Ovary of Zebrafish during Spawning Season: Ultrastructure and Immunohistochemical Profiles of Sox9 and Myostatin
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Histological Analysis
2.3. Immunohistochemical Detection
2.4. Semithin Sections and Transmission Electron Microscopy (TEM)
2.5. Digitally Colored TEM Images
2.6. Morphometrical Analysis
3. Results
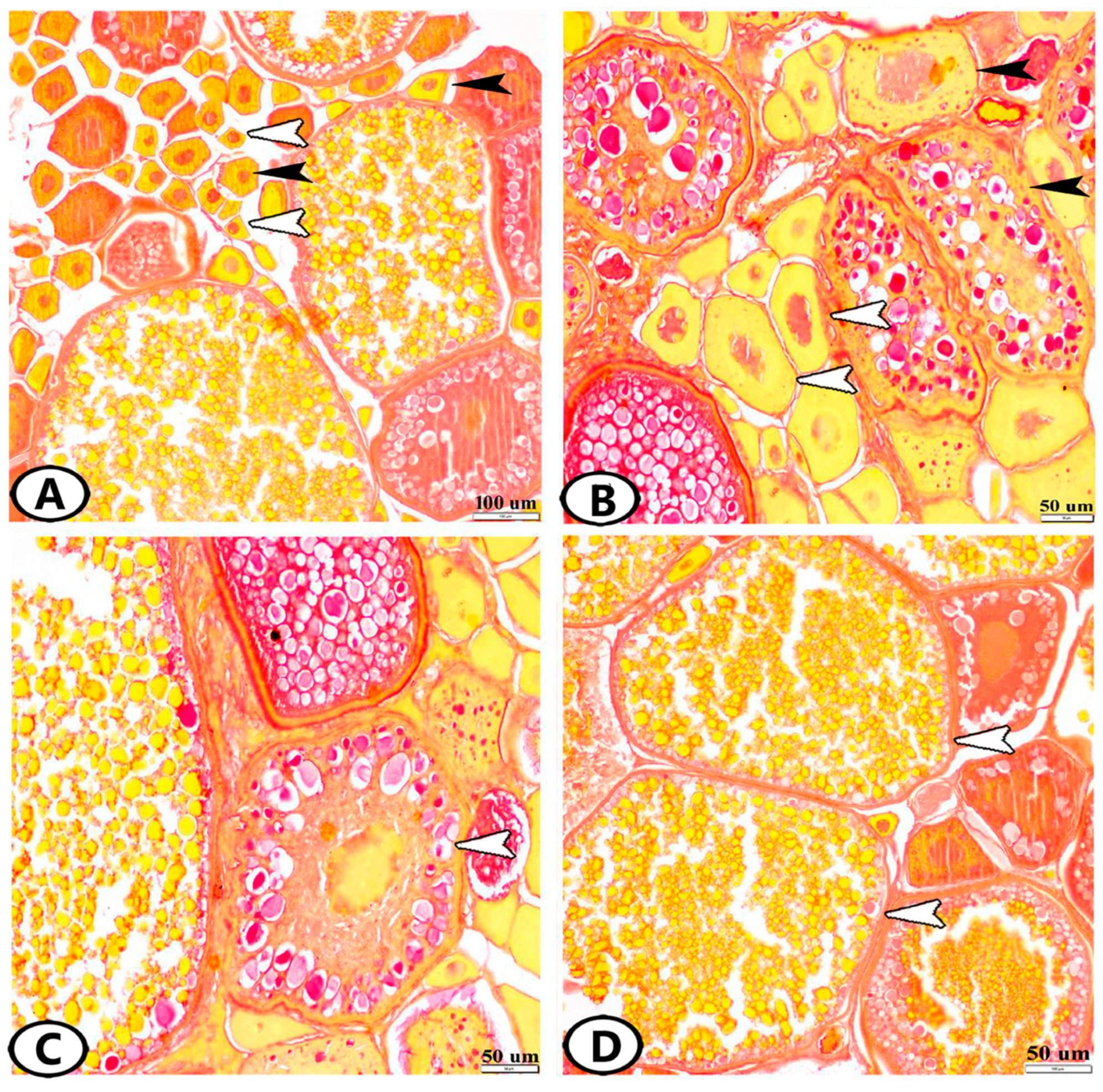
3.1. Histological Analysis
3.1.1. Follicular Development
3.1.2. Atretic Follicles
