Effect of Environmental and Farm-Associated Factors on Live Performance Parameters of Broilers Raised under Commercial Tropical Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Database and Statistical Software
2.2. Data Analysis 1: Performance and Environment
- T = Temperature;
- RH = Relative humidity.
2.3. Data Analysis 2: Farm Management Factors and Performance
2.4. Data Analysis 3: Prediction of Performance with ML
3. Results
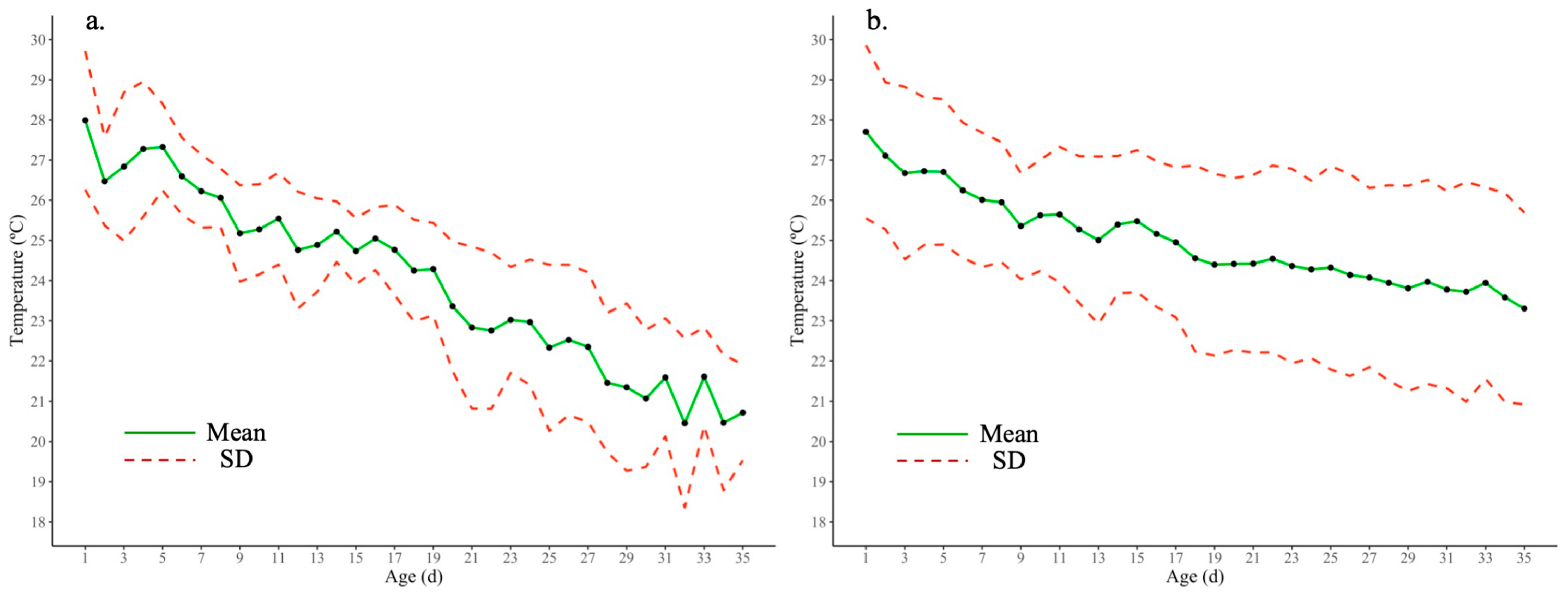
3.1. Data Analysis: Performance and Environment
3.2. Data Analysis 2: Farm Management and Infrastructure Factors and Performance
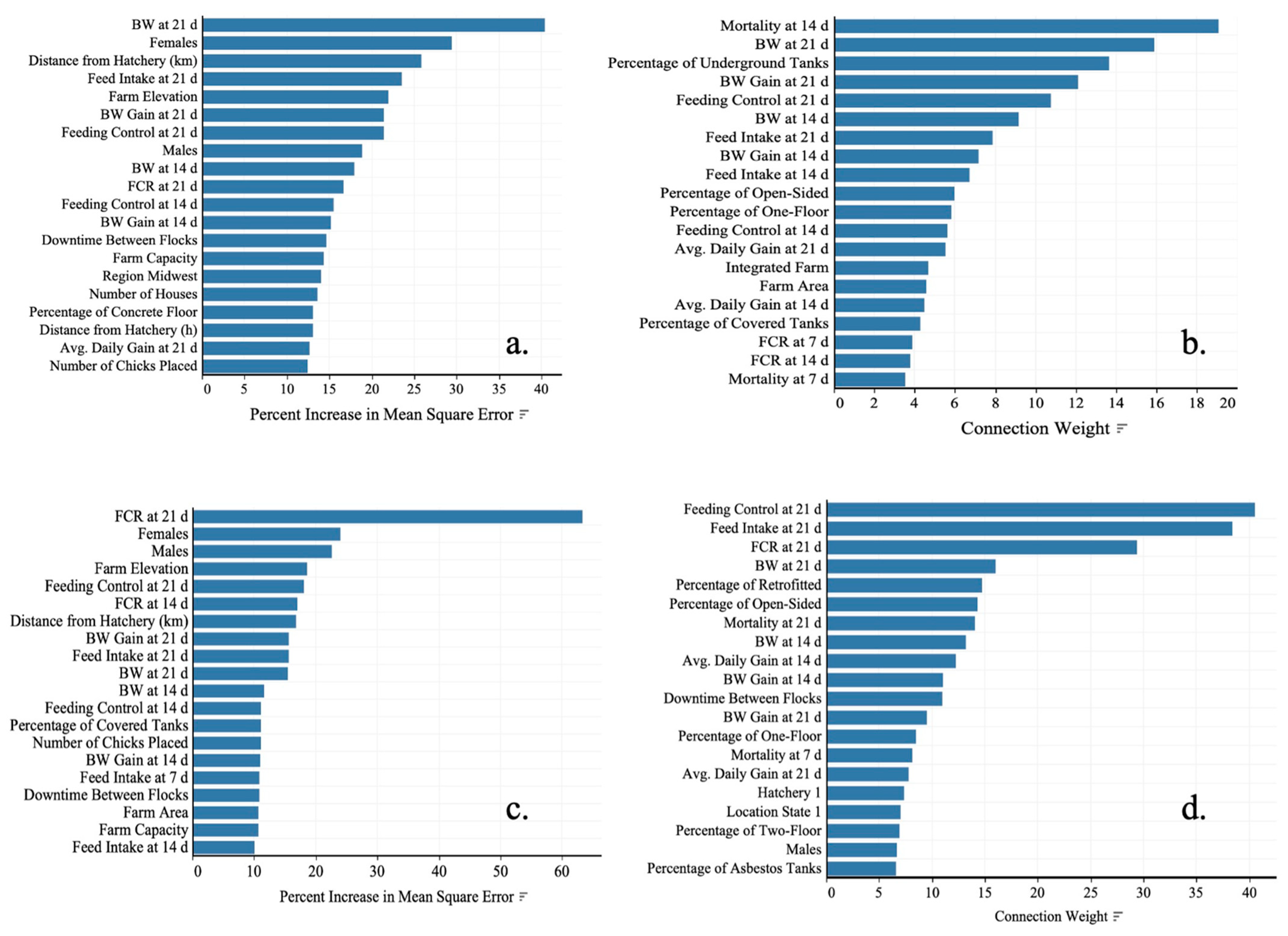
3.3. Data Analysis 3: Prediction of Performance with ML
4. Discussion
4.1. Broiler Performance and Environment
4.2. Farm Management and Infrastructure Factors and Performance
4.3. Prediction of Performance with ML
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuidhof, M.J.; Schneider, B.L.; Carney, V.L.; Korver, D.R.; Robinson, F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014, 93, 2970–2982. [Google Scholar] [CrossRef]
- Chen, X.Y.; Li, R.; Wang, M.; Geng, Z.Y. Identification of differentially expressed genes in hypothalamus of chicken during cold stress. Mol. Biol. Rep. 2014, 41, 2243–2248. [Google Scholar] [CrossRef]
- Yang, X.; Luo, Y.H.; Zeng, Q.F.; Zhang, K.Y.; Ding, X.M.; Bai, S.P.; Wang, J.P. Effects of low ambient temperatures and dietary vitamin C supplement on growth performance, blood parameters, and antioxidant capacity of 21-day-old broilers. Poult. Sci. 2014, 93, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Tallentire, C.W.; Leinonen, I.; Kyriazakis, I. Breeding for efficiency in the broiler chicken: A review. Agron. Sustain. Dev. 2016, 36, 66. [Google Scholar] [CrossRef]
- Mohammadalipour, R.; Rahmani, H.R.; Jahanian, R.; Riasi, A.; Mohammadalipour, M.; Nili, N. Effect of early feed restriction on physiological responses, performance and ascites incidence in broiler chickens raised in normal or cold environment. Animal 2017, 11, 219–226. [Google Scholar] [CrossRef]
- Zhou, H.J.; Kong, L.L.; Zhu, L.X.; Hu, X.Y.; Busye, J.; Song, Z.G. Effects of cold stress on growth performance, serum biochemistry, intestinal barrier molecules, and adenosine monophosphate-activated protein kinase in broilers. Animal 2021, 15, 100138. [Google Scholar] [CrossRef]
- Yousaf, A.; Jabbar, A.; Rajput, N.; Memon, A.; Shahnawaz, R.; Mukhtar, N.; Farooq, F.; Abbas, M.; Khalil, R. Effect of environmental heat stress on performance and carcass yield of broiler chicks. World Vet. J. 2019, 9, 26–30. [Google Scholar] [CrossRef]
- Kang, D.; Shim, K. Early heat exposure effect on the heat shock proteins in broilers under acute heat stress. Poult. Sci. 2021, 100, 100964. [Google Scholar] [CrossRef] [PubMed]
- Siegel, H.V.; Van Kampen, M. Energy relationships in growing chickens given daily injections of corticosterone. Br. Poult. Sci. 1984, 25, 477–485. [Google Scholar] [CrossRef]
- Faria Filho, D.E.; Rosa, P.S.; Vieira, B.S.; Macari, M.; Furlan, R.L. Protein levels and environmental temperature effects on carcass characteristics, performance, and nitrogen excretion of broiler chickens from 7 to 21 days of age. Braz. J. Poult. Sci. 2005, 7, 247–253. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383. [Google Scholar] [CrossRef]
- Cassuce, D.C.; Tinôco, I.D.; Baêta, F.C.; Zolnier, S.; Cecon, P.R.; Vieira, M.D. Thermal comfort temperature update for broiler chickens up to 21 days of age. Eng. Agríc. Jaboticabal. 2013, 33, 28–36. [Google Scholar] [CrossRef]
