The Science Underlying Giant Panda Conservation Translocations
Abstract
:Simple Summary
Abstract
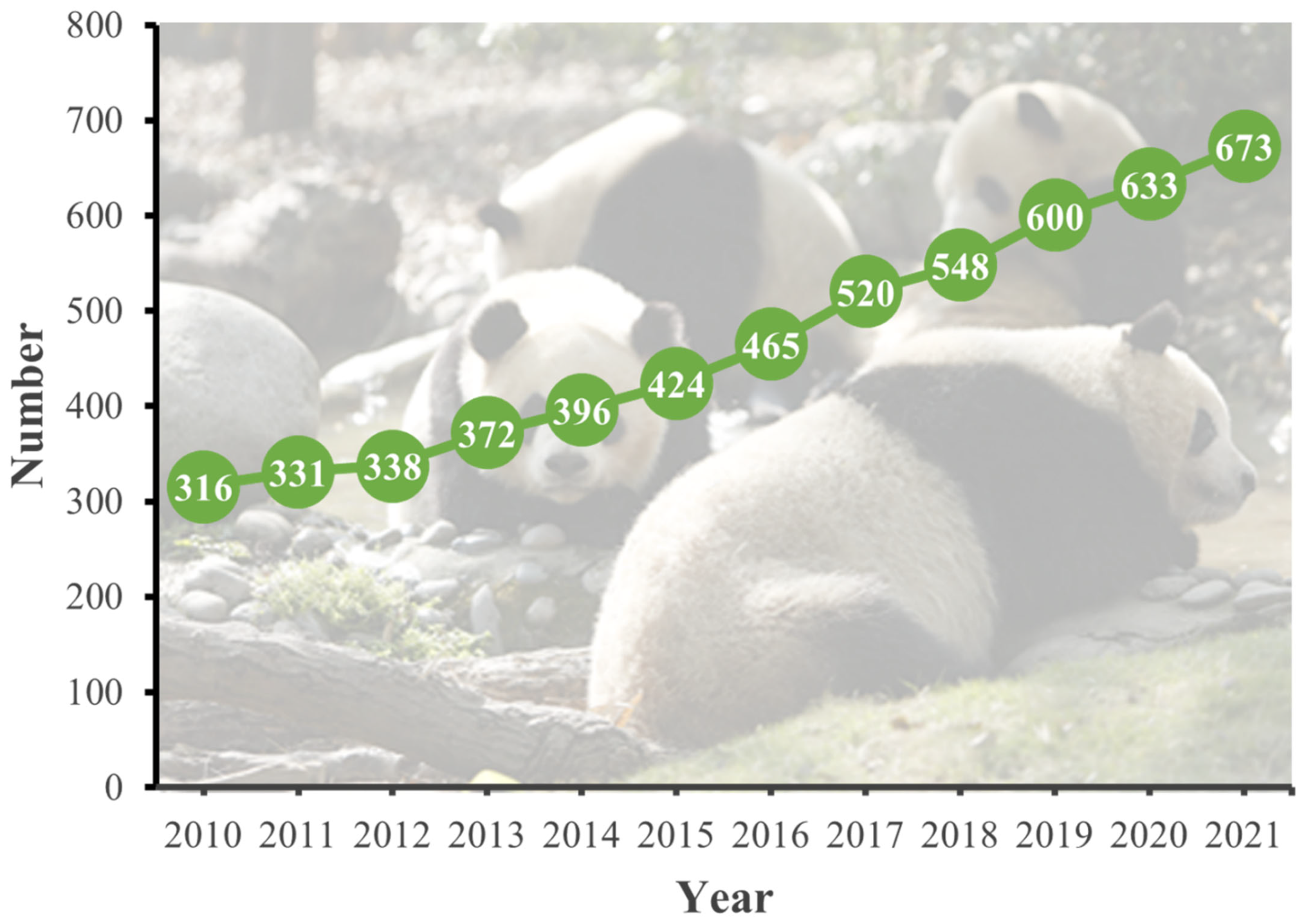
1. Introduction
2. Selecting Suitable Release Sites
3. Selecting Suitable Release Candidacy
3.1. Individual Source
3.2. Body Condition
3.3. Parasites and Viruses
3.4. Sex and Age
3.5. Behaviour
3.6. Genetic Consideration
4. Pre-Release Training of Candidate
5. Post-Release Monitoring of Released Individuals
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wagler, R. 6th mass extinction. In Encyclopedia of the Anthropocene; Dellasala, D.A., Goldstein, M.I., Eds.; Elsevier: Oxford, UK, 2018; pp. 9–12. [Google Scholar]
- Cafaro, P. Three ways to think about the sixth mass extinction. Biol. Conserv. 2015, 192, 387–393. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Y.; Yu, R.; Xu, X.; Xu, M.; Li, G.; Wang, W.; Yang, Y. China’s biodiversity conservation in the process of implementing the sustainable development goals (sdgs). J. Clean. Prod. 2022, 338, 130595. [Google Scholar] [CrossRef]
- State Forestry Administration of China. The Fourth National Giant Panda Survey; State Forestry Administration of China Press: Beijing, China, 2015. [Google Scholar]
- Wei, F.W.; Costanza, R.; Dai, Q.; Stoeckl, N.; Gu, X.D.; Farber, S.; Nie, Y.G.; Kubiszewski, I.; Hu, Y.B.; Swaisgood, R.; et al. The value of ecosystem services from giant panda reserves. Curr. Biol. 2018, 28, 2174. [Google Scholar] [CrossRef]
- Swaisgood, R.R.; Wang, D.; Wei, F. Panda downlisted but not out of the woods. Conserv. Lett. 2018, 11, e12355. [Google Scholar] [CrossRef]
- Qing, J.; Yang, Z.; He, K.; Zhang, Z.; Gu, X.; Yang, X.; Zhang, W.; Yang, B.; Qi, D.; Dai, Q. The minimum area requirements (mar) for giant panda: An empirical study. Sci. Rep. 2016, 6, 37715. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, X.; Nie, Y.; Huang, F.; Huang, Y.; Dai, Q.; Hu, Y.; Yang, Y.; Zhou, X.; Zhang, H. Reintroduction of the giant panda into the wild: A good start suggests a bright future. Biol. Conserv. 2018, 217, 181–186. [Google Scholar] [CrossRef]
- Hong, M.S.; Wei, W.; Tang, J.F.; Zhou, H.; Han, H.; Zhang, Z.J. Positive responses from giant pandas to the natural forest conservation programme based on slope utilisation. Glob. Ecol. Conserv. 2021, 27, e01616. [Google Scholar] [CrossRef]
