Epidemiological Investigation of Yak (Bos grunniens) Fascioliasis in the Pastoral Area of Qinghai–Tibet Plateau, China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin of Experimental Animals
2.2. Examination of Fasciola spp. Eggs in Feces
2.3. Examination and Identification of Adult Worms
2.4. Molecular Identification of Adult Worms
2.5. Prevalence of Fasciola spp.
2.6. Statistical Analysis
3. Results
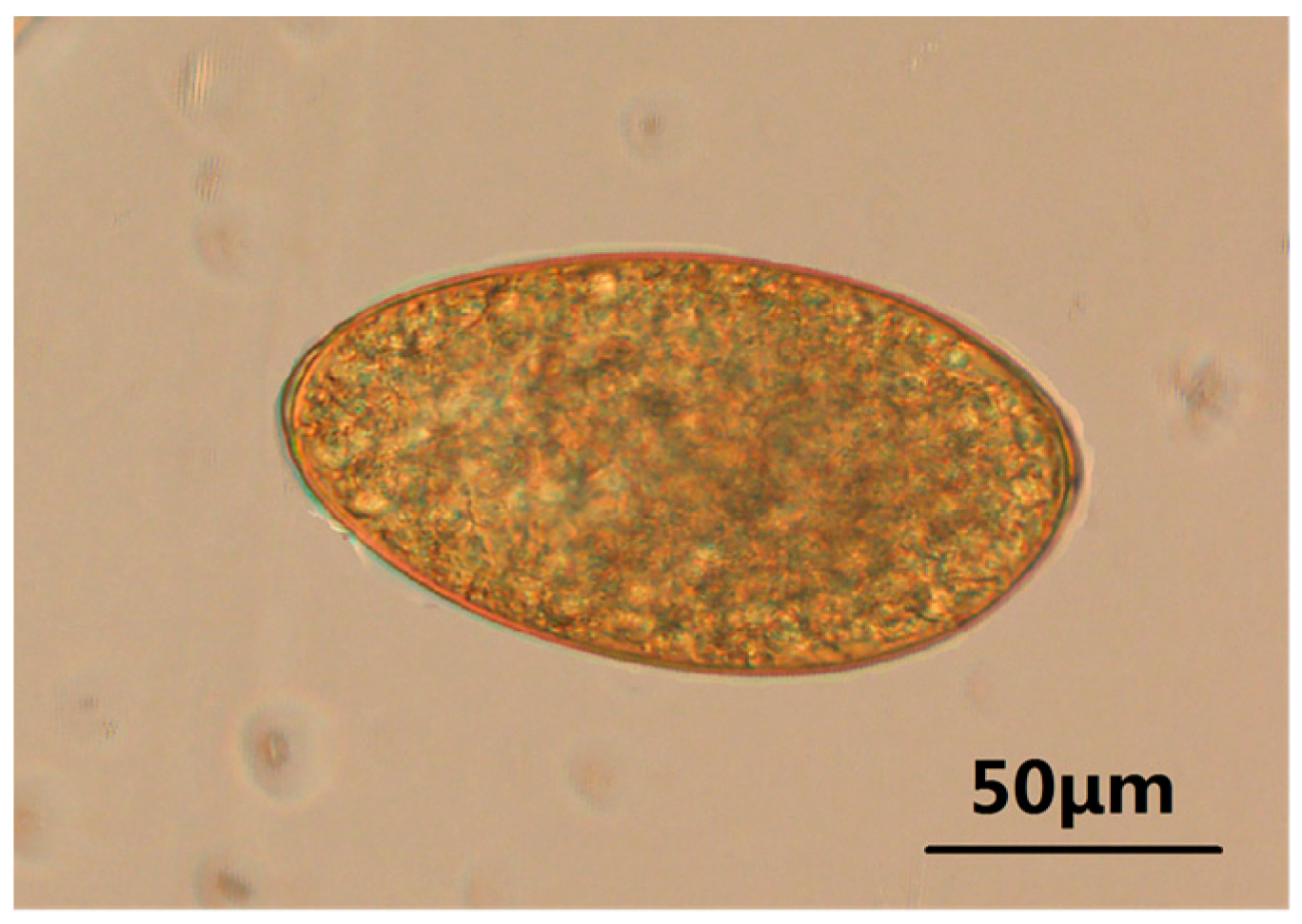
3.1. Identification of Eggs
3.2. Morphological and Molecular Identification of Adult Worms
3.3. Results of the Fecal Examination
3.3.1. Infection of Fasciola spp. by Fecal Examination
3.3.2. Fasciola spp. Infection in Yaks of Different Ages
3.3.3. Differences in Fasciola spp. Egg Infection in Yaks from Different Areas
3.3.4. Spatial Characteristics of Fasciola spp. Infection in Yaks
3.4. Autopsy Results
3.5. Assessment of Risk Factors for Fasciola spp. Infection of Yaks Based on Areas and Age Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.M. The WTO accession and China’s yak industry. J. Int. Trade 2002, 28, 16–18. (In Chinese) [Google Scholar]
- Niu, C.E.; Zhang, L.P.; Sun, J.F.; Gao, Y.Q.; Cao, J.M. Analysis on the current situation of yak resources and its product development and utilization. J. Anhui Agric. Sci. 2009, 37, 8003–8005. (In Chinese) [Google Scholar]
- Ma, J.; Zhu, Y.; Wang, Z.; Yu, X.; Hu, R.; Wang, X.; Cao, G.; Zou, H.; Shah, A.M.; Peng, Q.; et al. Comparing the bacterial community in the gastrointestinal tracts between growth-retarded and normal yaks on the Qinghai–Tibetan Plateau. Front. Microbiol. 2020, 11, 600516. [Google Scholar] [CrossRef]
- Caravedo, M.A.; Cabada, M.M. Human fascioliasis: Current epidemiological status and strategies for diagnosis, treatment, and control. Res. Rep. Trop. Med. 2020, 11, 149–158. [Google Scholar] [CrossRef]
- Arias, M.S.; Piñeiro, P.; Sánchez-Andrade, R.; Suárez, J.L.; Hillyer, G.V.; Díez-Baños, P.; Paz-Silva, A.; Morrondo, P. Relationship between exposure to Fasciola hepatica in roe deer (Capreolus capreolus) and cattle extensively reared in an endemic area. Res. Vet. Sci. 2013, 95, 1031–1035. [Google Scholar] [CrossRef]
- Torres, G.B.; Iwashita, A.T.; Vargas, C.M.; Luján, L.V.; Bianchi, H.A.; Casanova, R.T. Human fasciolasis and gastrointestinal compromise: Study of 277 patients in the Cayetano Heredia national hospital (1970–2002). Rev. Gastroenterol. Peru 2004, 24, 143–157. [Google Scholar]