- Candido, M.G.L.; Tinoco, I.d.F.F.; Pinto, F.d.A.d.C.; Santos, N.T.; Roberti, R.P. Determination of thermal comfort zone for early-stage broilers. Eng. Agric. Jaboticabal. 2016, 36, 760–767. [Google Scholar] [CrossRef]
- Naga Raja Kumari, K.; Narendra Nath, D. Ameliorative measures to counter heat stress in poultry. World’s Poult. Sci. J. 2018, 74, 117–130. [Google Scholar] [CrossRef]
- Goo, D.; Kim, J.H.; Park, G.H.; de los Reyes, J.B.; Kil, D.Y. Effect of heat stress and stocking density on growth performance, breast meat quality, and intestinal barrier function in broiler chickens. Animal 2019, 9, 107. [Google Scholar] [CrossRef]
- Awad, E.A.; Najaa, M.; Zulaikha, Z.A.; Zulkifli, I.; Soleimani, A.F. Effects of heat stress on growth performance, selected physiological and immunological parameters, caecal microflora, and meat quality in two broiler strains. Asian-Australas J. Anim. Sci. 2020, 33, 778–787. [Google Scholar] [CrossRef]
- Liu, L.; Ren, M.; Ren, K.; Jin, Y.; Yan, M. Heat stress impacts on broiler performance: A systematic review and meta-analysis. Poult. Sci. 2020, 96, 6205–6211. [Google Scholar] [CrossRef]
- Ipek, A.; Sahan, U. Effects of cold stress on broiler performance and ascites susceptibility. Asian-Aust. J. Anim. Sci. 2006, 19, 734–738. [Google Scholar] [CrossRef]
- Weaver, W.; Meijerhof, R. The effect of different levels of relative humidity and air movement on litter conditions, ammonia levels, growth, and carcass quality for broiler chickens. Poult. Sci. 1990, 70, 746–755. [Google Scholar] [CrossRef]
- Yahav, S. Relative humidity at moderate ambient temperatures: Its effect on male broiler chickens and turkeys. Br. Poult. Sci. 2000, 41, 94–100. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Zhang, M.; Feng, J. Effect of relative humidity at either acute or chronic moderate temperature on growth performance and droppings’ corticosterone metabolites of broilers. J. Integr. Agric. 2019, 18, 152–159. [Google Scholar] [CrossRef]
- Zulovich, J.M.; DeShazer, J.A. Estimating egg production declines at high environmental temperatures and humidities. ASAE Pap. 1990, 904021, 15. [Google Scholar]
- Xin, H.; DeShazer, J.A.; Beck, M.M. Responses of prefasted growing turkeys to acute heat exposure. Trans. ASAE 1992, 35, 315–318. [Google Scholar] [CrossRef]
- Brown-Brand, T.M.; Beck, M.M.; Schulte, D.D.; Parkhurst, A.M.; DeShazer, J.A. Temperature humidity index for growing tom turkeys. Proc. Trans. ASAE 1997, 40, 203–209. [Google Scholar] [CrossRef]
- Tao, X.; Xin, H. Acute synergistic effects of air temperature, humidity, and velocity on homeostasis of market–size broilers. Trans. ASAE 2003, 46, 491–497. [Google Scholar] [CrossRef]
- Chepete, H.J.; Chimbombi, E.; Tsheko, R. Production performance and temperature-humidity index of Cobb 500 broilers reared in open-sided naturally ventilated houses in Botswana. In Proceedings of the 7th International Symposium, Beijing, China, 18 May 2005. [Google Scholar] [CrossRef]
- Purswell, J.L.; Dozier III, W.A.; Olanrewaju, H.A.; Davis, J.D.; Xin, H.; Gates, R.S. Effect of temperature-humidity index on live performance in broiler chickens grown from 49 to 63 days of age. In Proceedings of the 9th International Livestock Environment Symposium, Valencia, Spain, 8–12 July 2012. [Google Scholar] [CrossRef]