- Bai, W.; Huang, Q.; Zhang, J.; Stabach, J.; Huang, J.; Yang, H.; Songer, M.; Connor, T.; Liu, J.; Zhou, S.; et al. Microhabitat selection by giant pandas. Biol. Conserv. 2020, 247, 108615. [Google Scholar] [CrossRef]
- Walters, J.R.; Derrickson, S.R.; Michael Fry, D.; Haig, S.M.; Marzluff, J.M.; Wunderle, J.M., Jr. Status of the california condor (Gymnogyps californianus) and efforts to achieve its recovery. Auk 2010, 127, 969–1001. [Google Scholar] [CrossRef]
- Frith, C. The Woodhen: A Flightless Island Bird Defying Extinction; CSIRO Publishing: Canberra, Australia, 2013. [Google Scholar]
- Bubac, C.M.; Johnson, A.C.; Fox, J.A.; Cullingham, C.I. Conservation translocations and post-release monitoring: Identifying trends in failures, biases, and challenges from around the world. Biol. Conserv. 2019, 238, 108239. [Google Scholar] [CrossRef]
- Armstrong, D.P.; Seddon, P.J. Directions in reintroduction biology. Trends Ecol. Evol. 2008, 23, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, S.; Wei, F.; Wang, H.; Li, M.; Hu, J. Translocation and discussion on reintroduction of captive giant panda. Acta Theriol. Sin. 2006, 26, 292. [Google Scholar]
- Kang, D. A review of the impacts of four identified major human disturbances on the habitat and habitat use of wild giant pandas from 2015 to 2020. Sci. Total Environ. 2021, 763, 142975. [Google Scholar] [CrossRef]
- Kong, L.Q.; Xu, W.H.; Xiao, Y.; Pimm, S.L.; Shi, H.; Ouyang, Z.Y. Spatial models of giant pandas under current and future conditions reveal extinction risks. Nat. Ecol. Evol. 2021, 5, 1309. [Google Scholar] [CrossRef] [PubMed]
- Lomolino, M.V.; Channell, R. Range collapse, re-introductions, and biogeographic guidelines for conservation. Conserv. Biol. 1998, 12, 481–484. [Google Scholar] [CrossRef]
- Baling, M.; Stuart-Fox, D.; Brunton, D.H.; Dale, J. Habitat suitability for conservation translocation: The importance of considering camouflage in cryptic species. Biol. Conserv. 2016, 203, 298–305. [Google Scholar] [CrossRef]
- Hu, J.C.; Schaller, G.B.; Pan, W.S.; Zhu, J. The Giant Panda of Wolong; Sichuan Science and Technology Press: Chengdu, China, 1985. [Google Scholar]
- Hull, V.; Zhang, J.D.; Huang, J.Y.; Zhou, S.Q.; Vina, A.; Shortridge, A.; Li, R.G.; Liu, D.A.; Xu, W.H.; Ouyang, Z.Y.; et al. Habitat use and selection by giant pandas. PLoS ONE 2016, 11, e0162266. [Google Scholar] [CrossRef]
