Assessing Optimal Cell Counts in Sperm Shape Abnormality Assays in Rodents
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
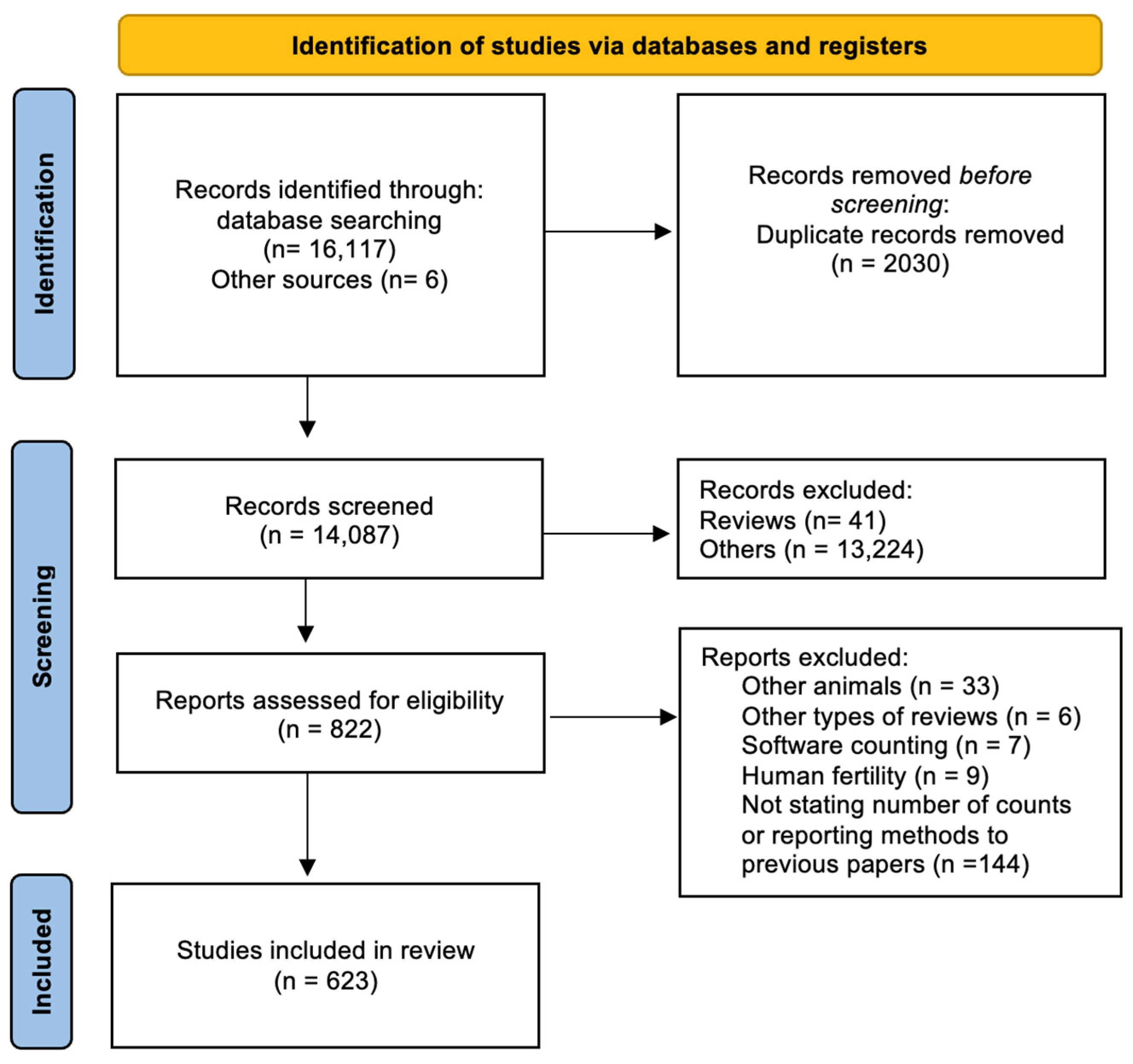
2.2. Literature Review
2.3. Sampling of Wild Rodents
2.4. Sperm Shape Abnormality Assay
2.5. Data Analyses
3. Results
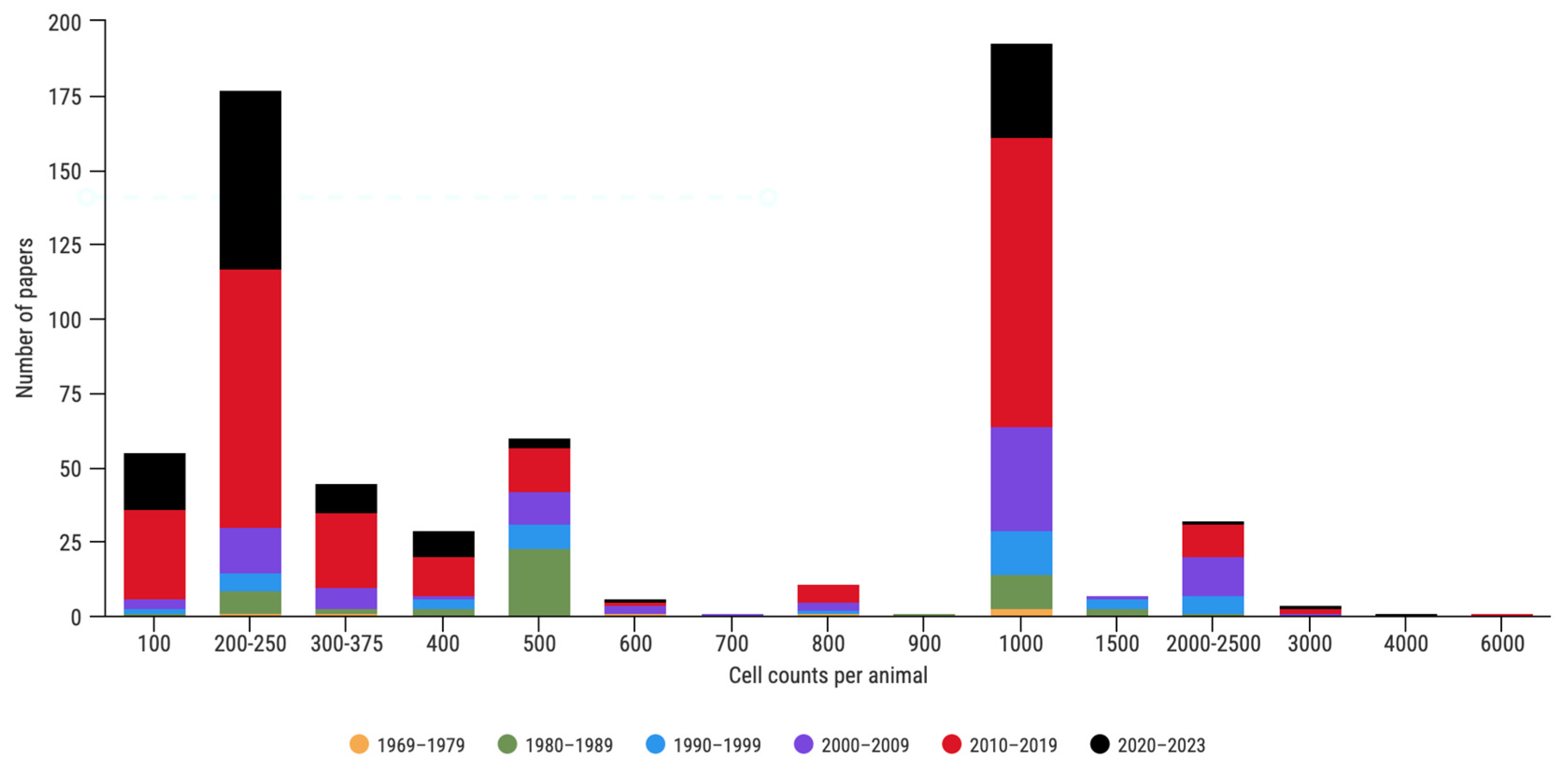
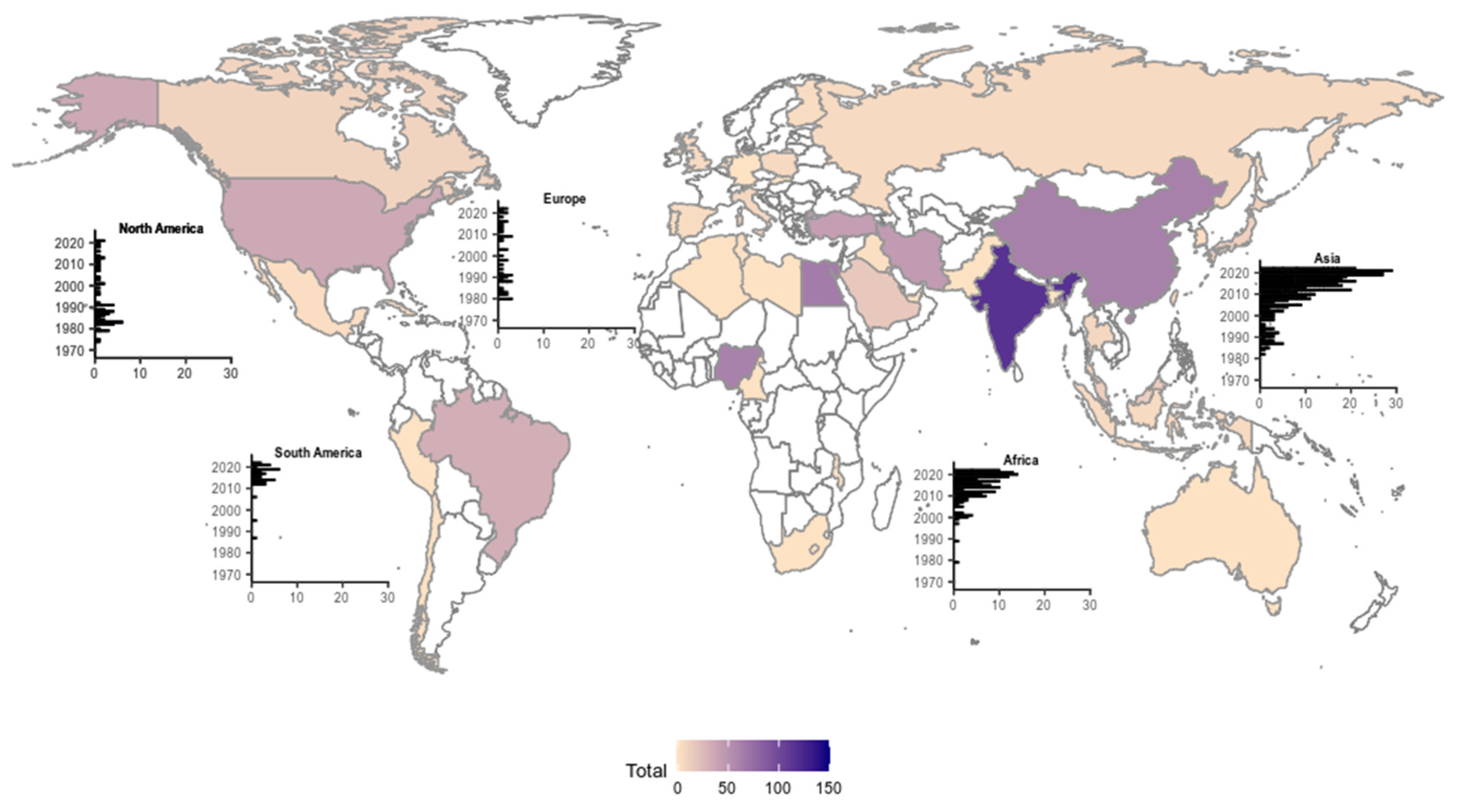
3.1. Literature Review
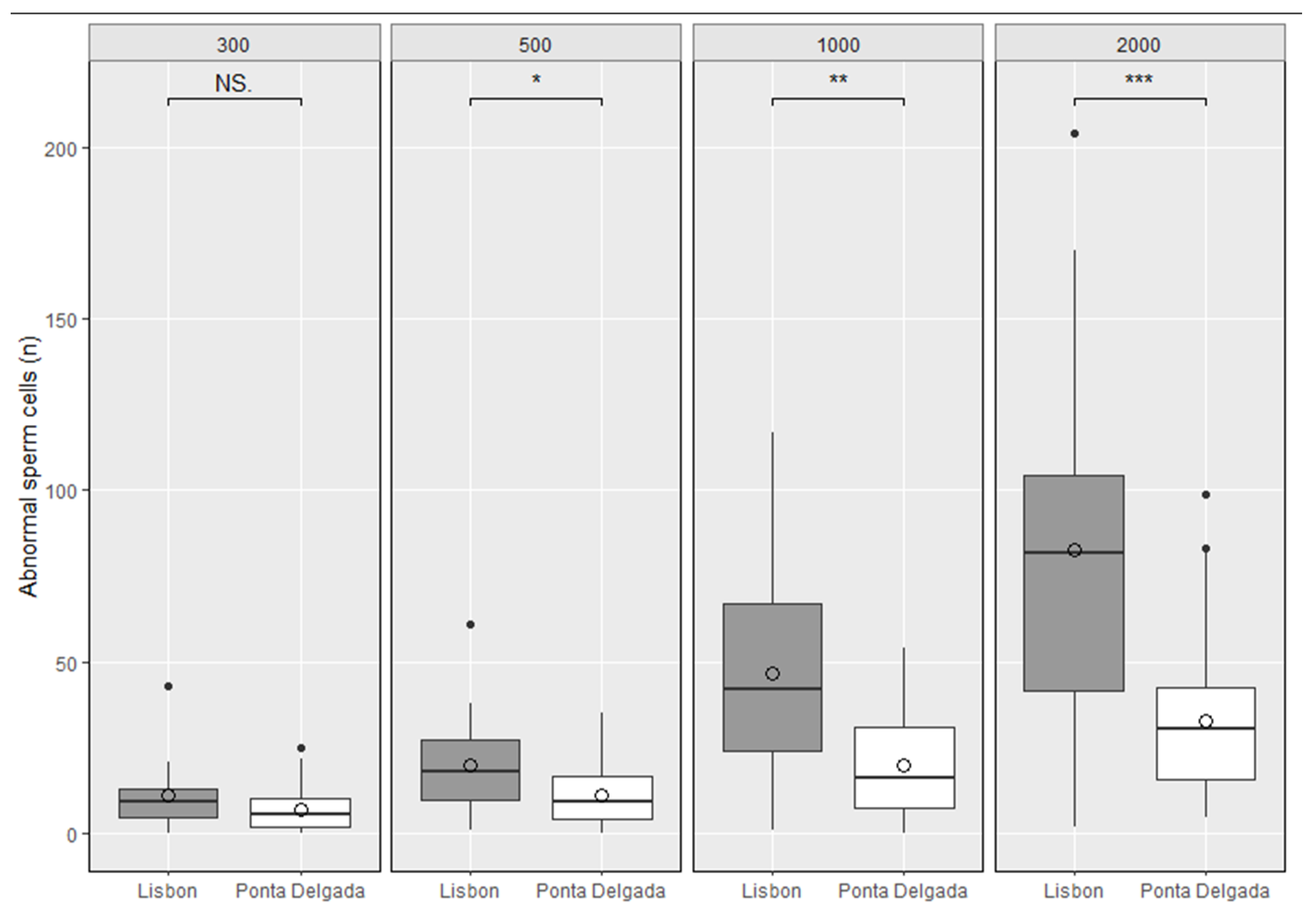
3.2. Sperm Shape Abnormalities–Case-Study with Wild Rodents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Turkez, H.; Arslan, M.E.; Ozdemir, O. Genotoxicity testing: Progress and prospects for the next decade. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Bhunya, S.P.; Pati, P.C. Genotoxicity of an inorganic pesticide, copper sulphate in mouse in vivo test system. Cytologia 1987, 52, 801–808. [Google Scholar] [CrossRef]
- Vega, S.G.; Guzman, P.; Garcia, L.; Espinosa, J.; Denava, C.C. Sperm shape abnormality and urine mutagenicity in mice treated with niclosamide. Mutat. Res. 1988, 204, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Rasgele, P.G.; Gokalp, F.D.; Kaya, S.T. Investigation of genotoxic effects of rhododendron honey using three mammalian bioassays in vivo. Drug Chem. Toxicol. 2022, 45, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Lähdetie, J. Genotoxicity assays of vinyl acetate and acetaldehyde in male germ cells of mice. Mutat. Res. 1990, 6, 389–390. [Google Scholar]
- Zhang, K.; Weng, H.; Yang, J.; Wu, C. Protective effect of Liuwei Dihuang Pill on cisplatin-induced reproductive toxicity and genotoxicity in male mice. J. Ethnopharm. 2020, 247, 112269. [Google Scholar] [CrossRef]
- Pal, B.B.; Bunya, S.P. Genotoxic effect of a preservative, sodium metabisulphite as revealed by mammalian in vivo bioassays. Cytologia 1992, 57, 455–461. [Google Scholar] [CrossRef][Green Version]
- Rabbani, S.I.; Sajid, S.; Mani, V.; Khan, O.; Salman, M.A.A. Inhibitory Effect of Salvadora persica (Miswak) against Cigarette Smoke-induced Mutagenicity and Sperm Abnormalities in Rats. Pharm. Res. 2019, 11, 384–388. [Google Scholar] [CrossRef]
- Das, S.; Langthasa, P.; Barhoi, D.; Upadhaya, P.; Giri, S. Effect of nutritional status on arsenic and smokeless tobacco-induced genotoxicity, sperm abnormality and oxidative stress in mice in vivo. Environ. Mol. Mutagen. 2018, 59, 386–400. [Google Scholar] [CrossRef]