- Bergoug, H.; Guinebretière, M.; Tong, Q.; Roulston, N.; Romanini, C.E.B.; Exadaktylos, V.; Berckmans, D.; Garain, P.; Demmers, T.G.M.; McGonnell, I.M.; et al. Effect of transportation duration of 1-day-old chicks on postplacement production performances and pododermatitis of broilers up to slaughter age. Poult. Sci. 2013, 92, 3300–3309. [Google Scholar] [CrossRef]
- Rachmawati, L.S.; Sjofjan, O.; Natsir, D.M.H. Effect of elevation altitude rearing and population on carcass quality of the broilers. J. Agric. Vet. Sci. 2016, 9, 36–41. [Google Scholar] [CrossRef]
- Toledo, T.D.S.D.; Pich, C.S.; Roll, A.A.; Dai Prá, M.A.; Leivas Leite, F.; Gonçalves Xavier, E.; Roll, V.F.B. The effect of litter materials on broiler performance: A systematic review and meta-analysis. Br. Poult. Sci. 2019, 60, 605–616. [Google Scholar] [CrossRef]
- Yerpes, M.; Llonch, P.; Manteca, X. Factors associated with cumulative first-week mortality in broiler chicks. Animal 2020, 10, 310. [Google Scholar] [CrossRef]
- Garcês, A.; Afonso, S.M.S.; Chilundo, A.; Jairoce, C.T.S. Evaluation of different litter materials for broiler production in a hot and humid environment: 2. Productive performance and carcass characteristics. Trop. Anim. Health Prod. 2017, 49, 369–374. [Google Scholar] [CrossRef]
- van Limbergen, T.; Sarrazin, S.; Chantziaras, I.; Dewulf, J.; Ducatelle, R.; Kyriazakis, I.; McMullin, P.; Mendez, J.; Niemi, J.K.; Papasolomontos, S.; et al. Risk factors for poor health and performance in European broiler production systems. BMC Vet. Res. 2020, 16, 287. [Google Scholar] [CrossRef]
- Pitesky, M.; Gendreau, J.; Bond, T.; Carrasco-Medanic, R. Data challenges and practical aspects of machine learning-based statistical methods for the analyses of poultry data to improve food safety and production efficiency. Cab. Rev. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Neethirajan, S. The role of sensors, big data and machine learning in modern animal farming. Sens. Bio-Sens. 2020, 29, 100367. [Google Scholar] [CrossRef]
- Wen, P.; Li, L.; Xue, H.; Jia, Y.; Gao, L.; Li, R.; Huo, L. Comprehensive evaluation method of the poultry house indoor environment based on grey relation analysis and analytic hierarchy process. Poult. Sci. 2021, 101, 101587. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Aviagen. Broiler Management Handbook; Aviagen, Ltd.: Hunstville, AL, USA, 2018. [Google Scholar]
- Berman, A.; Horovitz, T.; Kaim, M.; Gacitua, H. A comparison of THI indices leads to a sensible heat-based heat stress index for shaded cattle that aligns temperature and humidity stress. Int. J. Biometeorol. 2016, 60, 1453–1462. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J.H. Prototype methods and nearest-neighbors. In The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: New York, NY, USA, 2009; pp. 459–485. [Google Scholar]
- Rahimi, I.; Behmanesh, R. Improve poultry farm efficiency in Iran: Using combination neural networks, decision trees, and data envelopment analysis (DEA). Int. J. Oper. Res. 2012, 2, 69–84. [Google Scholar] [CrossRef]
- Bischl, B.; Lang, M.; Kotthoff, L.; Schiffner, J.; Richter, J.; Studerus, E.; Casalicchio, G.; Jones, Z.M. MLR: Machine Learning in R. J. Mach. Learn. Res. 2016, 17, 5938–5942. [Google Scholar]
- Kuhn, M.; Johnson, K. Data pre-processing. In Applied Predictive Modeling; Springer: New York, NY, USA, 2013; pp. 27–59. [Google Scholar]
- Wei, P.; Lu, Z.; Song, J. Variable importance analysis: A comprehensive review. Reliab. Eng. Syst. 2015, 142, 399–432. [Google Scholar] [CrossRef]