- Liu, Z.; Dayananda, B.; Jeffree, R.A.; Tian, C.; Zhang, Y.; Yu, B.; Zheng, Y.; Jing, Y.; Si, P.; Li, J. Giant panda distribution and habitat preference: The influence of sympatric large mammals. Glob. Ecol. Conserv. 2020, 24, e1221. [Google Scholar] [CrossRef]
- Wei, F.; Swaisgood, R.; Hu, Y.; Nie, Y.; Yan, L.; Zhang, Z.; Qi, D.; Zhu, L. Progress in the ecology and conservation of giant pandas. Conserv. Biol. 2015, 29, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Swaisgood, R.R.; Zhang, S.; Nordstrom, L.A.; Wang, H.; Gu, X.; Hu, J.; Wei, F. Old-growth forest is what giant pandas really need. Biol. Lett. 2011, 7, 403–406. [Google Scholar] [CrossRef]
- Zhiyun, O.; Jianguo, L.; Han, X.; Yingchun, T.; Hemin, Z. An assessment of giant panda habitat in wolong nature reserve. Acta Ecol. Sin. 2001, 21, 1869–1874. [Google Scholar]
- Lei, M.; Yuan, S.; Yang, Z.; Hong, M.; Yang, X.; Gu, X.; Huang, F.; Zhang, Z. Comparison of microhabitats and foraging strategies between the captive-born zhangxiang and wild giant pandas: Implications for future reintroduction. Environ. Sci. Pollut. Res. 2015, 22, 15089–15096. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Beck, B.; Travis, D.; Unwin, S.; Walkup, K. Best Practice Guidelines for the Re-Introduction of Great Apes; IUCN: Gland, Switzerland, 2007. [Google Scholar]
- Guo, J.; Chen, Y.; Hu, J. Population viability analysis of giant pandas in the yele nature reserve. J. Nat. Conserv. 2002, 10, 35–40. [Google Scholar] [CrossRef]
- Griffith, B.; Scott, J.M.; Carpenter, J.W.; Reed, C. Translocation as a species conservation tool: Status and strategy. Science 1989, 245, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Mathews, F.; Orros, M.; Mclaren, G.; Gelling, M.; Foster, R. Keeping fit on the ark: Assessing the suitability of captive-bred animals for release. Biol. Conserv. 2005, 121, 569–577. [Google Scholar] [CrossRef]
- Venesky, M.D.; Mendelson Iii, J.R.; Sears, B.F.; Stiling, P.; Rohr, J.R. Selecting for tolerance against pathogens and herbivores to enhance success of reintroduction and translocation. Conserv. Biol. 2012, 26, 586–592. [Google Scholar] [CrossRef]
- Bronson, E.; Deem, S.L.; Sanchez, C.; Murray, S. Serologic response to a canarypox-vectored canine distemper virus vaccine in the giant panda (Ailuropoda melanoleuca). J. Zoo Wildl. Med. 2007, 38, 363–366. [Google Scholar] [CrossRef]
