Canine Gallbladder Erosion/Ulcer and Hemocholecyst: Clinicopathological Characteristics of 14 Cases
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cases
2.2. Histopathology
2.3. Histochemistry
2.4. Immunohistochemistry
3. Results
3.1. Signalment, Duration between Clinical Onset and Cholecystectomy, Presence/Absence of Rupture, and Chief Complaint
3.2. Ultrasonography, Complete Blood Count, Blood Chemistry, and Lesions in Other Organs
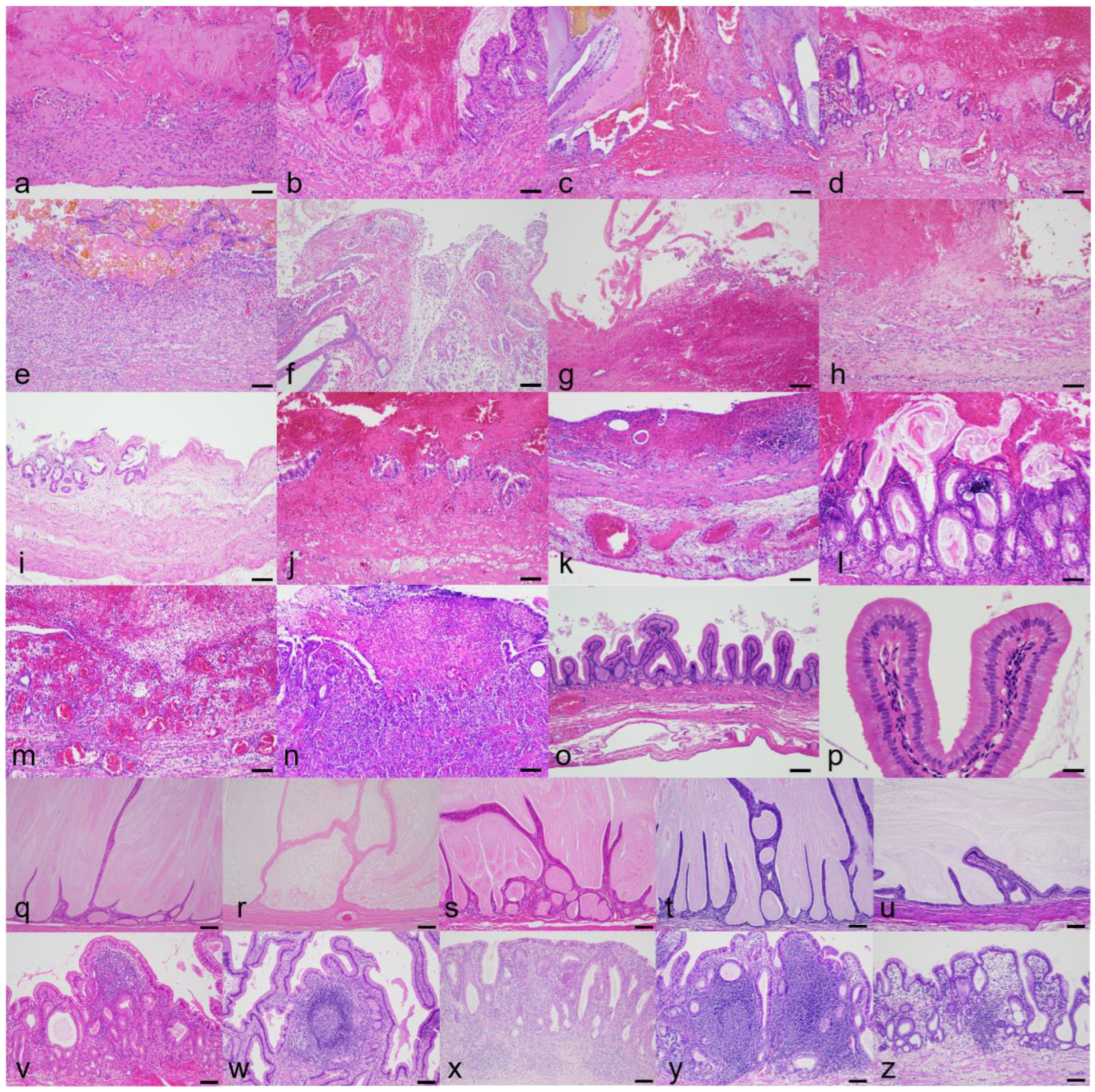
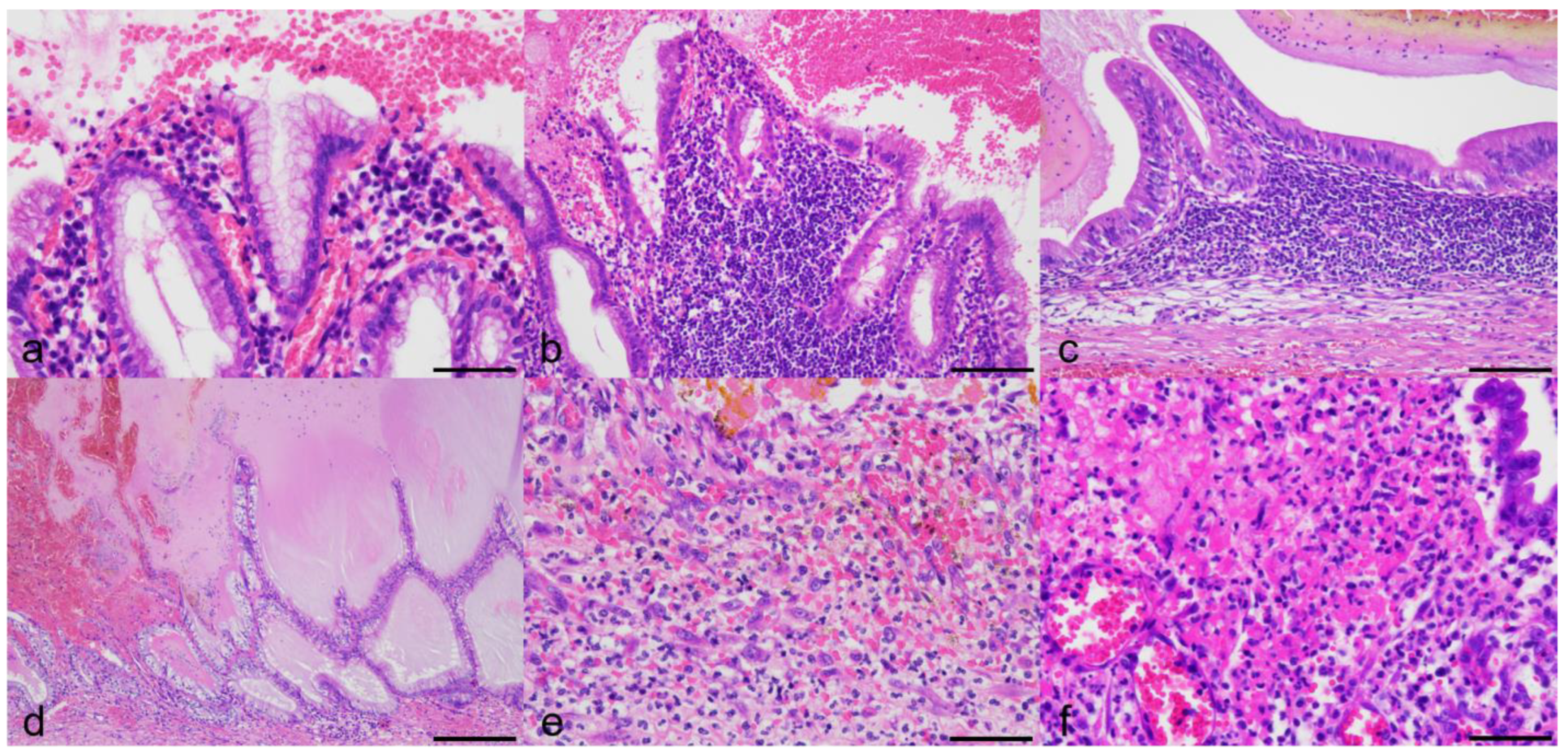
3.3. Histologic Findings
3.4. Bacterial Detection and Chemical Property of Mucus
3.5. Expression of COX-1 and COX-2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dooley, J.S.; Gurusamy, K.S.; Davidson, B.R. Chapter 14: Gallstones and Benign Biliary Disease. In Sherlock’s Diseases of the Liver and Biliary System, 13th ed.; Dooley, J.S., Lok, A.S.F., Garcia-Tsao, G., Pinzani, M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Jivegård, L.; Thornell, E.; Svanvik, J. Fluid secretion by gallbladder mucosa in experimental cholecystitis is influenced by intramural nerves. Dig. Dis. Sci. 1987, 32, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.M.; Brown, K.; Hammond, G.; Parkin, T.; Bouyssou, S.; Coia, M.; Nurra, G.; Ridyard, A.E. Cholelithiasis in the Dog: Prevalence, Clinical Presentation, and Outcome. J. Am. Anim. Hosp. Assoc. 2020, 56, 152. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.L.; Turek, B.J.; Brown, D.C.; Bradley, C.; Callahan Clark, J. Cholangitis and Cholangiohepatitis in Dogs: A Descriptive Study of 54 Cases Based on Histopathologic Diagnosis (2004–2014). J. Vet. Intern. Med. 2018, 32, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Crews, L.J.; Feeney, D.A.; Jessen, C.R.; Rose, N.D.; Matise, I. Clinical, ultrasonographic, and laboratory findings associated with gallbladder disease and rupture in dogs: 45 cases (1997–2007). J. Am. Vet. Med. Assoc. 2009, 234, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.M.; Stalker, M.J. Chapter 2: Liver and Biliary System. In Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals, 6th ed.; Maxie, M.G., Ed.; Elsevier Inc.: St. Louis, MO, USA, 2016; Volume 2. [Google Scholar]