- Gautam, R.; Singh, K.V.; Nirala, J.; Murmu, N.N.; Meena, R.; Rajamani, P. Oxidative stress-mediated alterations on sperm parameters in male Wistar rats exposed to 3G mobile phone radiation. Andrologia 2019, 51, e13201. [Google Scholar] [CrossRef]
- Saleem, U.; Zubair, S.; Riaz, A.; Anwar, F.; Ahmad, B. Effect of venlafaxine, pramipexole, and valsartan on spermatogenesis in male rats. ACS Omega 2020, 5, 20481–20490. [Google Scholar] [CrossRef] [PubMed]
- Hemavathi, E.; Rahiman, M.A. Toxicological effects of ziram, thiram, and dithane M-45 assessed by sperm shape abnormalities in mice. J. Toxicol. Environ. Health 1993, 38, 393–398. [Google Scholar] [CrossRef]
- Anitha, B.; Sudha, S.; Gopinath, P.M.; Durairaj, G. Genotoxicity evaluation of pyrazinamide in mice. Mutat. Res. 1994, 321, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.C.C.; Salvadori, D.M.F.; Oliveira, S.V.; Ribeiro, L.R. The action of the herbicide paraquat on somatic and germ-cells of mice. Mutat. Res. 1995, 328, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Tapisso, J.T.; Marques, C.C.; Mathias, M.L.; Ramalhinho, M.G. Induction of micronuclei and sister chromatid exchange in bone-marrow cells and abnormalities in sperm of Algerian mice (Mus spretus) expose to cadmium, lead and zinc. Mutat. Res. Gen. Toxicol. Environ. 2009, 678, 59–64. [Google Scholar] [CrossRef]
- Alimba, C.G.; Adewumi, O.O.; Binuyo, O.M.; Odeigah, P.G.C. Wild black rats (Rattus rattus Linnaeus, 1758) as zoomonitor of genotoxicity and systemic toxicity induced by hazardous emissions from Abule Egba unsanitary landfill, Lagos, Nigeria. Environ. Sci. Pollut. Res. 2021, 28, 10603–10621. [Google Scholar] [CrossRef]
- Møller, A.P.; Surai, P.; Mousseau, T.A. Antioxidants, radiation and mutation as revealed by sperm abnormality in barn swallows from Chernobyl. Proc. R. Soc. B 2005, 272, 247–252. [Google Scholar] [CrossRef]
- Chandra, P.; Khuda-Bukhsh, A.R. Genotoxic effects of cadmium chloride and azadirachtin treated singly and in combination in fish. Ecotox. Environ. Safe 2004, 58, 194–201. [Google Scholar] [CrossRef]
- Ieradi, L.A.; Cristaldi, M.; Mascanzoni, D.; Cardarelli, E.; Grossi, R.; Campanella, L. Genetic damage in urban mice exposed to traffic pollution. Environ. Pollut. 1996, 92, 323–328. [Google Scholar] [CrossRef]
