunol. 2017, 77, e12653. [Google Scholar] [CrossRef]
- Li, T.; Huang, S.; Lei, L.; Tao, S.; Xiong, Y.; Wu, G.; Hu, J.; Yuan, X.; Zhao, S.; Zuo, B.; et al. Intrauterine growth restriction alters nutrient metabolism in the intestine of porcine offspring. J. Anim. Sci. Biotechnol. 2021, 12, 15. [Google Scholar] [CrossRef]
- Darendeliler, F. IUGR: Genetic influences, metabolic problems, environmental associations/triggers, current and future management. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101260. [Google Scholar] [CrossRef]
- Sharma, D.; Shastri, S.; Sharma, P. Intrauterine growth restriction: Antenatal and postnatal aspects. Clin. Med. Insights Pediatr. 2016, 10, 67–83. [Google Scholar] [CrossRef]
- Aucott, S.W.; Donohue, P.K.; Northington, F.J. Increased morbidity in severe early intrauterine growth restriction. J. Perinatol. 2004, 24, 435–440. [Google Scholar] [CrossRef]
- Oksbjerg, N.; Nissen, P.M.; Therkildsen, M.; Møller, H.S.; Larsen, L.B.; Andersen, M.; Young, J.F. Meat Science and Muscle Biology Symposium: In utero nutrition related to fetal development, postnatal performance, and meat quality of pork. J. Anim. Sci. 2013, 91, 1443–1453. [Google Scholar] [CrossRef]
- Gatford, K.L.; Simmons, R.A. Prenatal programming of insulin secretion in intrauterine growth restriction. Clin. Obstet. Gynecol. 2013, 56, 520–528. [Google Scholar] [CrossRef]
- Huppertz, B.; Kadyrov, M.; Kingdom, J.C.P. Apoptosis and its role in the trophoblast. Am. J. Obstet. Gynecol. 2006, 195, 29–39. [Google Scholar] [CrossRef]
- Bowman, C.J.; Streck, R.D.; Chapin, R.E. Maternal-placental insulin-like growth factor (IGF) signaling and its importance to normal embryo-fetal development. Birth Defects Res. B Dev. Reprod. Toxicol. 2010, 89, 339–349. [Google Scholar] [CrossRef]
- Gurugubelli, K.R.; Vishnu, B.B. Molecular mechanisms of intrauterine growth restriction. J. Matern. Fetal Neonatal Med. 2018, 31, 2634–2640. [Google Scholar] [CrossRef]
- Calthorpe, R.J.; Poulter, C.; Smyth, A.R.; Sharkey, D.; Bhatt, J.; Jenkins, G.; Tatler, A.L. Complex roles of TGF-beta signaling pathways in lung development and bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 324, L285–L296. [Google Scholar] [CrossRef]
- Thorn, S.R.; Brown, L.D.; Rozance, P.J.; Hay, J.W.W.; Friedman, J.E. Increased hepatic glucose production in fetal sheep with intrauterine growth restriction is not suppressed by insulin. Diabetes 2013, 62, 65–73. [Google Scholar] [CrossRef]
- Inoue, H.; Nishio, H.; Takada, H.; Sakai, Y.; Nanishi, E.; Ochiai, M.; Onimaru, M.; Chen, S.J.; Matsui, T.; Hara, T. Activation of Nod1 signaling induces fetal growth restriction and death through fetal and maternal vasculopathy. J. Immunol. 2016, 196, 2779–2787. [Google Scholar] [CrossRef]