- Gookin, J.L.; Correa, M.T.; Peters, A.; Malueg, A.; Mathews, K.G.; Cullen, J.; Seiler, G. Association of Gallbladder Mucocele Histologic Diagnosis with Selected Drug Use in Dogs: A Matched Case-Control Study. J. Vet. Intern. Med. 2015, 29, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Gookin, J.L.; Mathews, K.G.; Cullen, J.; Seiler, G. Qualitative metabolomics profiling of serum and bile from dogs with gallbladder mucocele formation. PLoS ONE 2018, 13, e0191076. [Google Scholar] [CrossRef]
- Kesimer, M.; Cullen, J.; Cao, R.; Radicioni, G.; Mathews, K.G.; Seiler, G.; Gookin, J.L. Excess Secretion of Gel-Forming Mucins and Associated Innate Defense Proteins with Defective Mucin Un-Packaging Underpin Gallbladder Mucocele Formation in Dogs. PLoS ONE 2015, 10, e0138988. [Google Scholar] [CrossRef]
- Lawrence, Y.A.; Ruaux, C.G.; Nemanic, S.; Milovancev, M. Characterization, treatment, and outcome of bacterial cholecystitis and bactibilia in dogs. J. Am. Vet. Med. Assoc. 2015, 246, 982–989. [Google Scholar] [CrossRef]
- Mitsui, I.; Ohtsuki, S.; Uchida, K. Chronic Cholecystitis of Dogs: Clinicopathologic Features and Relationship with Liver. Animals 2021, 11, 3324. [Google Scholar] [CrossRef]
- Pike, F.S.; Berg, J.; King, N.W.; Penninck, D.G.; Webster, C.R. Gallbladder mucocele in dogs: 30 cases (2000–2002). J. Am. Vet. Med. Assoc. 2004, 224, 1615–1622. [Google Scholar] [CrossRef]
- Rogers, E.; Jaffey, J.A.; Graham, A.; Hostnik, E.T.; Jacobs, C.; Fox-Alvarez, W.; Van Eerde, E.; Arango, J.; Williams, F.; DeClue, A.E. Prevalence and impact of cholecystitis on outcome in dogs with gallbladder mucocele. J. Vet. Emerg. Crit. Care 2020, 30, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.D.; Tamborini, A.; Watson, P.J.; Bexfield, N.H. Clinical characteristics and histology of cholecystectomised dogs with nongravity-dependent biliary sludge: 16 cases (2014–2019). J Small Anim. Pract. 