- Kazemitabar, S.J.; Amini, A.A.; Bloniarz, A.; Talwalkar, A. Variable importance using decision trees. In Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4 December 2017. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Olden, J.D.; Joy, M.K.; Death, R.G. An accurate comparison of methods for quantifying variable importance in artificial neural networks using simulated data. Ecol. Modell. 2004, 178, 389–397. [Google Scholar] [CrossRef]
- Su, Y.; Li, S.; Xin, H.; Li, J.; Li, X.; Zhang, R.; Li, J.; Bao, J. Proper cold stimulation starting at an earlier age can enhance immunity and improve adaptability to cold stress in broilers. Poult. Sci. 2020, 99, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Bruzual, J.J.; Peak, S.; Brake, J.; Peebles, E. Effects of relative humidity during the last five days of incubation and brooding temperature on performance of broiler chicks from young broiler breeders. Poult. Sci. 2000, 79, 1385–1391. [Google Scholar] [CrossRef]
- Deaton, J.W.; Branton, S.L.; Simmons, J.D.; Lott, B.D. The effect of brooding temperature on broiler performance. Poult. Sci. 1996, 75, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Yahav, S.; Straschnow, A.; Plavnik, I.; Hurwitz, S. Effects of diurnally cycling versus constant temperatures on chicken growth and food intake. Br. Poult. Sci. 1996, 37, 43–54. [Google Scholar] [CrossRef]
- Angelo, J.C.; Gonzalez, E.; Kondo, N.; Anzai, N.H.; Cabral, M.M. Material de cama: Qualidade, quantidade e efeito sobre o desempenho de frangos de corte. R. Bras. Zootec. 1997, 26, 121–130. [Google Scholar]
- Araújo, J.S.; Oliveira, V.D.; Braga, G.C. Desempenho de frangos de corte criados em diferentes tipos de cama e taxa de lotação. Cienc. Anim. Bras. 2007, 8, 59–64. [Google Scholar]
- Ramadan, S.G.A.; Mahboub, H.D.; Helal, M.A.; Gaafar, K.M. Behaviour, welfare and performance of broiler chicks reared on different litter materials. Assiut Vet. Med. J. 2013, 59, 9–18. [Google Scholar] [CrossRef]
- Brito, D.A.P.; Brito, D.R.B.; Gomes, A.M.N.; Cunha, A.S.; Filho, U.A.S.; Pinheiro, A.A. Desempenho produtivo e rendimento de carcaça de frangos criados em diferentes materiais de cama aviária. Cienc. Anim. Bras. 2016, 17, 192–197. [Google Scholar] [CrossRef]
- Ramadan, S.G.A.; El-Khloya, S.Z. Do alternative litter materials affect performance, welfare and immune response of broiler chicks? Alex. J. Vet. Sci. 2017, 52, 133–141. [Google Scholar] [CrossRef]
- Llewellyn, G.C.; Sherertz, P.C.; Armstrong, C.W.; Miller, G.B., Jr.; Reynolds, J.D.; Kimbrough, T.D.; Bean, G.A.; Hagler, W.M., Jr.; Haney, C.A.; Trempus, C.S.; et al. Mycotoxigenic isolates and toxin production on buckwheat and rice hulls used as bedding materials. J. Ind. Microbiol. Biotechnol. 1988, 3, 351–356. [Google Scholar] [CrossRef]
- Abougabal, M.S. Possibility of broiler production on reused litter. Egypt. Poult. Sci. 2019, 39, 405–421. [Google Scholar] [CrossRef]
- Garcés-Gudino, J.; Merino, R.; Cevallos-Gordón, A.L. Litter reuse reduces Eimeria spp. oocyst counts and improves the performance in broiler chickens reared in a tropical zone in Ecuador. Europ. Poult. Sci. 2017, 82, 1–9. [Google Scholar] [CrossRef]
- Chapman, H.D. Anticoccidial drugs and their effects upon the development of immunity to Eimeria infections in poultry. Avian Pathol. 1999, 28, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Cressman, M.D.; Yu, Z.; Nelson, M.C.; Moeller, S.J.; Lilburn, M.S.; Zerby, H.N. Interrelations between the microbiotas in the litter and in the intestines of commercial broiler chickens. Appl. Environ. Microbiol. 2010, 76, 6572–6582. [Google Scholar] [CrossRef] [PubMed]