- Krsak, M.; Patel, N.U.; Poeschla, E.M. Case report: Hepatic fascioliasis in a young Afghani woman with severe wheezing, high-grade peripheral eosinophilia, and liver lesions: A brief literature review. Am. J. Trop. Med. Hyg. 2019, 100, 88–590. [Google Scholar] [CrossRef]
- Changwong, M.R.; Pinto, E.J.O.; Guzman, R.P.; Terashima, I.A.; Samalvides, C.F. Demographic and clinical aspects of hepatic fascioliasis between 2013–2010 in National Hospital Cayetano Heredia, Lima, Peru. Rev. Gastroenterol. Peru 2016, 36, 23–28. [Google Scholar]
- Machicado, C.; Machicado, J.D.; Maco, V.; Terashima, A.; Marcos, L.A. Association of Fasciola hepatica infection with liver fibrosis, cirrhosis, and cancer: A systematic review. PLoS Negl. Trop. Dis. 2016, 9, 28. [Google Scholar] [CrossRef]
- Fentie, T.; Erqou, S.; Gedefaw, M.; Desta, A. Epidemiology of human fascioliasis and intestinal parasitosis among schoolchildren in Lake Tana Basin, northwest Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 480–486. [Google Scholar] [CrossRef]
- Alemayehu, T.; Tariku, S.; Tesfaye, K. Fascioliasis complicated by acute necrotizing pancreatitis in an Ethiopian child—A case report on a rare complication of a rarely reported emerging disease. IJID Reg. 2022, 3, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.D. Study of dynamics and control of nematodes and trematodes in young yaks. Chin. Qinghai J. Anim. Vet. Sci. 1992, 22, 6–10. (In Chinese) [Google Scholar]
- Wu, F.; Li, T.M.; Guo, Y.C. Investigation of yak parasites in Suhurima region. Chin. Qinghai J. Anim. Vet. Sci. 1984, 5, 116. (In Chinese) [Google Scholar]
- Ma, C. Study on sustainable development strategie of Tianzhu white yak industry. Domest. Anim. Ecol. 2008, 29, 133–135. [Google Scholar]
- Zhang, X.X.; Feng, S.Y.; Ma, J.G.; Zheng, W.B.; Yin, M.Y.; Qin, S.Y.; Zhou, D.H.; Zhao, Q.; Zhu, X.Q. Seroprevalence and risk factors of fascioliasis in yaks, Bos grunniens, from three counties of Gansu province, China. Korean J. Parasitol. 2017, 55, 89–93. [Google Scholar] [CrossRef][Green Version]