2021, 62, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.J. Hemocholecyst: A neglected cause of gastrointestinal hemorrhage. Ann. Intern. Med. 1961, 55, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.W.; Enns, R. Hemobilia. Curr. Gastroenterol. Rep. 2010, 12, 121–129. [Google Scholar] [CrossRef]
- Lippo, N.J.; Williams, J.E.; Brawer, R.S.; Sobel, K.E. Acute hemobilia and hemocholecyst in 2 dogs with gallbladder carcinoid. J. Vet. Intern. Med. 2008, 22, 1249–1252. [Google Scholar] [CrossRef]
- Holt, D.E.; Mehler, S.; Mayhew, P.D.; Hendrick, M.J. Canine gallbladder infarction: 12 cases (1993–2003). Vet. Pathol. 2004, 41, 416–418. [Google Scholar] [CrossRef]
- Parekh, J.; Corvera, C.U. Hemorrhagic cholecystitis. Arch. Surg. 2010, 145, 202–204. [Google Scholar] [CrossRef]
- Tarazi, M.; Tomalieh, F.T.; Sweeney, A.; Sumner, D.; Abdulaal, Y. Literature review and case series of haemorrhagic cholecystitis. J. Surg. Case Rep. 2019, 2019, rjy360. [Google Scholar] [CrossRef]
- Yap, C.R.; Puri, R. A Rare Case of Acute Pancreatitis in the Setting of Hemorrhagic Cholecystitis. Cureus 2022, 14, e22546. [Google Scholar] [CrossRef]
- Jessurun, J.; Pambuccian, S. Chapter 37: Infectious and Inflammatory Disorders of the Gallbladder and Extrahepatic Biliary Tract. In Surgical Pathology of the GI Tract, Liver, Biliary Tract, and Pancreas, 3rd ed.; Odze, R.D., Goldblum, J.R., Eds.; Elsevier Inc.: Philadelphia, PA, USA, 2015. [Google Scholar]
- Jivegård, L.; Thune, A.; Svanvik, J. Intraluminal prostaglandin E2 affects gallbladder function by activation of intramural nerves in the anaesthetized cat. Acta Physiol. Scand. 1988, 132, 549–555. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.K.; Kim, M.H.; Seo, D.W.; Min, Y.I. Cyclooxygenase-2 mediates mucin secretion from epithelial cells of lipopolysaccharide-treated canine gallbladder. Dig. Dis. Sci. 2003, 48, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Kuver, R.; Savard, C.; Oda, D.; Lee, S.P. PGE generates intracellular cAMP and accelerates mucin secretion by cultured dog gallbladder epithelial cells. Am. J. Physiol. 1994, 267, G998–G1003. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, B.; Delbro, D.; Hedin, L.; Friman, S.; Andius, S.; Svanvik, J. Role of cyclooxygenase-2 for fluid secretion by the inflamed gallbladder mucosa. J. Gastrointest. Surg. 1998, 2, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Jivegård, L.; Thornell, E.; Björck, S.; Svanvik, J. The effects of morphine and enkephaline on gallbladder function in experimental cholecystitis. Inhibition of inflammatory gallbladder secretion. Scand. J. Gastroenterol. 1985, 20, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Longo, W.E.; Panesar, N.; Mazuski, J.E.; Kaminski, D. Synthetic pathways of gallbladder mucosal prostanoids: The role of cyclooxygenase-1 and 2. Prostaglandins Leukot Essent Fat. Acids 1999, 60, 77–85. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Chapter 3: Inflammation and Repair. In Robbins & Cotran Pathologic Basis of Disease, 10th ed.; Kumar, V., Abbas, A.K., Aster, J.C., Eds.; Elsevier Inc.: Philadelphia, PA, USA, 2021. [Google Scholar]
- Miratashi Yazdi, S.A.; Nazar, E.; Vesali, B. IgG4-related cholecystitis misinterpreted as gallbladder cancer, a case report. Ann. Med. Surg. 2022, 77, 103615. [Google Scholar] [CrossRef]
- Porter, K.R.; Singh, C.; Neychev, V. Endosalpingiosis of the Gallbladder: A Unique Complication of Ruptured Ectopic Pregnancy. Cureus 2019, 11, e5393. [Google Scholar] [CrossRef]
- Share of Pet Dogs Castrated or Spayed in Japan as of October 2022. Statista. Available online: https://www.statista.com/statistics/1252986/japan-pet-dogs-neutering-ratio/ (accessed on 5 April 2023).