- Tzschoppe, A.; Struwe, E.; Rascher, W.; Dörr, H.G.; Schild, R.L.; Goecke, T.W.; Beckmann, M.W.; Hofner, B.; Kratzsch, J.; Dötsch, J. Intrauterine growth restriction (IUGR) is associated with increased leptin synthesis and binding capability in neonates. Clin. Endocrinol. 2011, 74, 459–466. [Google Scholar] [CrossRef]
- Hardy, D.B. Maternal undernutrition and long-term effects on hepatic function. In Diet, Nutrition, and Fetal Programming; Springer International Publishing: Cham, Switzerland, 2017; pp. 107–120. [Google Scholar]
- Yates, D.T.; Cadaret, C.N.; Beede, K.A.; Riley, H.E.; Macko, A.R.; Anderson, M.J.; Camacho, L.E.; Limesand, S.W. Intrauterine growth-restricted sheep fetuses exhibit smaller hindlimb muscle fibers and lower proportions of insulin-sensitive Type I fibers near term. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R1020–R1029. [Google Scholar] [CrossRef]
- Chen, X.; Fahy, A.L.; Green, A.S.; Anderson, M.J.; Rhoads, R.P.; Limesand, S.W. β2-adrenergic receptor desensitization in perirenal adipose tissue in fetuses and lambs with placental insufficiency-induced intrauterine growth restriction. J. Physiol. 2010, 588, 3539–3549. [Google Scholar] [CrossRef]
- Wang, K.C.; Brooks, D.A.; Botting, K.J.; Morrison, J.L. IGF-2R-mediated signaling results in hypertrophy of cultured cardiomyocytes from fetal sheep. Biol. Reprod. 2012, 86, 183. [Google Scholar] [CrossRef]
- Lassala, A.; Bazer, F.W.; Cudd, T.A.; Datta, S.; Keisler, D.H.; Satterfield, M.C.; Spencer, T.E.; Wu, G. Parenteral administration of L-arginine enhances fetal survival and growth in sheep carrying multiple fetuses. J. Nutr. 2011, 141, 849–855. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.I.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, G.; Sahin, F. A survey on feature selection methods. Comput. Electr. Eng. 2014, 40, 16–28. [Google Scholar] [CrossRef]
- Basu, S.; Kumbier, K.; Brown, J.B.; Yu, B. Iterative random forests to discover predictive and stable high-order interactions. Proc. Natl. Acad. Sci. USA 2018, 115, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Hepp, T.; Schmid, M.; Gefeller, O.; Waldmann, E.; Mayr, A. Approaches to Regularized Regression—A Comparison between Gradient Boosting and the Lasso. Methods Inf. Med. 2016, 55, 422–430. [Google Scholar] [CrossRef]
- Noble, W.S. What is a support vector machine? Nat. Biotechnol. 2006, 24, 1565–1567. [Google Scholar] [CrossRef]
- Paul, A.; Mukherjee, D.P.; Das, P.; Gangopadhyay, A.; Chintha, A.R.; Kundu, S. Improved random forest for classification. IEEE Trans. Image Process. 2018, 27, 4012–4024. [Google Scholar] [CrossRef]