- Geng, Y.; Shen, F.; Wu, W.; Zhang, L.; Luo, L.; Fan, Z.; Hou, R.; Yue, B.; Zhang, X. First demonstration of giant panda’s immune response to canine distemper vaccine. Dev. Comp. Immunol. 2020, 102, 103489. [Google Scholar] [CrossRef]
- Dai, Z.; Wang, H.; Feng, Z.; Ma, L.; Yang, S.; Shen, Q.; Wang, X.; Zhou, T.; Zhang, W. Identification of a novel circovirus in blood sample of giant pandas (Ailuropoda melanoleuca). Infect. Genet. Evol. 2021, 95, 105077. [Google Scholar] [CrossRef]
- Zhang, J.; Daszak, P.; Huang, H.; Yang, G.; Kilpatrick, A.M.; Zhang, S. Parasite threat to panda conservation. EcoHealth 2008, 5, 6–9. [Google Scholar] [CrossRef]
- Zhu, Y.; Grueber, C.; Li, Y.; He, M.; Hu, L.; He, K.; Liu, H.; Zhang, H.; Wu, H. Mhc-associated baylisascaris schroederi load informs the giant panda reintroduction program. Int. J. Parasitol. Parasites Wildl. 2020, 12, 113–120. [Google Scholar] [CrossRef]
- Qin, Q.; Li, D.; Zhang, H.; Hou, R.; Zhang, Z.; Zhang, C.; Zhang, J.; Wei, F. Serosurvey of selected viruses in captive giant pandas (Ailuropoda melanoleuca) in China. Vet. Microbiol. 2010, 142, 199–204. [Google Scholar] [CrossRef] [PubMed]
- IUCN. Guidelines for Reintroductions and Other Conservation Translocations; IUCN Species Survival Commission: Gland, Switzerland, 2013; 57p. [Google Scholar]
- Zhan, X.J.; Zhang, Z.J.; Wu, H.; Goossens, B.; Li, M.; Jiang, S.W.; Bruford, M.W.; Wei, F.W. Molecular analysis of dispersal in giant pandas. Mol. Ecol. 2007, 16, 3792–3800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Swaisgood, R.R.; Wu, H.; Li, M.; Yong, Y.; Hu, J.; Wei, F. Factors predicting den use by maternal giant pandas. J. Wildl. Manag. 2007, 71, 2694–2698. [Google Scholar] [CrossRef]
- Howe, R.W.; Davis, G.J.; Mosca, V. The demographic significance of ‘sink’ populations. Biol. Conserv. 1991, 57, 239–255. [Google Scholar] [CrossRef]
- Hong, M.S.; Wei, W.; Zhou, H.; Tang, J.F.; Han, H.; Zhang, Z.J. Creative conservation in china: Releasing captive giant pandas into the wild. Environ. Sci. Pollut. Res. 2019, 26, 31548–31549. [Google Scholar] [CrossRef]
- Swaisgood, R.R.; Lindburg, D.G.; White, A.M.; Hemin, Z.; Xiaoping, Z. Chemical communication in giant pandas. Giant Pandas Biol. Conserv. 2004, 106. [Google Scholar] [CrossRef]