- Smith, J. Broiler production without antibiotics: United States field perspectives. Anim. Feed Sci. Technol. 2019, 250, 93–98. [Google Scholar] [CrossRef]
- Decuypere, E.; Tona, K.; Bruggeman, V.; Bamelis, F. The day-old chick: A crucial hinge between breeders and broilers. World’s Poult. Sci. J. 2001, 57, 127–138. [Google Scholar] [CrossRef]
- Oviedo-Rondón, E.O.; Wineland, M.J.; Small, J.; Cutchin, H.; McElroy, A.; Barri, A.; Martin, S. Effect of incubation temperatures and chick transportation conditions on bone development and leg health. J. Appl. Poult. Res. 2009, 18, 671–678. [Google Scholar] [CrossRef]
- Abreu, L.H.P.; Junior, T.Y.; Campos, A.T.; Bahuti, M.; Fassani, E. Cloacal and surface temperatures of broilers subject to thermal stress. Eng. Agric. 2017, 37, 877–886. [Google Scholar] [CrossRef]
- Yerpes, M.; Llonch, P.; Manteca, X. Effect of environmental conditions during transport on chick weight loss and mortality. Poult. Sci. 2021, 100, 129–137. [Google Scholar] [CrossRef]
- Moharrery, A.; Kargar, A. Artificial neural network for prediction of plasma hormones, liver enzymes and performance in broilers. J. Anim. Feed Sci. 2007, 16, 293–304. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, T.; Zheng, H.; Huang, J.; Cuan, K. Pose estimation and behavior classification of broiler chickens based on deep neural networks. Comput. Electron. Agric. 2021, 180, 105863. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Y.; Porter, Z.; Purswell, J. Automated measurement of broiler stretching beahviors under four stocking densities via faster region-based convolutional neural network. Animal 2021, 15, 100059. [Google Scholar] [CrossRef]
- Işık, Y.; Kayabaşı, A. Automatic classification of healthy and sick broilers in terms of avian influenza by using neural networks. Müh. Bil. Ve Araş. Derg. 2022, 24, 212–226. [Google Scholar] [CrossRef]
- Cuan, K.; Zhang, T.; Huang, J.; Fang, C.; Guan, Y. Detection of avian influenza-infected chickens based on a chicken sound convolutional neural network. Comput. Electron. Agric. 2020, 178, 105688. [Google Scholar] [CrossRef]
- You, J.; van der Klein, S.A.; Lou, E.; Zuidhof, M.J. Application of random forest classification to predict daily oviposition events in broiler breeders fed by precision feeding system. Comput. Electron. Agric. 2020, 175, 105526. [Google Scholar] [CrossRef]
- You, J.; Lou, E.; Afrouziyeh, M.; Zukiwsky, N.M.; Zuidhof, M.J. A supervised machine learning method to detect anomalous real-time broiler breeder body weight data recorded by a precision feeding system. Comput. Electron. Agric. 2021, 185, 106171. [Google Scholar] [CrossRef]
- You, J.; Lou, E.; Afrouziyeh, M.; Zukiwsky, N.M.; Zuidhof, M.J. Using an artificial neural network to predict the probability of oviposition events of precision-fed broiler breeder hens. Poult. Sci. 2021, 100, 101187. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Gao, M.; Zhang, M.; Lv, M.; Wang, G. Research on recognition method of broiler overlapping sounds based on random forest and confidence interval. Comput. Electron. Agric. 2023, 209, 107801. [Google Scholar] [CrossRef]
- Cahyaningtyas, C.; Manongga, D.; Sembiring, I. Algorithm comparison and feature selection for classification of broiler chicken harvest. J. Tek. Inform. (JUTIF) 2022, 3, 1717–1727. [Google Scholar] [CrossRef]