- Wyrobek, A.J.; Bruce, W.R. Chemical induction of sperm abnormalities in mice. Proc. Natl. Acad. Sci. USA 1975, 72, 4425–4429. [Google Scholar] [CrossRef]
- Wyrobek, A.J.; Gordon, L.A.; Burkhart, J.G.; Francis, M.W.; Kapp, R.W., Jr.; Letz, G.; Malling, H.V.; Topham, J.C.; Whorton, M.D. An evaluation of the mouse sperm morphology test and other sperm tests in nonhuman mammals: A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat. Res. 1983, 115, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.M.; Zimmerman, S.; Raj, A.Y. Effects of cannabinoids an spermatogenesis in mice. In Marihuana and Medicine, 1st ed.; Nahas, G.G., Sutin, K.M., Harvey, D.J., Agurell, S., Eds.; Humana Press: New York, NY, USA, 1999; Volume 1, pp. 347–358. [Google Scholar]
- Fahmy, M.A. Evaluation of the genotoxicity of spiramycine in male mice. Cytologia 2001, 66, 205–214. [Google Scholar] [CrossRef][Green Version]
- Quinto, I.; De Marinis, E.; De Dominicis, G.; Della Morte, R.; Staiano, N. Induction of sperm abnormalities in mice by ifosfamide and trofosfamide. Mutat. Res. 1988, 201, 113–116. [Google Scholar] [CrossRef]
- Elelaimy, I.A.; Ibrahim, H.M.; Elsayad, R.I. Genotoxicity of anticancer drug Azathioprine (Imuran): Role of Omega-3 (ω-3) oil as protective agent. J. Appl. Pharma. Sci. 2012, 4, 14–23. [Google Scholar]
- Amein, K.A.; Hamdy, M.M.; El-Eman, R.A.; Firkhy, H.; Osman, M.D. The effect of pioglitazone on genomic instability in induced diabetic rats. Res. J. Appl. Biotech. 2016, 2, 68–80. [Google Scholar] [CrossRef]
- Bruce, W.R.; Furrer, R.; Wyrobek, A.J. Abnormalities in shape of murine sperm after acute testicular x-irradiation. Mutat. Res. 1974, 23, 381–386. [Google Scholar] [CrossRef]
- Filler, R. Methods for Evaluation of Rat Epididymal Sperm Morphology. Male Reprod. Toxicol. 1993, 11, 334–343. [Google Scholar]
- Choudhury, R.C.; Jagdale, M.B.; Misra, S. Potential transmission of the cytogenetic effects of cisplatin in the male germline cells of Swiss mice. J. Chemother. 2000, 12, 352–359. [Google Scholar] [CrossRef]
- Rabbani, S.I.; Devi, K.; Khanam, S. Protective role of glibenclamide against nicotinamide- streptozotocin-induced nuclear damage in diabetic Wistar rats. J. Pharm. Pharmacother. 2010, 1, 18–23. [Google Scholar] [CrossRef]