- Gao, X.; Zhang, L.H.; Tong, X.L.; Zhang, H.; Mehmood, K.; Jiang, X.; Li, J.K. Epidemiological survey of fasciolosis in yaks and sheep living on the Qinghai-Tibet plateau, China. Acta Trop. 2020, 201, 105212. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhu, Q.; Peng, C.; Zhang, J.; Wang, M.; Zhang, J.J.; Ding, J.H.; Zhou, X.L. Change in autumn vegetation phenology and the climate controls from 1982 to 2012 on the Qinghai-Tibet Plateau. Front. Plant Sci. 2020, 10, 1677. [Google Scholar] [CrossRef]
- Zhan, N.; Liu, W.; Ye, T.; Li, H.; Chen, S.; Ma, H. High-resolution livestock seasonal distribution data on the Qinghai-Tibet Plateau in 2020. Sci. Data 2023, 10, 142. [Google Scholar] [CrossRef]
- NY/T1950-2010; Technical Specification for the Diagnosis of Fasciolosis. Agricultural Industry Standard of the People’s Republic of China, Ministry of Agriculture: Beijing, China, 2010. (In Chinese)
- Nielsen, M.K. What makes a good fecal egg count technique? Vet. Parasitol. 2021, 296, 109509. [Google Scholar] [CrossRef] [PubMed]
- Bam, J.; Thüer, S.; Holinger, M.; Oberhansli, T.; Leubin, M.; Leiber, F.; Werne, S. Performance and parasitological parameters of steers sequentially grazed with lambs. Vet. Parasitol. 2022, 302, 109645. [Google Scholar] [CrossRef]
- Wang, C.A.; Wang, D.W.; Wei, F.Q. Preliminary investigation on the infection of Fasciola hepatica in yak in Nyingchi region. Tibet. Med. J. 1996, 17, 62–63. (In Chinese) [Google Scholar]
- Mima, D.Z.; She, Y.X.; Qiong, R. Parasitoid fauna of yak in Nyingchi, Tibet. Chin. J. Vet. Med. 2001, 37, 23. (In Chinese) [Google Scholar]
- Ma, R.L.; Cai, J.S.; Li, L.F.; Ma, Z.Q.; Zhao, Q.B.; He, S.D.; Che, F.M.; Ni, M. Investigation on the parasite flora of Haiyan yak in Qinghai Province. Chin. J. Vet. Med. 2011, 47, 52–53. (In Chinese) [Google Scholar]
- Chai, Z.M. Investigation of fluke infection in yak liver in Xinghai County. Ani. Hus. Vet. Med. 2013, 45, 112. (In Chinese) [Google Scholar]
- Cai, J.S.; Li, L.F.; Ma, R.l.; Zhao, Q.B.; Ma, Z.Q.; Hu, G.W.; Li, J.; Yang, Q.X.; Wen, J.M.; Cao, X.F.; et al. Qinghai qilian area of cattle and sheep parasite fauna investigation report. Chin. J. Vet. Med. 2013, 49, 48–49. (In Chinese) [Google Scholar]
- He, F.F.; Li, C.H.; Lei, M.T.; Tong, X.J.; Cai, J.Z. Investigation on parasitic diseases of yaks in Xinghai County, Hainan Prefecture, Qinghai Province. Anim. Husb. Vet. Med. 2015, 47, 107–111. (In Chinese) [Google Scholar]