| Variable | Category | Northwest | Midwest | East | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| --------------------------------------- (farms 1) --------------------------------------- | |||||||
| Farms | 42 | 48.84 | 17 | 19.77 | 27 | 31.40 | |
| Technification level 2 | High | 7 | 8.14 | 9 | 10.47 | 10 | 11.63 |
| Low | 35 | 40.70 | 8 | 9.30 | 17 | 19.77 | |
| Chick source | Hatchery 1 | 42 | 48.84 | 0 | 0.00 | 0 | 0.00 |
| Hatchery 2 | 0 | 0.00 | 17 | 19.77 | 27 | 31.40 | |
| Litter type | Rice hulls | 0 | 0.00 | 1 | 1.16 | 27 | 31.40 |
| Wood shavings | 42 | 48.84 | 16 | 18.60 | 0 | 0.00 | |
| --------------------------------------- (houses 3) --------------------------------------- | |||||||
| Houses | 367 | 49.80 | 153 | 20.76 | 217 | 29.44 | |
| Type of house | Open-sided | 358 | 48.31 | 118 | 15.92 | 194 | 26.18 |
| Retrofitted | 15 | 2.02 | 21 | 2.83 | 23 | 3.10 | |
| Controlled | 0 | 0.00 | 12 | 1.62 | 0 | 0.00 | |
| House stories 4 | 1 | 262 | 35.74 | 143 | 19.51 | 217 | 29.60 |
| 2 | 101 | 13.78 | 10 | 1.36 | 0 | 0.00 | |
| House floor type | Soil | 273 | 37.24 | 61 | 8.32 | 96 | 13.10 |
| Concrete | 90 | 12.28 | 92 | 12.55 | 121 | 16.51 | |
| Water storage system | Covered | 202 | 27.41 | 118 | 16.01 | 148 | 20.08 |
| Uncovered | 157 | 21.30 | 41 | 5.56 | 69 | 9.36 | |
| Underground | 2 | 0.27 | 0 | 0.00 | 0 | 0.00 | |
| Variable | Northwest | Midwest | East | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | |
| Altitude (m.a.s.l.) | 1588 | 398 | 1050 | 2353 | 1184 | 300 | 915 | 1955 | 1184 | 466 | 99 | 2253 |
| Litter reuse cycles (# times) | 1.9 | 1.85 | 0 | 12 | 5.58 | 4.15 | 0 | 12 | 0.44 | 0.57 | 0 | 2 |
| Distance from the hatchery (km) | 39.1 | 18.6 | 5 | 80 | 188.8 | 29.4 | 132 | 245 | 444.6 | 40 | 358 | 531 |
| Distance from the hatchery (h) | 1.5 | 0.6 | 0.1 | 3 | 5.4 | 0.7 | 4 | 6.5 | 8.3 | 1.1 | 6 | 11 |
| Downtime between flocks (d) | 14.1 | 0.4 | 14 | 16 | 16.3 | 1.4 | 14 | 21 | 13.0 | 1.6 | 11 | 20 |
| Farm area (m2) | 6435 | 4607 | 1620 | 24,992 | 12,655 | 8761 | 1440 | 31,680 | 7795 | 9238 | 1240 | 48,956 |
| Age | Live Performance | Relative Humidity (%) | Age of Exposure (d) | r | p-Value Correlation | n | Intercept | Estimate | R2 | R2 Adj | RMSE | p-Value LinearRegression |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | FCR | <50 | 0 to 7 | −0.51 | <0.001 | 46 | 0.936 | −0.002 | 0.26 | 0.24 | 0.06 | <0.001 |
| 21 | Week mortality | <50 | 14 to 21 | −0.46 | 0.015 | 28 | 0.155 | −0.004 | 0.21 | 0.18 | 0.07 | 0.015 |
| 21 | Cumulative mortality | <50 | 14 to 21 | −0.45 | 0.015 | 29 | 0.209 | −0.003 | 0.20 | 0.17 | 0.06 | 0.015 |
| 21 | FCR | <50 or >75 | 14 to 21 | 0.41 | <0.001 | 69 | 1.277 | 0.001 | 0.17 | 0.16 | 0.06 | <0.001 |
| 28 | FCR | <50 | 21 to 28 | 0.41 | 0.035 | 27 | 1.360 | 0.003 | 0.17 | 0.13 | 0.06 | 0.035 |
| 28 | FCR | >75 | 0 to 28 | 0.41 | 0.001 | 68 | 1.384 | 0.0002 | 0.17 | 0.16 | 0.06 | 0.001 |
| 28 | FCR | <50 or >75 | 0 to 28 | 0.41 | <0.001 | 70 | 1.372 | 0.0002 | 0.17 | 0.16 | 0.06 | <0.001 |
| Item | Category/Cluster Mean | SD | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | BW | FCR | Mortality | n | BW | FCR | Mortality | |||
| Flocks | --(g)-- | -(g/g)- | -- (%) -- | flocks | -- (g) -- | -(g/g)- | --(%)-- | |||
| Region | Northwest | 381 | 1836 c | 1.461 c | 2.98 a | 385 | 1729 b | 1.514 c | 2.68 ab | |
| Midwest | 148 | 1947 a | 1.493 b | 2.52 b | 154 | 1751 a | 1.533 b | 2.27 b | ||