- Nie, Y.; Swaisgood, R.R.; Zhang, Z.; Hu, Y.; Ma, Y.; Wei, F. Giant panda scent-marking strategies in the wild: Role of season, sex and marking surface. Anim. Behav. 2012, 84, 39–44. [Google Scholar] [CrossRef]
- Zhou, W.; Nie, Y.; Hu, Y.; Swaisgood, R.R.; Zhang, Y.; Liu, D.; Wei, F. Seasonal and reproductive variation in chemical constituents of scent signals in wild giant pandas. Sci. China Life Sci. 2019, 62, 648–660. [Google Scholar] [CrossRef]
- Li, S.; Mcshea, W.J.; Wang, D.J.; Gu, X.D.; Zhang, X.F.; Zhang, L.; Shen, X.L. Retreat of large carnivores across the giant panda distribution range. Nat. Ecol. Evol. 2020, 4, 1327. [Google Scholar] [CrossRef] [PubMed]
- Griffin, A.S.; Blumstein, D.T.; Evans, C.S. Training captive-bred or translocated animals to avoid predators. Conserv. Biol. 2000, 14, 1317–1326. [Google Scholar] [CrossRef]
- Du, Y.; Huang, Y.; Zhang, H.; Li, D.; Yang, B.; Wei, M.; Zhou, Y.; Liu, Y. Innate predator recognition in giant pandas. Zool. Sci. 2012, 29, 67–70. [Google Scholar] [CrossRef]
- van Heezik, Y.; Seddon, P.J.; Maloney, R.F. Helping reintroduced houbara bustards avoid predation: Effective anti-predator training and the predictive value of pre-release behaviour. Anim. Conserv. Forum 1999, 2, 155–163. [Google Scholar] [CrossRef]
- Wei, F.; Hu, Y.; Zhu, L.; Bruford, M.W.; Zhan, X.; Zhang, L. Black and white and read all over: The past, present and future of giant panda genetics. Mol. Ecol. 2012, 21, 5660–5674. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Zhang, Z.; He, W.; Yue, B.; Zhang, A.; Zhang, L.; Hou, R.; Wang, C.; Watanabe, T. Microsatellite variability reveals the necessity for genetic input from wild giant pandas (Ailuropoda melanoleuca) into the captive population. Mol. Ecol. 2009, 18, 1061–1070. [Google Scholar] [CrossRef]
- Frankham, R.; Ballou, S.E.J.D.; Briscoe, D.A.; Ballou, J.D. Introduction to Conservation Genetics; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Tallmon, D.A.; Luikart, G.; Waples, R.S. The alluring simplicity and complex reality of genetic rescue. Trends Ecol. Evol. 2004, 19, 489–496. [Google Scholar] [CrossRef]
- Shan, L.; Hu, Y.; Zhu, L.; Yan, L.; Wang, C.; Li, D.; Jin, X.; Zhang, C.; Wei, F. Large-scale genetic survey provides insights into the captive management and reintroduction of giant pandas. Mol. Biol. Evol. 2014, 31, 2663–2671. [Google Scholar] [CrossRef]