- Lei, M.T.; Cai, J.Z.; Li, C.H.; Li, J.; Li, Z.X.C.R.; Huang, B.; Chen, Z.G. Investigation on parasitic diseases of Qilian Yak in Qinghai Province. Chin. J. Vet. Med. 2016, 52, 44–46. (In Chinese) [Google Scholar]
- Chai, Z.M. Prevalence of Fasciola hepatica infection in yaks in Xinghai. Anim. Husb. Vet. Med. 2013, 2, 112. (In Chinese) [Google Scholar]
- Yang, Y.F.; Wang, H.N.; Yang, G.Y.; Luo, G.R.; Yuan, B.; Yang, F. Epidemiological survey and cotrol method on parasitic diseases of yak and Tibet sheep on the West-North Sichuan grassland. Chin. Sichuan Anim. Vet. Sci. 2003, S1, 33–34. (In Chinese) [Google Scholar]
- Qin, S.Y.; Yin, M.Y.; Song, G.Y.; Tan, Q.D.; Wang, J.L.; Zhou, D.H. Prevalence of gastrointestinal parasites in free-range yaks (Bos grunniens) in Gansu Province, Northwest China. BMC Vet. Res. 2019, 15, 410. [Google Scholar] [CrossRef] [PubMed]
- RangaRao, G.S.; Sharma, R.L.; Hemaprasanth. Parasitic infections of Indian yak Bos (poephagus) grunniens—An overview. Vet. Parasitol. 1994, 53, 75–82. [Google Scholar] [CrossRef]
- Acharya, K.P.; Nirmal, B.K.; Kaphle, K.; Mahato, M.K.; Yadav, G.P.; Rana, H.B.; Kaphle, K. Prevalence of gastrointestinal and liver parasites in yaks in the cold desert area of lower Mustang, Nepal. Asian Pac. J. Trop. Dis. 2016, 6, 147–150. [Google Scholar] [CrossRef]
- Kong, X.Y.; Gan, F.B.; Gao, X.; Zhang, W.H.; Cai, J.S.; Li, J.K. Yak fasciola gigantica observation and PCR identification of trematode. Chin. Hubei Anim. Vet. Sci. 2019, 40, 5–6. (In Chinese) [Google Scholar]
- Gao, X.; Wang, D.; Zhang, Z.; Quan, C.; Zhou, S.; Li, K.; Li, Y.; Zhao, S.; Kong, X.; Kulyar, M.F.; et al. Genetic characterization and phylogenetic analysis of fasciola species isolated from yaks on Qinghai-Tibet Plateau, China. Front. Vet. Sci. 2022, 12, 824785. [Google Scholar] [CrossRef]





| Areas | Geographic Coordinates | Altitude (m) | Annual Precipitation (mm) | Fecal Test (Number) | Autopsy (Number) |
|---|---|---|---|---|---|
| Gonghe | E 100°62′66.23″, N 36°28′87.03″ | above 3200 | 371.0 | 96 | 22 |
| Xinghai | E 99°58′57.18″, N 35°35′18.25″ | above 3306 | 360.0 | 98 | 21 |
| Guinan | E 100°13′–101°31′, N 35°09′–36°08′ | about 3100 | 488.0 | 96 | 22 |
| Qilian | E 100°26′40.41″, N 38°03′44.15″ | above 2717 | 406.7 | 97 | 26 |
| Gangcha | E 100°08′45.00″, N 37°19′31.69″ | above 3700 | 345.0 | 96 | 23 |