| East | 669 | 1882 b | 1.515 a | 2.85 a | 670 | 1673 c | 1.557 a | 2.71 a | ||
| SEM ± | 4 | 0.003 | 0.10 | 4 | 0.003 | 0.09 | ||||
| CV % | 4.1 | 3.3 | 26.6 | 4.3 | 2.9 | 27.3 | ||||
| Technification level | High | 497 | 1889 a | 1.507 a | 2.94 | 502 | 1690 b | 1.550 a | 2.72 a | |
| Low | 687 | 1866 b | 1.487 b | 2.79 | 694 | 1708 a | 1.533 b | 2.54 b | ||
| SEM ± | 3 | 0.002 | 0.07 | 3 | 0.002 | 0.07 | ||||
| CV % | 4.4 | 3.6 | 26.8 | 4.7 | 3.1 | 28.2 | ||||
| Altitude (m.a.s.l.) | 1041 | 349 | 376 | 1873 b | 1.522 b | 2.69 b | 359 | 1730 b | 1.519 b | 1.96 c |
| 1446 | 498 | 172 | 1792 c | 1.577 a | 3.82 a | 645 | 1660 c | 1.573 a | 3.06 a | |
| 1670 | 434 | 650 | 1898 a | 1.457 c | 2.71 b | 205 | 1774 a | 1.475 c | 2.43 b | |
| SEM ± | 4 | 0.003 | 0.09 | 4 | 0.002 | 0.09 | ||||
| CV % | 4.0 | 2.2 | 26.1 | 3.9 | 2.0 | 27.4 | ||||
| Source of variation | -------------------------------------- p-value -------------------------------------- | |||||||||
| Region | <0.001 | <0.001 | 0.019 | <0.001 | <0.001 | 0.003 | ||||
| Type of administration | <0.001 | <0.001 | 0.087 | <0.001 | <0.001 | 0.008 | ||||
| Altitude | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Litter type | Rice hulls | 672 | 1883 a | 1.514 a | 2.85 | 676 | 1674 b | 1.556 a | 2.69 a | |
| Wood shaving | 526 | 1867 b | 1.470 b | 2.87 | 533 | 1733 a | 1.519 b | 2.54 b | ||
| SEM ± | 3 | 0.002 | 0.07 | 3 | 0.002 | 0.07 | ||||
| CV % | 4.4 | 3.4 | 26.8 | 4.3 | 2.9 | 28.9 | ||||
| Litter reuse cycles number | 0.8 | 0.7 | 348 | 1819 b | 1.556 a | 3.47 a | 797 | 1671 c | 1.561 a | 2.89 a |
| 1.3 | 1.2 | 767 | 1898 a | 1.465 c | 2.59 c | 297 | 1769 a | 1.481 c | 2.10 b | |
| 5.8 | 2.2 | 32 | 1898 a | 1.564 a | 3.41 ab | 69 | 1722 b | 1.562 a | 2.10 b | |
| 12.0 | 0.0 | 51 | 1898 a | 1.479 b | 2.53 bc | 46 | 1718 b | 1.522 b | 2.24 b | |
| SEM ± | 8 | 0.004 | 0.17 | 6 | 0.003 | 0.15 | ||||
| CV % | 4.0 | 2.4 | 26.0 | 4.0 | 2.3 | 28.1 | ||||
| Downtime between flocks (d) | 12.7 | 1.0 | 314 | 1822 c | 1.562 a | 3.44 a | 678 | 1662 c | 1.569 a | 2.94 a |
| 13.5 | 1.2 | 662 | 1906 a | 1.457 c | 2.51 b | 150 | 1778 a | 1.466 c | 2.05 b | |
| 15.0 | 1.6 | 222 | 1859 b | 1.510 b | 3.11 a | 381 | 1735 b | 1.517 b | 2.31 b | |
| SEM ± | 4 | 0.002 | 0.09 | 4 | 0.002 | 0.09 | ||||
| CV % | 4.0 | 2.2 | 25.9 | 3.9 | 2.1 | 28.1 | ||||
| Source of variation | ---------------------------------------- p-value ------------------------------------- | |||||||||
| Litter type | 0.001 | <0.001 | 0.657 | <0.001 | <0.001 | 0.011 | ||||
| Litter reuse cycle number | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Downtime between flocks | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Item | Category/Cluster Mean | SD | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | BW | FCR | Mortality | n | BW | FCR | Mortality | |||
| Flocks | --(g)-- | -(g/g)- | -- (%) -- | Flocks | -- (g) -- | -(g/g)- | --(%)-- | |||
| Chick source | Hatchery 1 | 381 | 1836 b | 1.461 b | 3.00 | 385 | 1729 a | 1.514 b | 2.65 | |
| Hatchery 2 | 817 | 1894 a | 1.511 a | 2.79 | 824 | 1687 b | 1.552 a | 2.62 | ||
| SEM ± | 3 | 0.003 | 0.07 | 3 | 0.002 | 0.07 | ||||
| CV % | 4.2 | 3.4 | 26.8 | 4.5 | 3.0 | 0.1 | ||||
| Distance from hatchery (km) | 73 | 71 | 461 | 1875 b | 1.455 c | 2.71 b | 322 | 1758 a | 1.491 c | 2.18 b |
| 356 | 169 | 257 | 1812 c | 1.572 a | 3.54 a | 722 | 1665 c | 1.570 a | 2.92 a | |
| 454 | 64 | 480 | 1909 a | 1.492 b | 2.65 b | 165 | 1740 b | 1.503 b | 2.21 b | |