- Zhao, S.C.; Zheng, P.P.; Dong, S.S.; Zhan, X.J.; Wu, Q.; Guo, X.S.; Hu, Y.B.; He, W.M.; Zhang, S.N.; Fan, W.; et al. Whole-genome sequencing of giant pandas provides insights into demographic history and local adaptation. Nat. Genet. 2013, 45, 67–99. [Google Scholar] [CrossRef]
- Kleiman, D.G. Reintroduction of captive mammals for conservation. Bioscience 1989, 39, 152–161. [Google Scholar] [CrossRef]
- Yao, R.; Xu, L.; Hu, T.; Chen, H.; Qi, D.; Gu, X.; Yang, X.; Yang, Z.; Zhu, L. The “wildness” of the giant panda gut microbiome and its relevance to effective translocation. Glob. Ecol. Conserv. 2019, 18, e644. [Google Scholar] [CrossRef]
- Zhang, M.C.; Huang, Y.; Hong, M.S.; Zhou, S.Q.; Huang, J.Y.; Li, D.S.; Li, R.G.; Liu, D.; Zhou, X.P.; Zhang, H.M. Impacts of man-made provisioned food on learned cub behaviours of giant pandas in pre-release reintroduction training. Folia Zool. 2017, 66, 58–66. [Google Scholar] [CrossRef]
- Wolf, C.M.; Garland, T., Jr.; Griffith, B. Predictors of avian and mammalian translocation success: Reanalysis with phylogenetically independent contrasts. Biol. Conserv. 1998, 86, 243–255. [Google Scholar] [CrossRef]
- Wang, L.; Ding, R.Z.; Zhai, Y.H.; Zhang, Q.L.; Tang, W.; Zheng, N.N.; Hua, G. Giant panda identification. IEEE Trans. Image Process. 2021, 30, 2837–2849. [Google Scholar] [CrossRef]
- Zhu, Z.; Pan, S.; Wei, B.; Liu, H.; Zhou, Z.; Huang, X.; Luo, Y.; Zhou, L.; Zhang, S.; Ma, X.; et al. High prevalence of multi-drug resistances and diversity of mobile genetic elements in escherichia coli isolates from captive giant pandas. Ecotoxicol. Environ. Saf. 2020, 198, 110681. [Google Scholar] [CrossRef]
- Wei, W.; Swaisgood, R.R.; Dai, Q.; Yang, Z.; Yuan, S.; Owen, M.A.; Pilfold, N.W.; Yang, X.; Gu, X.; Zhou, H.; et al. Giant panda distributional and habitat-use shifts in a changing landscape. Conserv. Lett. 2018, 11, e12575. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, T.; Deng, S.; Zang, Z.; Zhao, Z.; Xie, Z.; Xu, W.; Shen, G. Forest-cover change rather than climate change determined giant panda’s population persistence. Biol. Conserv. 2022, 265, 109436. [Google Scholar] [CrossRef]