- Vasquez, M.M.; Hu, C.; Roe, D.J.; Chen, Z.; Halonen, M.; Guerra, S. Least absolute shrinkage and selection operator type methods for the identification of serum biomarkers of overweight and obesity: Simulation and application. BMC Med. Res. Methodol. 2016, 16, 154. [Google Scholar] [CrossRef]
- Wilkerson, M.D.; Hayes, D.N. ConsensusClusterPlus: A class discovery tool with confidence assessments and item tracking. Bioinformatics 2010, 26, 1572–1573. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Q.; Li, B.; Wang, D.; Wang, L.; Zhou, Y.L. m6A regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Mol. Cancer 2020, 19, 53. [Google Scholar] [CrossRef] [PubMed]
- Hagel-Bradway, S.; Tatakis, D.N.; Dziak, R. Prostaglandin-induced changes in calcium uptake and cAMP production in osteoblast-like cells: Role of protein kinase C. Calcif. Tissue Int. 1991, 48, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, P.; Tang, J.; Wang, R.; Wang, X.; Wang, F.; Ruan, J.; Yu, S.; Tang, J.; Huang, R.; et al. m6A regulator-mediated tumour infiltration and methylation modification in cervical cancer microenvironment. Front. Immunol. 2022, 13, 888650. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liang, C.; Li, J. A signal recognition particle-related joint model of lasso regression, SVM-RFE and artificial neural network for the diagnosis of systemic sclerosis-associated pulmonary hypertension. Front. Genet. 2022, 13, 1078200. [Google Scholar] [CrossRef]
- Beck, M.W. NeuralNetTools: Visualization and analysis tools for neural networks. J. Stat. Softw. 2018, 85, 1. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Sthle, L.; Wold, S. Analysis of variance (ANOVA). Chemom. Intell. Lab. Syst. 1989, 6, 259–272. [Google Scholar] [CrossRef]
- Lin, G.; Wang, X.; Wu, G.; Feng, C.; Zhou, H.; Li, D.; Wang, J. Improving amino acid nutrition to prevent intrauterine growth restriction in mammals. Amino Acids 2014, 46, 1605–1623. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Astiz, S.; Parraguez, V.H.; Garcia-Contreras, C.; Vazquez-Gomez, M. Empowering translational research in fetal growth restriction: Sheep and swine animal models. Curr. Pharm. Biotechnol. 2016, 17, 848–855. [Google Scholar] [CrossRef]
- Brown, L.D.; Hay, J.W.W. Impact of placental insufficiency on fetal skeletal muscle growth. Mol. Cell. Endocrinol. 2016, 435, 69–77. [Google Scholar] [CrossRef]
- Carrera, S.; Senra, J.; Acosta, M.I.; Althubiti, M.; Hammond, E.M.; Verdier, P.J.D.; Macip, S. The role of the HIF-1α transcription factor in increased cell division at physiological oxygen tensions. PLoS ONE 2014, 9, e97938. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, D.; Fang, Q.; Zhong, M. Aberrant hydroxymethylation of ANGPTL4 is associated with selective intrauterine growth restriction in monochorionic twin pregnancies. Epigenetics 2020, 15, 887–899. [Google Scholar] [CrossRef]