- Okano, T.; Ishiniwa, H.; Onuma, M.; Shindo, J.; Yokohata, Y.; Tamaoki, M. Effects of environmental radiation on testes and spermatogenesis in wild large Japanese field mice (Apodemus speciosus) from Fukushima. Sci. Rep. 2016, 6, 23601. [Google Scholar] [CrossRef]
- OECD (Organisation for Economic Cooperation and Development). Test No. 416: Two-Generation Reproduction Toxicity. In OECD—Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2001. [Google Scholar] [CrossRef]
- Tablado, L.; Perez-Sanchez, F.; Nunez, J.; Nunez, M.; Soler, C. Effects of exposure to static magnetic fields on the morphology and morphometry of mouse epididymal sperm. Bioelectromag 1998, 19, 377–383. [Google Scholar] [CrossRef]
- Hassanane, M.S.; Abdalla, E.S.A.; EI-Fiky, S.; Amer, M.A.; Hamdy, A. Mutagenicity of the Mycotoxin diacetoxyscirpenol on somatic and germ cells of mice. Mycotoxin. Res. 2000, 16, 53–64. [Google Scholar] [CrossRef]
- Fernandes, F.H.; Bustos-Obregonb, E.; Salvadori, D.M.L. Disperse Red 1 (textile dye) induces cytotoxic and genotoxic effects in mouse germ cells. Reprod. Toxicol. 2015, 53, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, M.A.; Abd-Alla, H.I.; Hassan, E.E.; Hassan, Z.M.; Sweelam, H.T.M. Genotoxicity and sperm defects induced by 5-FU in male mice and the possible protective role of Pentas lanceolata—Iridoids. Mutat. Res. Gen. Toxicol. Environ. 2020, 850, 503145. [Google Scholar] [CrossRef] [PubMed]
- Rijsselaere, T.; Van Soom, A.; Hoflack, G.; Maes, D.; de Kruif, A. Automated sperm morphometry and morphology analysis of canine semen by the Hamilton-Thorne analyser. Theriogenology 2004, 62, 1292–1306. [Google Scholar] [CrossRef]
- Lu, J.C.; Huang, Y.F.; Lü, N.Q. Computer-aided sperm analysis: Past, present and future. Andrologia 2014, 46, 329–338. [Google Scholar] [CrossRef]
- Odeigah, P.G.C. Sperm head abnormalities and dominant lethal effects of formaldehyde in albino rats. Mutat. Res. Gen. Toxicol. Environ. 1997, 389, 141–148. [Google Scholar] [CrossRef]
- Singla, N.; Kaur, G.; Babbar, B.K.; Sandhu, B.S. Potential of triptolide in reproductive management of the house rat, Rattus rattus. Integr. Zool. 2013, 8, 260–276. [Google Scholar] [CrossRef]