- Tamborini, A.; Jahns, H.; McAllister, H.; Kent, A.; Harris, B.; Procoli, F.; Allenspach, K.; Hall, E.; Day, M.; Watson, P.; et al. Bacterial Cholangitis, Cholecystitis, or both in Dogs. J. Vet. Intern. Med. 2016, 30, 1046–1055. [Google Scholar] [CrossRef]
- Aguirre, A. Chapter 288: Diseases of the Gallbladder and Extrahepatic Biliary System. In Textbook of Veterinary Internal Medicine, 8th ed.; Ettinger, S.J., Feldman, E.C., Côté, Eds.; Elsevier Inc.: St. Louis, MO, USA, 2017; Volume 2. [Google Scholar]
- Davies, N.H.; Yu, D. Chapter 34: Imaging of the Liver and Diagnostic Approach of Space-Occupying Lesions. In Sherlock’s Diseases of the Liver and Biliary System, 13th ed.; Dooley, J.S., Lok, A.S.F., Garcia-Tsao, G., Pinzani, M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Katta, T.; Tavakoli, K. Necrotizing Cholecystitis in the Gallbladder: A Case Report. Cureus 2022, 14, e21368. [Google Scholar] [CrossRef]
- Jones, M.W.; Gnanapandithan, K.; Panneerselvam, D.; Ferguson, T. Chronic Cholecystitis; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470236/ (accessed on 5 April 2023).
- Kakimoto, T.; Kanemoto, H.; Fukushima, K.; Ohno, K.; Tsujimoto, H. Bile acid composition of gallbladder contents in dogs with gallbladder mucocele and biliary sludge. Am. J. Vet. Res. 2017, 78, 223–229. [Google Scholar] [CrossRef]
- Mizutani, S.; Torisu, S.; Kaneko, Y.; Yamamoto, S.; Fujimoto, S.; Ong, B.H.E.; Naganobu, K. Retrospective analysis of canine gallbladder contents in biliary sludge and gallbladder mucoceles. J. Vet. Med. Sci. 2017, 79, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.; Pan, J.; Zhou, B.; Yu, C.; Li, Y.; Chen, W.; Shen, Z. Helicobacter Pylori infection of the gallbladder and the risk of chronic cholecystitis and cholelithiasis: A systematic review and meta-analysis. Helicobacter 2018, 23, e12457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Guan, W.B.; Wang, J.D.; Zhang, Y.; Gong, W.; Quan, Z.W. A comparative study of clinicopathological features between chronic cholecystitis patients with and without Helicobacter pylori infection in gallbladder mucosa. PLoS ONE 2013, 8, e70265. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, M.; Saito, T.; Ohno, H.; Midorikawa, S.; Sanji, T.; Handa, Y.; Morita, S.; Yoshida, H.; Tsurui, M.; Misaka, R.; et al. Bacteria closely resembling Helicobacter pylori detected immunohistologically and genetically in resected gallbladder mucosa. J. Gastroenterol. 1996, 31, 294–298. [Google Scholar] [CrossRef]
- Patnayak, R.; Reddy, V.; Jena, A.; Gavini, S.; Thota, A.; Nandyala, R.; Chowhan, A.K. Helicobacter pylori in Cholecystectomy Specimens-Morphological and Immunohistochemical Assessment. J. Clin. Diagn. Res. 2016, 10, EC01–EC03. [Google Scholar] [CrossRef]