- Nakamura, Y. Isolation of p53-target genes and their functional analysis. Cancer Sci. 2004, 95, 7–11. [Google Scholar] [CrossRef]
- Baserga, M.; Hale, M.A.; Ke, X.; Wang, Z.M.; Yu, X.; Callaway, C.W.; McKnight, R.A.; Lane, R.H. Uteroplacental insufficiency increases p53 phosphorylation without triggering the p53-MDM2 functional circuit response in the IUGR rat kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R412–R418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, X.; Zhang, L.; Lu, Y.; Deng, J. Abnormal meiosis progression and DNA damage response are associated with the development of intrauterine growth restriction. J. Cell. Physiol. 2020, 235, 9204–9213. [Google Scholar]
- Cho, C. Testicular and epididymal ADAMs: Expression and function during fertilization. Nat. Rev. Urol. 2012, 9, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.W.; Huang, Y.K.; Kuo, T.T.; Liu, J.P.; Sher, Y.P. An overview of ADAM9: Structure, activation, and regulation in human diseases. Int. J. Mol. Sci. 2020, 21, 7790. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.I.Y.; Ibrahim, M.E.; Khalil, E.A.G. High altitude and pre-eclampsia: Adaptation or protection. Med. Hypotheses 2017, 104, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Lakhssassi, K.; Sarto, M.P.; Lahoz, B.; Alabart, J.L.; Folch, J.; Serrano, M.; Calvo, J.H. Blood transcriptome of Rasa Aragonesa rams with different sexual behavior phenotype reveals CRYL1 and SORCS2 as genes associated with this trait. J. Anim. Sci. 2023, 101, skad098. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Ding, G.; Zhou, Y.; Zhu, H.; Jiang, H. Downregulation of Crystallin Lambda 1 is a New Independent Prognostic Marker in Clear Cell Renal Cell Carcinoma. Pharmacogenom. Pers. Med. 2022, 10, 857–866. [Google Scholar] [CrossRef]
- Thurston, T.L.; Ryzhakov, G.; Bloor, S.; von Muhlinen, N.; Randow, F. The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat. Immunol. 2009, 10, 1215–1221. [Google Scholar] [CrossRef]
- Verlhac, P.; Grégoire, I.P.; Azocar, O.; Petkova, D.S.; Baguet, J.; Viret, C.; Faure, M. Autophagy receptor NDP52 regulates pathogen-containing autophagosome maturation. Cell Host Microbe 2015, 17, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chen, H.; Chen, Q.; Wang, C.; Liang, L. Compound hemizygous variants in SERPINA7 gene cause thyroxine-binding globulin deficiency. Mol. Genet. Genom. Med. 2021, 9, e1571. [Google Scholar] [CrossRef] [PubMed]