- Hafemeister, C. How Many Cells. 2019. Available online: https://satijalab.org/howmanycells (accessed on 31 May 2023).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed effects models using lme4. J. Stat. Soft. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- OECD (Organisation for Economic Cooperation and Development). Test No. 401: Guideline for Testing of Chemicals. In Acute Oral Toxicity OECD—Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 1987. [Google Scholar] [CrossRef]
- Klimisch, H.J.; Andreae, M.; Tillmann, U. A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data. Reg. Toxicol. Pharm. 1997, 25, 1–5. [Google Scholar] [CrossRef]
- Barlow, S.M.; Greig, J.B.; Bridges, J.W.; Carere, A.; Carpy, A.J.M.; Galli, C.L.; Kleiner, J.; Knudsen, I.; Koëter, H.B.W.M.; Levy, L.S.; et al. Hazard identification by methods of animal-based toxicology. Food Chem. Toxicol. 2002, 40, 145–191. [Google Scholar] [CrossRef] [PubMed]
- Narayana, K.; D’Souza, U.J.A.; Rao, K.P.S. Ribavirin-induced sperm shape abnormalities in Wistar rat. Mutat. Res. 2002, 513, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.K.; Narayana, K.; Bairy, K.L.; D’Souza, U.J.A.; Samuel, V.P.; Gopalakrishna, K. Dacarbazine induces genotoxic and cytotoxic germ cell damage with concomitant decrease in testosterone and increase in lactate dehydrogenase concentration in the testis. Mutat. Res. 2006, 607, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Topham, J.C. Chemically-induced transmissible abnormalities in sperm-head shape. Mutat. Res. 1980, 70, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Sager, D.; Girard, D.; Nelson, D. Early postnatal exposure to PCBs: Sperm function in rats. Environ. Toxicol. Chem. Int. J. 1991, 10, 737–746. [Google Scholar] [CrossRef]
- Aikawa, H.; Koyama, S.; Matsuda, M.; Nakahashi, K.; Akazome, Y.; Mori, T. Relief effect of vitamin A on the decreased motility of sperm and the increased incidence of malformed sperm in mice exposed neonatally to bisphenol A. Cell Tissue Res. 2004, 315, 119–124. [Google Scholar] [CrossRef]
- Vaamonde, D.; Diaz, A.; Rodriguez, I. Preliminary results of trans-resveratrol as an effective protector against exercise-induced morphology abnormalities on mice sperm. Fertil. Steril. 2011, 96, S166–S167. [Google Scholar] [CrossRef]