| SEM ± | 4 | 0.002 | 0.09 | 4 | 0.002 | 0.09 | ||||
| CV % | 4.0 | 2.3 | 26.2 | 3.9 | 2.0 | 28.1 | ||||
| Distance from hatchery (h) | 2.1 | 1.6 | 429 | 1873 a | 1.449 c | 2.73 b | 258 | 1755 a | 1.486 c | 2.21 b |
| 7.0 | 2.9 | 247 | 1808 b | 1.573 a | 3.58 a | 714 | 1663 b | 1.571 a | 2.93 a | |
| 8.2 | 1.8 | 522 | 1909 a | 1.496 b | 2.63 b | 237 | 1750 a | 1.507 b | 2.16 b | |
| SEM ± | 4 | 0.002 | 3.85 | 4 | 0.002 | 3.85 | ||||
| CV % | 4.0 | 2.2 | 26.2 | 3.9 | 2.0 | 28.1 | ||||
| Source of variation | ---------------------------------------- p-value ---------------------------------------- | |||||||||
| Chick source | <0.001 | <0.001 | 0.146 | <0.001 | <0.001 | 0.349 | ||||
| Distance from the hatchery (km) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Distance from the hatchery (h) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Variable | Sum Sq | Contribution (%) 1 | F-Value | p-Value |
|---|---|---|---|---|
| BW | ||||
| Females | 13,108,737.0 | 89.27 | 2325.7 | <0.001 |
| Feed Intake 14 d | 646,918.0 | 4.41 | 114.8 | <0.001 |
| BW Gain at 7 d | 267,502.8 | 1.82 | 47.5 | <0.001 |
| FCR at 21 d | 209,950.5 | 1.43 | 37.2 | <0.001 |
| Percentage of Retrofitted | 200,095.7 | 1.36 | 35.5 | <0.001 |
| Percentage of Open-Sided | 119,749.4 | 0.82 | 21.2 | <0.001 |
| Region | 79,587.1 | 0.54 | 14.1 | <0.001 |
| Percentage of Underground Tanks | 51,800.2 | 0.35 | 9.2 | 0.002 |
| FCR | ||||
| FCR at 21 d | 0.41 | 42.41 | 162.21 | <0.001 |
| Downtime Between Flocks | 0.18 | 18.67 | 71.41 | <0.001 |
| Feed Intake 14 d | 0.17 | 17.63 | 67.43 | <0.001 |
| Percentage of Retrofitted | 0.07 | 7.34 | 28.09 | <0.001 |
| Percentage of Underground Tanks | 0.04 | 3.94 | 15.06 | <0.001 |
| Percentage of Open-Sided | 0.03 | 2.69 | 10.29 | 0.001 |
| Percentage of Concrete Floor | 0.02 | 2.56 | 9.80 | 0.002 |
| Feed Intake 21 d | 0.02 | 2.22 | 8.51 | 0.004 |
| Month at Placement | 0.01 | 1.47 | 5.62 | 0.018 |
| Mortality at 21 d | 0.01 | 1.06 | 4.04 | 0.045 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintana-Ospina, G.A.; Alfaro-Wisaquillo, M.C.; Oviedo-Rondon, E.O.; Ruiz-Ramirez, J.R.; Bernal-Arango, L.C.; Martinez-Bernal, G.D. Effect of Environmental and Farm-Associated Factors on Live Performance Parameters of Broilers Raised under Commercial Tropical Conditions. Animals 2023, 13, 3312. https://doi.org/10.3390/ani13213312
Quintana-Ospina GA, Alfaro-Wisaquillo MC, Oviedo-Rondon EO, Ruiz-Ramirez JR, Bernal-Arango LC, Martinez-Bernal GD. Effect of Environmental and Farm-Associated Factors on Live Performance Parameters of Broilers Raised under Commercial Tropical Conditions. Animals. 2023; 13(21):3312. https://doi.org/10.3390/ani13213312
Chicago/Turabian StyleQuintana-Ospina, Gustavo A., Maria C. Alfaro-Wisaquillo, Edgar O. Oviedo-Rondon, Juan R. Ruiz-Ramirez, Luis C. Bernal-Arango, and Gustavo D. Martinez-Bernal. 2023. "Effect of Environmental and Farm-Associated Factors on Live Performance Parameters of Broilers Raised under Commercial Tropical Conditions" Animals 13, no. 21: 3312. https://doi.org/10.3390/ani13213312
APA StyleQuintana-Ospina, G. A., Alfaro-Wisaquillo, M. C., Oviedo-Rondon, E. O., Ruiz-Ramirez, J. R., Bernal-Arango, L. C., & Martinez-Bernal, G. D. (2023). Effect of Environmental and Farm-Associated Factors on Live Performance Parameters of Broilers Raised under Commercial Tropical Conditions. Animals, 13(21), 3312. https://doi.org/10.3390/ani13213312