| Haiyan | E 100°59′39.95″, N 36°53′40.81″ | above 3008 | 447.4 | 96 | 22 |
| Dulan | E 98°06′09.74″, N 36°18′27.40″ | above 3041 | 213.4 | 96 | 24 |
| Wulan | E 98°48′67.39″, N 36°93′57.48″ | above 3100 | 210.4 | 97 | 26 |
| Dari | E 99°39′3.99″, N 33°45′12.55″ | above 3900 | 531.5 | 96 | 20 |
| Maduo | E 97°19′15.99″, N 34°57′10.55″ | above 4290 | 585.5 | 96 | 20 |
| Yushu | E 97°00′87.85″, N 32°99′31.07″ | above 3681 | 487.0 | 97 | 22 |
| Chengduo | E 97°06′39.00″, N 33°22′09.18″ | above 3851 | 500.0 | 96 | 20 |
| Nangqian | E 96°48′58″, N31°32′20″ | above 3650 | 527.3 | 96 | 22 |
| Henan | E 102°08′56.36″, N 34°29′16.96″ | above 3520 | 597.1 | 96 | 20 |
| Zeku | E 100°34′–102°08′, N 34°45′–35°32′ | above 3650 | 564.9 | 96 | 20 |
| Datong | E 101°38′00.28″, N 37°25′23.12″ | above 2950 | 547.2 | 97 | 32 |
| Total | 1542 | 242 |
| Number | Slaughterhouse |
|---|---|
| 1 | Qinghai Lake meat industry Co., Ltd., slaughterhouse (Gonghe, China) |
| 2 | Xinghai County green grass source food Co., Ltd., slaughterhouse (Xinghai, China) |
| 3 | Guinan County Lvjiayuan cattle and sheep slaughterhouse (Guinan, China) |
| 4 | Qilian Yida animal products Co., Ltd., slaughterhouse (Qilian, China) |
| 5 | Gangcha County Yipin animal products Co., Ltd., slaughterhouse (Gangcha, China) |
| 6 | Haiyan County Huaxia cattle and sheep slaughterhouse (Haiyan, China) |
| 7 | Qinghai Kaitai agriculture and animal husbandry Co., Ltd., slaughterhouse (Dulan, China) |
| 8 | Wulan County Hengcheng beef and mutton slaughterhouse (Wulan, China) |
| 9 | Guoluo Haoyun designated Cattle and Sheep Co., Ltd. (Dari, China) |
| 10 | Guoluo Jin Grassland yak slaughtering and processing Co., Ltd. (Maduo, China) |
| 11 | Zhiduo County meat food Co., Ltd., slaughterhouse (Zhiduo, China) |
| 12 | Chengduo County plateau yak livestock products Co., Ltd., slaughterhouse (Chengduo, China) |
| 13 | Yushu Muyuan meat industry Co., Ltd., slaughterhouse (Yushu, China) |
| 14 | Sanjiang Ranch Co., Ltd., slaughterhouse (Henan, China) |
| 15 | Qinghai Northwest Hong organic resources development Co., Ltd., cattle and sheep slaughterhouse (Zeku, China) |
| 16 | Qinghai Datong Jinlu Industry and Trade Co., Ltd., slaughterhouse (Datong, China) |
| Areas | Number of Investigated Yaks | Positive Rate (%) | Mean Intensity (epg) |
|---|---|---|---|