- Faisal, K.; Akbarsha, M.A. Observations on Dag-like defect of spermatozoa induced by treatment of the phytotherapeutic quassia amara/quassin in the mouse model. Andrologia 2021, 53, e14046. [Google Scholar] [CrossRef]
- Akinboro, A.; Bakare, A.A. Spermatotoxic, cytotoxic and genotoxic evaluation of aqueous extract of Ocimum gratissimum in albino mice. J. Med. Arom. Plant 2013, 4, 10–14. [Google Scholar]
- Radhi, M.J.; Al-Bairuty, G.A.A.L. Effect of Zinc oxide nanoparticles (ZnO-NPs) on weights of some reproductive organs and sperm abnormalities in the tail of epididymis of albino mice. J. Pharm. Sci. Res. 2019, 11, 243–246. [Google Scholar]
- Abd-Elrazek, A.M.; El-dash, H.A.; Said, N.I. The role of propolis against paclitaxel-induced oligospermia, sperm abnormality, oxidative stress and DNA damage in testes of male rats. Andrologia 2020, 52, e13394. [Google Scholar] [CrossRef] [PubMed]
- Rahimah, S.B.; Dananjaya, R.; Wydiastuti, E.; Feriandi, Y. Protective effect of ethanolic extract of white oyster mushroom on morphological rat sperm damage due to cigarette smoke exposure. Ind. J. Med. Health 2021, 12, 257–266. [Google Scholar] [CrossRef]
- Wurlina, W.; Mustofa, I.; Meles, D.K.; Safitri, E.; Susilowati, S.; Mulyati, S.; Utomo, B.; Utama, S. α-Tocopherol restores semen quality in rats exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Vet. World 2022, 15, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.I.; Lim, J.O.; Pak, S.W.; Lee, S.J.; Shin, I.S.; Moon, C.; Heo, J.; Kim, J.C. Exposure to China dust exacerbates testicular toxicity induced by cyclophosphamide in mice. Toxicol. Res. 2023, 39, 115–125. [Google Scholar] [CrossRef]
- Seed, J.; Chapin, R.S.; Clegg, E.D.; Dostal, L.A.; Foote, R.H.; HuR, M.E.; Klinefelter, G.R.; Makris, S.L.; Peerreault, S.D.; Schrader, S.; et al. Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: A consensus report. Reprod. Toxicol. 1996, 10, 237–244. [Google Scholar] [CrossRef]
- Radwan, A.; Sakr, M.M. Review of Egypt Science and Technology System: SWOT analysis. Entrep. Sustain. Issues 2017, 5, 204–211. [Google Scholar] [CrossRef]
- United Nations. World Economic Situation and Prospects. 2023. Available online: https://www.un.org/development/desa/dpad/publication/world-economic-situation-and-prospects-2023/ (accessed on 3 February 2023).