- Gawandi, S.; Jothivel, K.; Kulkarni, S. Identification of a novel mutation in thyroxine-binding globulin (TBG) gene associated with TBG-deficiency and its effect on the thyroid function. J. Endocrinol. Investig. 2022, 45, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.; Boland, M.R. Enabling pregnant women and their physicians to make informed medication decisions using artificial intelligence. J. Pharmacokinet. Pharmacodyn. 2020, 47, 305–318. [Google Scholar] [CrossRef]
- Ueyama, H.; Kato, Y.; Akazawa, Y.; Yatagai, N.; Komori, H.; Takeda, T.; Matsumoto, K.; Ueda, K.; Matsumoto, K.; Hojo, M.; et al. Application of artificial intelligence using a convolutional neural network for diagnosis of early gastric cancer based on magnifying endoscopy with narrow-band imaging. J. Gastroenterol. Hepatol. 2021, 36, 482–489. [Google Scholar] [CrossRef]
- Wuestemann, J.; Hupfeld, S.; Kupitz, D.; Genseke, P.; Schenke, S.; Pech, M.; Kreissl, M.C.; Grosser, O.S. Analysis of bone scans in various tumor entities using a deep-learning-based artificial neural network algorithm-evaluation of diagnostic performance. Cancers 2020, 12, 2654. [Google Scholar] [CrossRef]

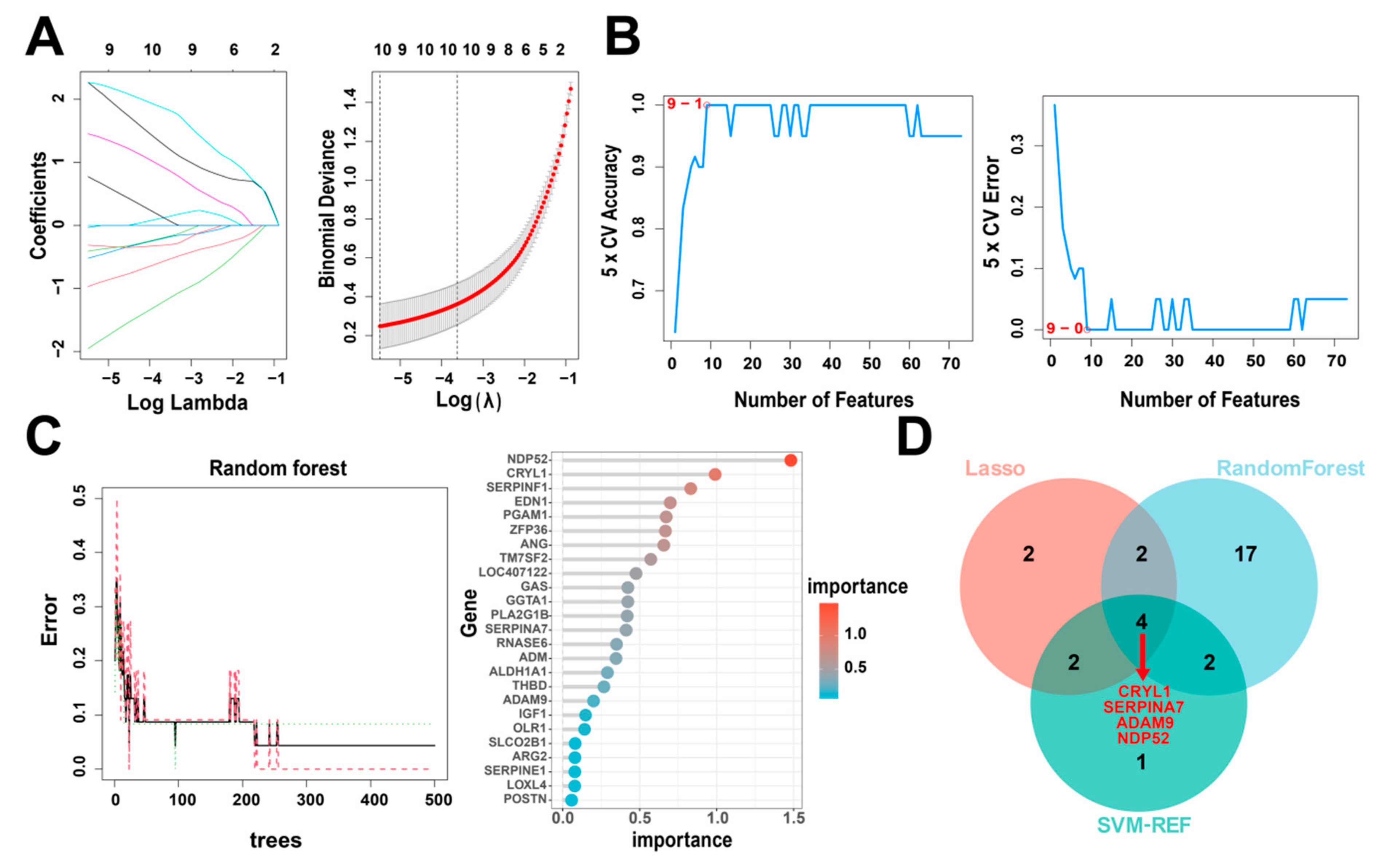

| Methods | Genes |
|---|---|
| Lasso | CRYL1, TM7SF2, SERPINA7, POSTN, ADAM9, CKM, LGALS1, CYB5, CCNB2, and NDP52 |
| RandomForest | NDP52, CRYL1, SERPINF1, EDN1, PGAM1, ZFP36, ANG, TM7SF2, LOC407122, GGTA1, GAS, PLA2G1B, SERPINA7, RNASE6, ADM, ALDH1A1, THBD, ADAM9, IGF1, OLR1, SLCO2B1, ARG2, SERPINE1, LOXL4, and POSTN |
| SVM-REF | SERPINA7, CRYL1, NCAM1, NDP52, ZFP36, ADAM9. CYB5, GGTA1, and CKM |
| Training Set | Test Set | ||||
|---|---|---|---|---|---|
| Normal | IUGR | Normal | IUGR | ||
| Prediction | Normal | 10 | 0 | 2 | 1 |
| IUGR | 0 | 7 | 0 | 3 | |
| Normal accuracy | 1 | 1 | |||
| IUGR accuracy | 1 | 0.75 | |||
| AUC | 1 | 0.875 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Chen, S.; Qiao, L.; Zhang, S.; Liu, Q.; Yang, K.; Pan, Y.; Liu, J.; Liu, W. Four Markers Useful for the Distinction of Intrauterine Growth Restriction in Sheep. Animals 2023, 13, 3305. https://doi.org/10.3390/ani13213305
Wang W, Chen S, Qiao L, Zhang S, Liu Q, Yang K, Pan Y, Liu J, Liu W. Four Markers Useful for the Distinction of Intrauterine Growth Restriction in Sheep. Animals. 2023; 13(21):3305. https://doi.org/10.3390/ani13213305
Chicago/Turabian StyleWang, Wannian, Sijia Chen, Liying Qiao, Siying Zhang, Qiaoxia Liu, Kaijie Yang, Yangyang Pan, Jianhua Liu, and Wenzhong Liu. 2023. "Four Markers Useful for the Distinction of Intrauterine Growth Restriction in Sheep" Animals 13, no. 21: 3305. https://doi.org/10.3390/ani13213305
APA StyleWang, W., Chen, S., Qiao, L., Zhang, S., Liu, Q., Yang, K., Pan, Y., Liu, J., & Liu, W. (2023). Four Markers Useful for the Distinction of Intrauterine Growth Restriction in Sheep. Animals, 13(21), 3305. https://doi.org/10.3390/ani13213305