| Gonghe | 96 | 17.71 (17/96) | 53.3 (29–79) |
| Xinghai | 98 | 23.47 (23/98) | 46.3 (21–66) |
| Guinan | 96 | 27.08 (26/96) | 45.6 (20–70) |
| Qilian | 97 | 17.53 (17/97) | 49.7 (23–76) |
| Gangcha | 96 | 37.50 (36/96) | 57.9 (30–112) |
| Haiyan | 96 | 32.29 (31/96) | 53.8 (28–97) |
| Dulan | 96 | 38.54 (37/96) | 55.7 (27–82) |
| Wulan | 97 | 40.21 (39/97) | 63.1 (36–103) |
| Dari | 96 | 0 | 0 |
| Maduo | 96 | 0 | 0 |
| Zhiduo | 97 | 0 | 0 |
| Chengduo | 96 | 0 | 0 |
| Nangqian | 96 | 19.79 (19/96) | 45.1 (18–84) |
| Henan | 96 | 22.92 (22/96) | 48.1 (32–59) |
| Zeku | 96 | 0 | 0 |
| Datong | 97 | 0 | 0 |
| Total | 1542 | 17.32 (267/1542) | 51.9 (18–112) |
| Areas | 0–1 (<1 Year Old) | 1–2 (≥1 Year Old and <3 Years Old) | Over 3 Years (≥3 Years Old) | |||
|---|---|---|---|---|---|---|
| Number | Mean Intensity (Epg) | Number | Mean Intensity (Epg) | Number | Mean Intensity (Epg) | |
| Gonghe | 32 | 47.4 (29–62) | 32 | 53.2 (37–64) | 30 | 59.3 (34–79) |
| Xinghai | 33 | 42.3 (21–58) | 32 | 46.6 (30–59) | 31 | 50.1 (28–66) |
| Guinan | 32 | 39.5 (20–62) | 32 | 44.8 (27–70) | 31 | 52.6 (36–68) |
| Qilian | 32 | 43.9 (23–53) | 32 | 50.3 (33–68) | 30 | 54.8 (33–76) |
| Gangcha | 33 | 50.2 (30–92) | 32 | 57.4 (38–89) | 31 | 66.1 (29–112) |
| Haiyan | 33 | 45.7 (28–71) | 32 | 53.0 (39–82) | 32 | 62.6 (40–97) |
| Dulan | 32 | 48.9 (27–54) | 32 | 57.7 (31–69) | 32 | 60.4 (39–82) |
| Wulan | 34 | 55.1 (36–79) | 32 | 63.2 (39–93) | 32 | 71.0 (43–103) |
| Dari | 32 | 0 | 32 | 0 | 32 | 0 |
| Maduo | 32 | 0 | 32 | 0 | 32 | 0 |
| Zhiduo | 33 | 0 | 32 | 0 | 32 | 0 |
| Chengduo | 32 | 0 | 32 | 0 | 32 | 0 |
| Nangqian | 32 | 38.7 (18–54) | 32 | 45.5 (25–61) | 31 | 51.2 (37–84) |
| Henan | 32 | 43.5 (32–54) | 32 | 48.5 (41–58) | 32 | 52.7 (43–65) |
| Zeku | 0 | 0 | 0 | 0 | 0 | 0 |
| Datong | 33 | 0 | 33 | 0 | 32 | 0 |
| Total | 515 | 45.5 (18–92) | 513 | 52.0 (23–93) | 514 | 58.1 (28–112) |
| Infection rate | 9.90% | 16.18% | 25.88% | |||
| Study Areas | Number of Yaks Examined | Prevalence (%) | Mean Intensity (Number of Worms) |
|---|---|---|---|
| Gonghe | 22 | 22.73 (5/22) | 9.2 (3–22) |
| Xinghai | 21 | 28.57 (6/21) | 17.5 (4–37) |
| Guinan | 22 | 27.27 (6/22) | 14.2 (6–26) |
| Qilian | 26 | 19.23 (5/26) | 11.6 (6–18) |
| Gangcha | 23 | 39.13 (9/23) | 30.6 (7–46) |
| Haiyan | 22 | 31.82 (7/22) | 22.7 (3–21) |