- You, J.B.; McCallum, C.; Wang, Y.; Riordon, J.; Nosrati, R.; Sinton, D. Machine learning for sperm selection. Nat. Rev. Urol. 2021, 18, 387–403. [Google Scholar] [CrossRef]
- Alquézar-Baeta, C.; Gimeno-Martos, S.; Miguel-Jiménez, S.; Santolaria, P.; Yániz, J.; Palacín, I.; Casao, A.; Cébrian-Pérez, J.A.; Muiño-Blanco, T.; Pérez-Pé, R. OpenCASA: A new open-source and scalable tool for sperm quality analysis. PLoS Comp. Biol. 2019, 15, e1006691. [Google Scholar] [CrossRef]
- Kidd, S.A.; Eskenazi, B.; Wyrobek, A.J. Effects of male age on semen quality and fertility: A review of the literature. Fertil. Steril. 2001, 75, 237–248. [Google Scholar] [CrossRef]
- Turturro, A.; Witt, W.W.; Lewis, S.; Hass, B.S.; Lipman, R.D.; Hart, R.W. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 1999, 54, B492–B501. [Google Scholar] [CrossRef]
- Calhoun, J.B. The Ecology and Sociology of the Norway Rat, No. 1008; Public Health Service: Bethesda, MD, USA, 1963; p. 288.
- Miller III, M.C.; Mohrenweiser, H.W.; Bell, D.A. Genetic variability in susceptibility and response to toxicants. Toxicol. Lett. 2001, 120, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Klöting, I.; Nitschke, C.; van den Brandt, J. Impact of genetic profiles on experimental studies: Outbred versus wild rats. Toxicol. Appl. Pharmacol. 2003, 189, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Makino, S.; Kimura, H.; Ota, T.; Furuhashi, T.; Nagamura, Y. Sperm motion analysis in rats treated with adriamycin and its applicability to male reproductive toxicity studies. J. Toxicol. Sci. 2001, 26, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, J.; Hashimoto, K. Reproductive toxicity of acrylamide and related compounds in mice—Effects on fertility and sperm morphology. Arch. Toxicol. 1986, 59, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Chemes, H.E.; Rawe, V.Y. Sperm pathology: A step beyond descriptive morphology. Origin, characterization and fertility potential of abnormal sperm phenotypes in infertile men. Hum. Reprod. Update 2003, 9, 405–428. [Google Scholar] [CrossRef]
- Enciso, M.; Cisale, H.; Johnston, S.D.; Sarasa, J.; Fernández, J.L.; Gosálvez, J. Major morphological sperm abnormalities in the bull are related to sperm DNA damage. Theriogenology 2011, 76, 23–32. [Google Scholar] [CrossRef]
- García-Vázquez, F.A.; Gadea, J.; Matás, C.; Holt, W.V. Importance of sperm morphology during sperm transport and fertilization in mammals. Asian J. Androl. 2016, 18, 844. [Google Scholar] [CrossRef]
- Ostermeier, G.C.; Sargeant, G.A.; Yandell, B.S.; Evenson, D.P.; Parrish, J.J. Relationship of bull fertility to sperm nuclear shape. J. Androl. 2001, 22, 595–603. [Google Scholar]
- Beckman, L.; Nordenson, I. Interaction between some common genotoxic agents. Hum. Hered. 1986, 36, 397–401. [Google Scholar] [CrossRef]
- Rodríguez, M.C.; Dupont-Courtade, L.; Oueslati, W. Air pollution and urban structure linkages: Evidence from European cities. Renew. Sustain. Energy Rev. 2016, 53, 1–9. [Google Scholar] [CrossRef]
- Tannenbaum, L.V.; Thran, B.H.; Williams, K.J. Demonstrating ecological receptor health at contaminated sites with wild rodent sperm parameters. Arch. Environ. Contam. Toxicol. 2007, 53, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, G.Y.; Davydova, Y.A. Effect of industrial pollution of the environment on the frequency of abnormal spermatozoa in the bank vole, Myodes glareolus. Russ. J. Ecol. 2018, 49, 459–463. [Google Scholar] [CrossRef]
- Silva-Júnior, F.M.R.; Monarca, R.I.; Dias, D.; Ramalhinho, M.G.; Mathias, M.L.; Muccillo-Baisch, A.L. Geno- and Cyto-toxicity in Free-Living Rodent Mus spretus Exposed to Simulated Onshore Oil Spill. Bull. Environ. Contam. Toxicol. 2013, 91, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Ieradi, L.A.; Cristaldi, M.; Amaddeo, D.; Lillini, E.; Nuti, M. Wild rodents of Pontine Islands as bioindicators of environmental quality/I Roditori selvatici delle isole Pontine come bioindicatori della qualità ambientale. Hystrix Ital. J. Mammal. 1992, 4, 41–49. [Google Scholar]