- Amorim, I.; Smet, A.; Alves, O.; Teixeira, S.; Saraiva, A.L.; Taulescu, M.; Reis, C.; Haesebrouck, F.; Gärtner, F. Presence and significance of Helicobacter spp. in the gastric mucosa of Portuguese dogs. Gut Pathog. 2015, 7, 12. [Google Scholar] [CrossRef]
- Prachasilpchai, W.; Nuanualsuwan, S.; Chatsuwan, T.; Techangamsuwan, S.; Wangnaitham, S.; Sailasuta, A. Diagnosis of Helicobacter spp. infection in canine stomach. J. Vet. Sci. 2007, 8, 139–145. [Google Scholar] [CrossRef]
- Lin, S.J.; Arruda, B.; Burrough, E. Alteration of Colonic Mucin Composition and Cytokine Expression in Acute Swine Dysentery. Vet. Pathol. 2021, 58, 531–541. [Google Scholar] [CrossRef]



| Antibody | Host | Type | Dilution | Source | Catalog Number |
|---|---|---|---|---|---|
| COX-1 | Rabbit | Polyclonal | 1:200 | Proteintech Group, Inc., Rosemont, IL, USA | 13393-1-AP |
| COX-2 | Rabbit | Polyclonal | 1:200 | Proteintech Group, Inc., Rosemont, IL, USA | 12375-1-AP |
| Helicobacter | Rabbit | Polyclonal | RTU a | Nichirei Biosciences Inc., Tokyo, Japan | 413151 |
| Cases | WS d | Giemsa | HID-AB h pH2.5 | COX-1 | COX-2 | Helicobacter |
|---|---|---|---|---|---|---|
| GU a 1 | C f | Mixed | P | |||
| GU2 | C, CB g | Su i | ||||
| GU3 | Mixed | |||||
| GU4 | C, CB | Two-tone j | ||||
| GU5 | R, CB | Si k | ||||
| GU6 | C, CB | Su | P l | P | ||
| GU7 | Su | P | ||||
| GU8 | Su | P | ||||
| GU9 | Unstained | |||||
| GU10 | Su | |||||
| GU11 | Mixed | P | ||||
| GU12 | C | Mixed | ||||
| GU13 | C | Mixed | P | P | ||
| GU14 | C | Two-tone | P | P | ||
| GM b 1 | CB | Mixed | P | P | ||
| GM2 | C, CB | Su | P | |||
| GM3 | C, CB | Mixed | P | P | ||
| GM4 | R e | R | Su | P | P | P |
| GM5 | C, CB | Si | P | P | ||
| CC c 1 | Su | P | P | |||
| CC2 | Su | P | P | |||
| CC3 | Mixed | P | P | |||
| CC4 | Mixed | P | P | |||
| CC5 | Mixed | P | P | |||
| Clinical control | Su |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsui, I.; Uchida, K. Canine Gallbladder Erosion/Ulcer and Hemocholecyst: Clinicopathological Characteristics of 14 Cases. Animals 2023, 13, 3335. https://doi.org/10.3390/ani13213335
Mitsui I, Uchida K. Canine Gallbladder Erosion/Ulcer and Hemocholecyst: Clinicopathological Characteristics of 14 Cases. Animals. 2023; 13(21):3335. https://doi.org/10.3390/ani13213335
Chicago/Turabian StyleMitsui, Ikki, and Kazuyuki Uchida. 2023. "Canine Gallbladder Erosion/Ulcer and Hemocholecyst: Clinicopathological Characteristics of 14 Cases" Animals 13, no. 21: 3335. https://doi.org/10.3390/ani13213335
APA StyleMitsui, I., & Uchida, K. (2023). Canine Gallbladder Erosion/Ulcer and Hemocholecyst: Clinicopathological Characteristics of 14 Cases. Animals, 13(21), 3335. https://doi.org/10.3390/ani13213335