- Wei, W.; Nie, Y.; Zhang, Z.; Hu, Y.; Yan, L.; Qi, D.; Li, X.; Wei, F. Hunting bamboo: Foraging patch selection and utilization by giant pandas and implications for conservation. Biol. Conserv. 2015, 186, 260–267. [Google Scholar] [CrossRef]
- Wei, W.; Swaisgood, R.R.; Owen, M.A.; Pilfold, N.W.; Han, H.; Hong, M.; Zhou, H.; Wei, F.; Nie, Y.; Zhang, Z. The role of den quality in giant panda conservation. Biol. Conserv. 2019, 231, 189–196. [Google Scholar] [CrossRef]
- Sun, X.; Long, Z.; Jia, J. A multi-scale maxent approach to model habitat suitability for the giant pandas in the qionglai mountain, china. Glob. Ecol. Conserv. 2021, 30, e1766. [Google Scholar] [CrossRef]
- Morris, S.D.; Brook, B.W.; Moseby, K.E.; Johnson, C.N. Factors affecting success of conservation translocations of terrestrial vertebrates: A global systematic review. Glob. Ecol. Conserv. 2021, 28, e1630. [Google Scholar] [CrossRef]
- Mccarthy, M.A.; Armstrong, D.P.; Runge, M.C. Adaptive management of reintroduction. Reintroduction Biol. Integr. Sci. Manag. 2012, 12, 256. [Google Scholar]
- Allen, C.R.; Fontaine, J.J.; Pope, K.L.; Garmestani, A.S. Adaptive management for a turbulent future. J. Environ. Manag. 2011, 92, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
| Name | Gender | Source | Release Year | Release Site | Survival Status |
|---|---|---|---|---|---|
| Shenglin 1 | Female | Wild-born | 2005 | Longxi-hongkou NR | Still alive |
| Xiangxiang | Male | Captive-born | 2006 | Wolong NR | Died (fights and injuries) |
| Luxin | Female | Wild-born | 2009 | Liziping NR | Still alive (produced offspring) |
| Taotao | Male | Captive-born | 2012 | Liziping NR | Still alive |
| Zhangxiang | Female | Captive-born | 2013 | Liziping NR | Still alive |
| Xuexue | Female | Captive-born | 2014 | Liziping NR | Died (conditional pathogenic infections) |
| Huajiao | Female | Captive-born | 2015 | Liziping NR | Still alive |
| Hesheng | Male | Captive-born | 2016 | Liziping NR | Died (attacked by unknown animal) |
| Huayan | Female | Captive-born | 2016 | Liziping NR | Still alive |
| Zhangmeng | Female | Captive-born | 2016 | Liziping NR | Still alive |
| Yingxue | Female | Captive-born | 2017 | Liziping NR | Still alive |
| Baxi | Male | Captive-born | 2017 | Liziping NR | Still alive |
| Qinxin | Female | Captive-born | 2018 | Longxi-hongkou NR | Still alive |
| Xiaohetao | Female | Captive-born | 2018 | Longxi-hongkou NR | Still alive |