- van der Horst, G. Status of sperm functionality assessment in wildlife species: From fish to primates. Animals 2021, 11, 1491. [Google Scholar] [CrossRef]
- Roldan, E.R.; Gomendio, M. Sperm and Conservation. In Sperm Biology, 1st ed.; Birkhead, T.R., Pitnik, S., Hosken, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 539–564. [Google Scholar]
| Class | Description | Picture |
|---|---|---|
| Normal sperm | Head accented by a marked hook, leading to a comma-like form. | 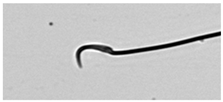 |
| Short hook/Banana | Shortening of the hook, leading to a banana-like form. | 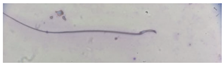 |
| Hook at wrong angle | Shows a crooked hook. |  |
| Straight/no hook | Similar to a straight line. | 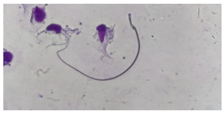 |
| Triangular | Similar to a triangle/Arrow. | 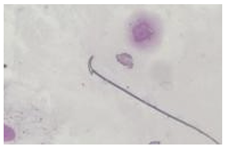 |
| Amorphous | Altered and indefinite form of the sperm head. | 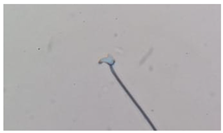 |
| Swollen acrosome | Enlargement of the basal area of the sperm head. | 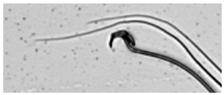 |
| Acute curvature | Pronounced hook. |  |
| Swollen hook | Enlargement of the hook tip. | 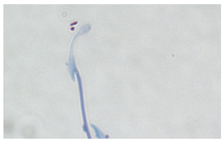 |
| Probability of Abnormality | Number of Cell Counts Needed | |
|---|---|---|
| Power 95% | Power 99% | |
| 0.01 | 1693 | 1985 |
| 0.02 | 848 | 986 |
| 0.05 | 329 | 387 |
| 0.1 | 158 | 221 |
| Sperm Abnormality (Total/Classes) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Abnormalities (All Individuals) | Short Hook/ Banana | Hook Angle | Straight Hook | Triangular | Amorphous | Swollen Acrosome | Acute Curvature | Swollen Hook | |||||||||||
| Cell Counts | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Lisbon (n = 16) | 300 | 177 | 3.7 | 144 | 81.4 | 8 | 4.5 | 1 | 0.6 | 18 | 10.2 | 0 | 0.0 | 1 | 0.6 | 4 | 2.3 | 1 | 0.6 |
| 500 | 323 | 4.0 | 255 | 78.9 | 18 | 5.6 | 1 | 0.3 | 33 | 10.2 | 2 | 0.6 | 2 | 0.6 | 8 | 2.5 | 4 | 1.2 | |
| 1000 | 751 | 4.7 | 577 | 76.8 | 49 | 6.5 | 1 | 0.1 | 85 | 11.3 | 5 | 0.7 | 3 | 0.4 | 23 | 3.1 | 8 | 1.1 | |
| 2000 | 1319 | 4.1 | 996 | 75.5 | 94 | 7.1 | 4 | 0.3 | 165 | 12.5 | 8 | 0.6 | 3 | 0.2 | 41 | 3.1 | 8 | 0.6 | |
| Ponta Delgada (n = 34) | 300 | 246 | 2.4 | 191 | 77.6 | 41 | 16.7 | 2 | 0.8 | 4 | 1.6 | 0 | 0.0 | 0 | 0.0 | 8 | 3.3 | 0 | 0.0 |
| 500 | 396 | 2.3 | 304 | 76.8 | 70 | 17.7 | 5 | 1.3 | 5 | 1.3 | 0 | 0.0 | 1 | 0.3 | 10 | 2.5 | 1 | 0.3 | |
| 1000 | 684 | 2.0 | 530 | 77.5 | 109 | 15.9 | 11 | 1.6 | 12 | 1.8 | 0 | 0.0 | 1 | 0.1 | 18 | 2.6 | 3 | 0.4 | |
| 2000 | 1141 | 1.7 | 899 | 78.8 | 152 | 13.3 | 24 | 2.1 | 23 | 2.0 | 2 | 0.2 | 1 | 0.1 | 34 | 3.0 | 6 | 0.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso, E.; Mathias, M.d.L.; Monarca, R.I.; Gabriel, S.I. Assessing Optimal Cell Counts in Sperm Shape Abnormality Assays in Rodents. Animals 2023, 13, 3324. https://doi.org/10.3390/ani13213324
Cardoso E, Mathias MdL, Monarca RI, Gabriel SI. Assessing Optimal Cell Counts in Sperm Shape Abnormality Assays in Rodents. Animals. 2023; 13(21):3324. https://doi.org/10.3390/ani13213324
Chicago/Turabian StyleCardoso, Elizandra, Maria da Luz Mathias, Rita I. Monarca, and Sofia I. Gabriel. 2023. "Assessing Optimal Cell Counts in Sperm Shape Abnormality Assays in Rodents" Animals 13, no. 21: 3324. https://doi.org/10.3390/ani13213324
APA StyleCardoso, E., Mathias, M. d. L., Monarca, R. I., & Gabriel, S. I. (2023). Assessing Optimal Cell Counts in Sperm Shape Abnormality Assays in Rodents. Animals, 13(21), 3324. https://doi.org/10.3390/ani13213324