| Dulan | 24 | 37.50 (9/24) | 28.3 (4–34) |
| Wulan | 26 | 38.46 (10/26) | 28.6 (9–39) |
| Dari | 20 | 0 | 0 |
| Maduo | 20 | 0 | 0 |
| Yushu | 22 | 0 | 0 |
| Chengduo | 20 | 0 | 0 |
| Nangqian | 22 | 22.73 (5/22) | 20.1 (7–43) |
| Henan | 20 | 20.00 (4/20) | 16.8 (12–36) |
| Zeku | 20 | 0 | 0 |
| Datong | 32 | 0 | 0 |
| Total | 242 | 27.27 (66/242) | 21.2 (3–46) |
| Factor | Group | n | Prevalence (%) | OR | 95% CI for the OR | p-Value | |
|---|---|---|---|---|---|---|---|
| Upper Limit | Lower Limit | ||||||
| Age | 1 year | 322 | 15.84 | 1.000 | |||
| 2 years | 320 | 25.94 | 1.895 | 1.275 | 2.816 | 0.002 | |
| Over 3 years | 322 | 41.30 | 3.921 | 2.681 | 5.736 | <0.001 | |
| Areas | Qilian | 97 | 17.53 | 1.000 | |||
| Gonghe | 96 | 17.71 | 1.007 | 0.472 | 2.146 | 0.986 | |
| Xinghai | 98 | 23.47 | 1.459 | 0.711 | 2.993 | 0.303 | |
| Guinan | 96 | 27.08 | 1.789 | 0.881 | 3.632 | 0.108 | |
| Gangcha | 96 | 37.50 | 2.985 | 1.503 | 5.927 | 0.002 | |
| Haiyan | 96 | 32.29 | 2.334 | 1.166 | 4.675 | 0.017 | |
| Dulan | 96 | 38.54 | 3.130 | 1.578 | 6.207 | 0.001 | |
| Wulan | 97 | 40.21 | 3.348 | 1.693 | 6.619 | 0.001 | |
| Nangqian | 96 | 19.79 | 1.162 | 0.553 | 2.440 | 0.692 | |
| Henan | 96 | 22.92 | 1.413 | 0.685 | 2.917 | 0.349 | |
| Total | 964 | 27.70 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Q.; Lei, M.; Li, C.; Cai, J.; Ma, D.; Zhang, H. Epidemiological Investigation of Yak (Bos grunniens) Fascioliasis in the Pastoral Area of Qinghai–Tibet Plateau, China. Animals 2023, 13, 3330. https://doi.org/10.3390/ani13213330
Cai Q, Lei M, Li C, Cai J, Ma D, Zhang H. Epidemiological Investigation of Yak (Bos grunniens) Fascioliasis in the Pastoral Area of Qinghai–Tibet Plateau, China. Animals. 2023; 13(21):3330. https://doi.org/10.3390/ani13213330
Chicago/Turabian StyleCai, Qijian, Mengtong Lei, Chunhua Li, Jinzhong Cai, Doudou Ma, and Houshuang Zhang. 2023. "Epidemiological Investigation of Yak (Bos grunniens) Fascioliasis in the Pastoral Area of Qinghai–Tibet Plateau, China" Animals 13, no. 21: 3330. https://doi.org/10.3390/ani13213330
APA StyleCai, Q., Lei, M., Li, C., Cai, J., Ma, D., & Zhang, H. (2023). Epidemiological Investigation of Yak (Bos grunniens) Fascioliasis in the Pastoral Area of Qinghai–Tibet Plateau, China. Animals, 13(21), 3330. https://doi.org/10.3390/ani13213330