| Tangtang | Female | Wild-born | 2021 | Foping NR | Still alive |
| Research Topic | Number of Papers from Different Sources | ||
|---|---|---|---|
| Science Direct | Web of Science | CNKI | |
| Wild giant panda | 1471 | 282 | 653 |
| Captive giant panda | 551 | 234 | 637 |
| Giant panda | 3330 | 1398 | 9462 |
| Giant panda population | 2036 | 347 | 1144 |
| Giant panda landscape | 625 | 98 | 46 |
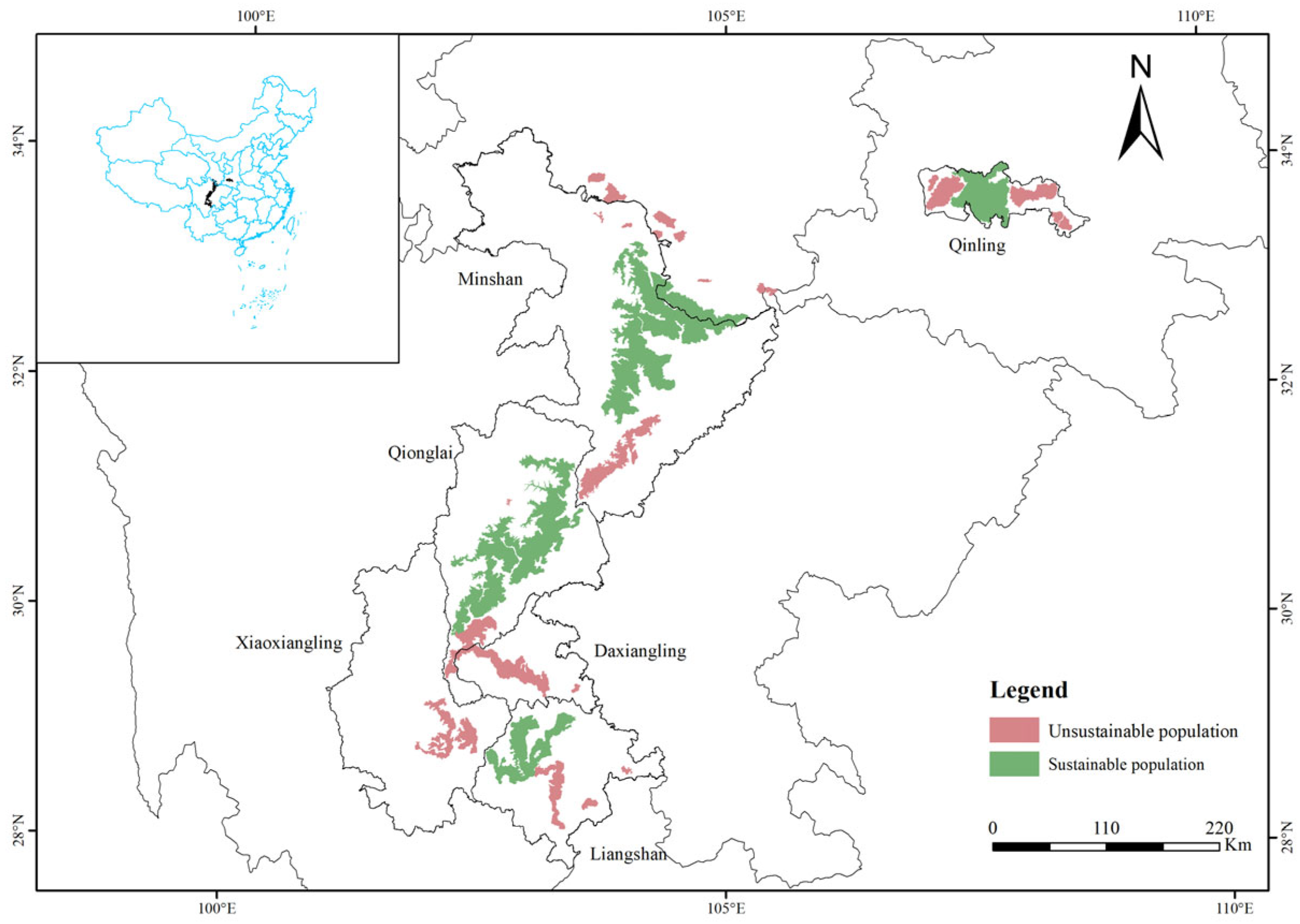
| Mountain Ranges | Populations Name | Number of Extant Giant Pandas | Habitat Area (km2) | Suggested Mitigation Measures |
|---|---|---|---|---|
| Qinling | Qinling A | 7 | 211.41 | 1 |
| Qinling B | 20 | 594.22 | 1 | |
| Qinling D | 36 | 641.01 | 1 | |
| Qinling E | 3 | 90.47 | 1 + 2 | |
| Qinling F | 4 | 128.45 | 1 + 2 | |
| Minshan | Minshan A | 4 | 135.93 | 1 + 2 |
| Minshan B | 9 | 238.81 | 1 | |
| Minshan C | 3 | 204.89 | 1 | |
| Minshan D | 1 | 56.00 | 2 | |
| Minshan E | 2 | 93.39 | 2 | |
| Minshan F | 2 | 36.07 | 2 | |
| Minshan H | 2 | 34.06 | 2 | |
| Minshan I | 1 | 33.69 | 2 | |
| Minshan L | 35 | 1363.99 | 1 | |
| Qionglai | Qionglai D | 29 | 800.44 | 1 |
| Qionglai E | 1 | 17.27 | 2 | |
| Daxiangling | Daxiangling A | 4 | 205.75 | 1 |
| Daxiangling B | 32 | 979.16 | 1 | |
| Daxiangling C | 2 | 43.78 | 2 | |
| Xiaoxiangling | Xiaoxiangling A | 21 | 442.31 | 1 |
| Xiaoxiangling B | 9 | 751.33 | 1 | |
| Liangshan | Liangshan B | 22 | 528.41 | 1 |
| Liangshan C | 3 | 173.60 | 1 | |
| Liangshan D | 4 | 107.59 | 1 + 2 | |
| Liangshan E | 3 | 48.12 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wei, W.; Yuan, F.; Cao, D.; Zhang, Z. The Science Underlying Giant Panda Conservation Translocations. Animals 2023, 13, 3332. https://doi.org/10.3390/ani13213332
Wang Y, Wei W, Yuan F, Cao D, Zhang Z. The Science Underlying Giant Panda Conservation Translocations. Animals. 2023; 13(21):3332. https://doi.org/10.3390/ani13213332
Chicago/Turabian StyleWang, Yue, Wei Wei, Feiyun Yuan, Dandan Cao, and Zejun Zhang. 2023. "The Science Underlying Giant Panda Conservation Translocations" Animals 13, no. 21: 3332. https://doi.org/10.3390/ani13213332
APA StyleWang, Y., Wei, W., Yuan, F., Cao, D., & Zhang, Z. (2023). The Science Underlying Giant Panda Conservation Translocations. Animals, 13(21), 3332. https://doi.org/10.3390/ani13213332