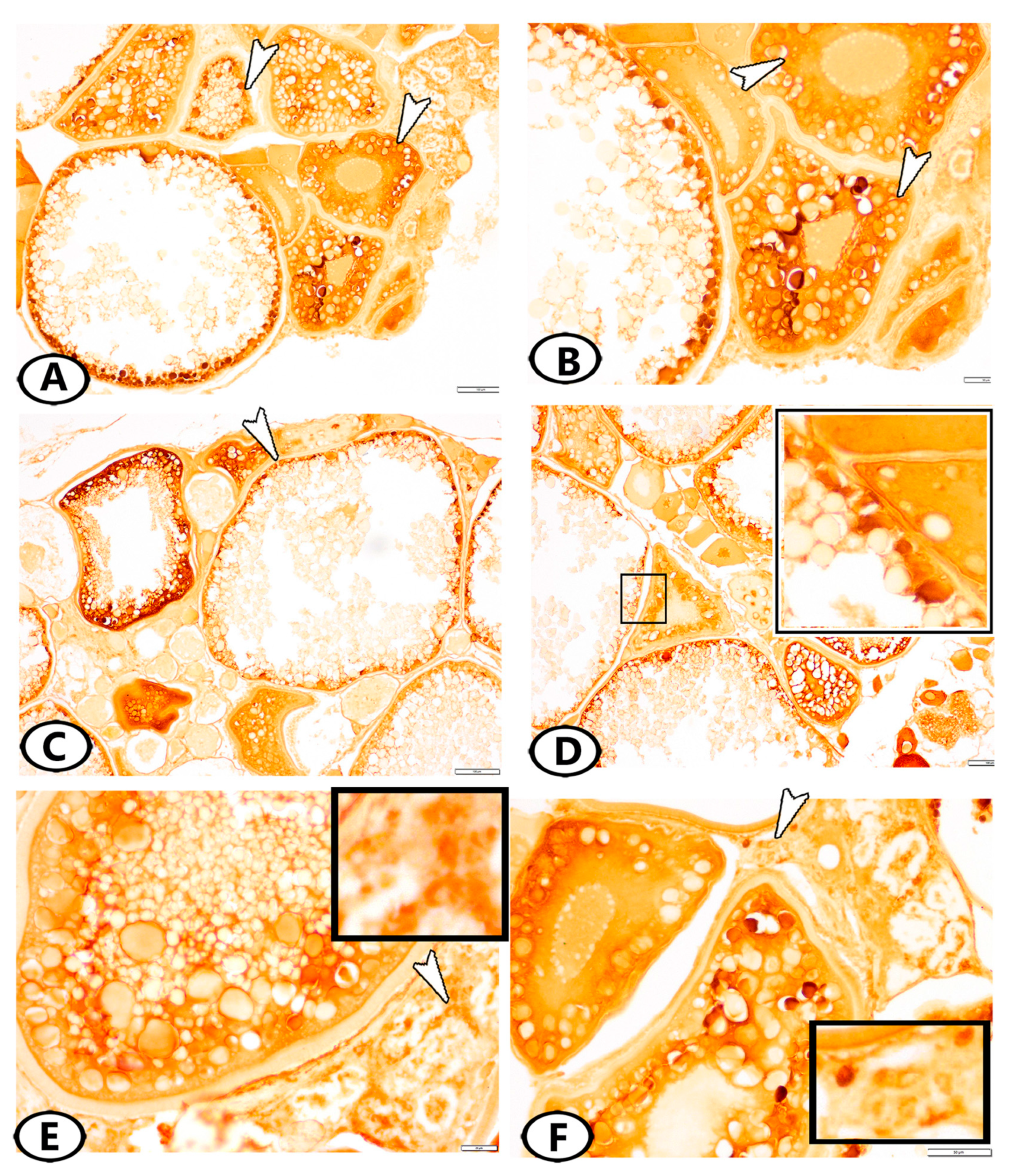
3.2. Immunohistochemistry
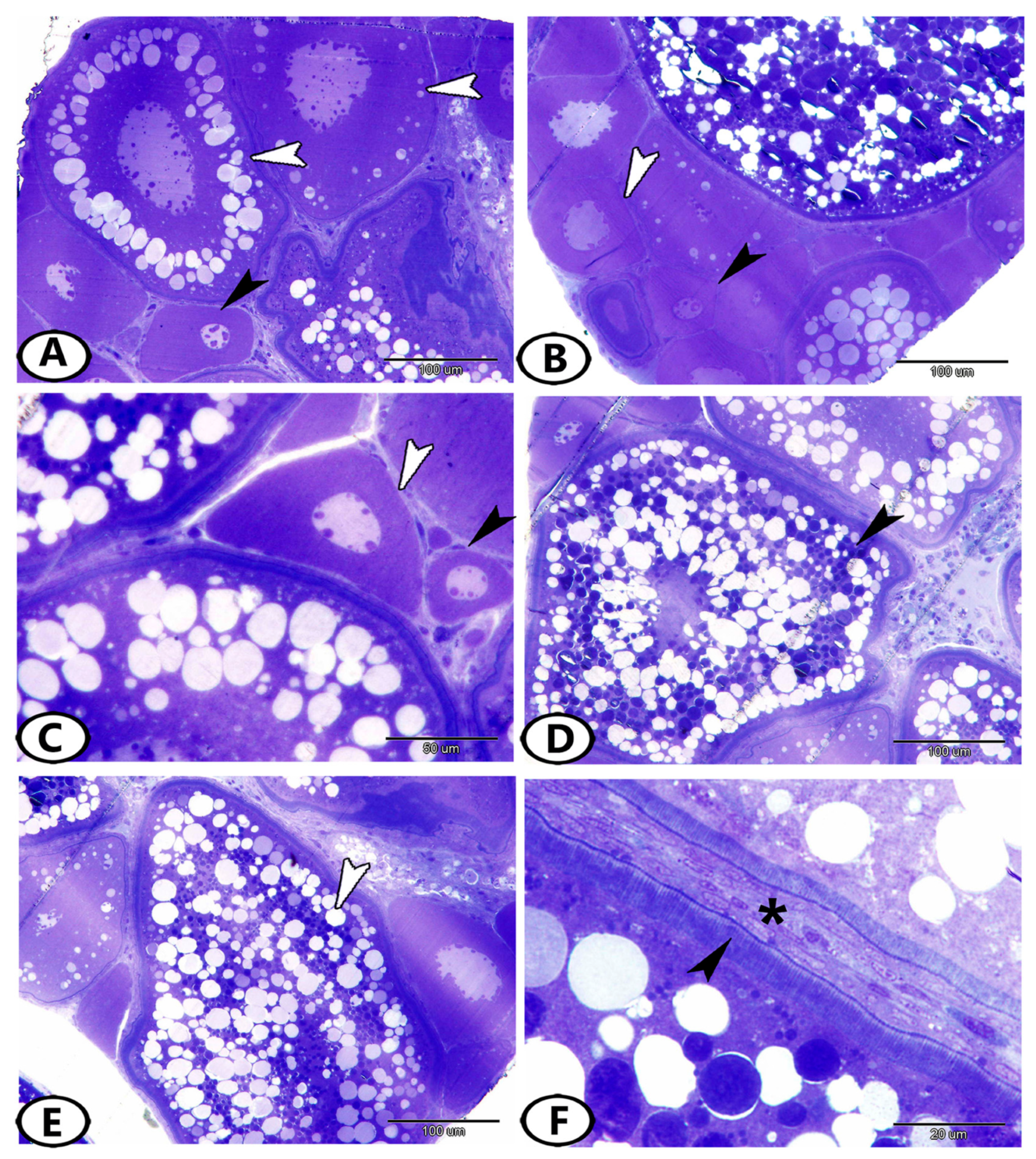
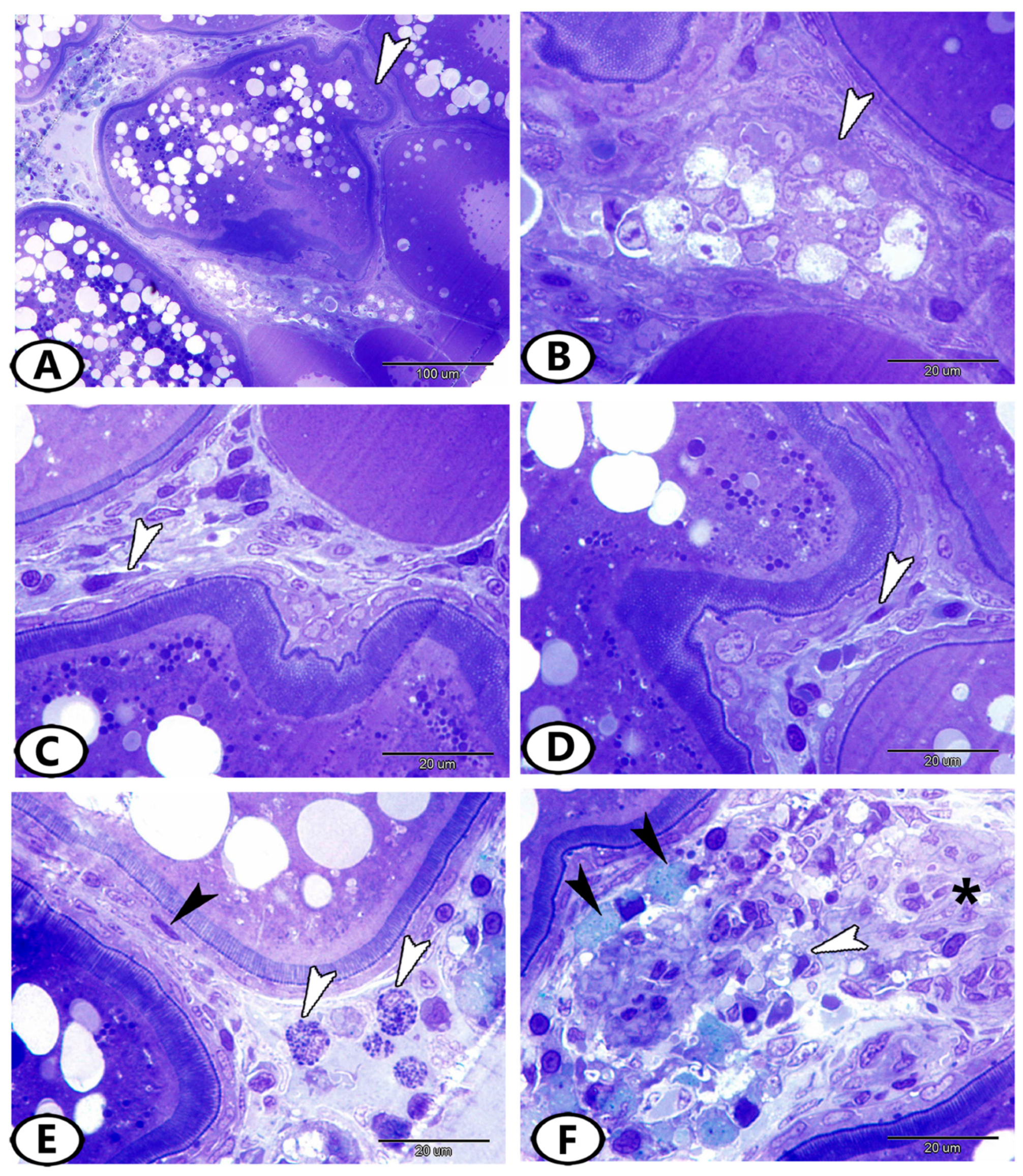
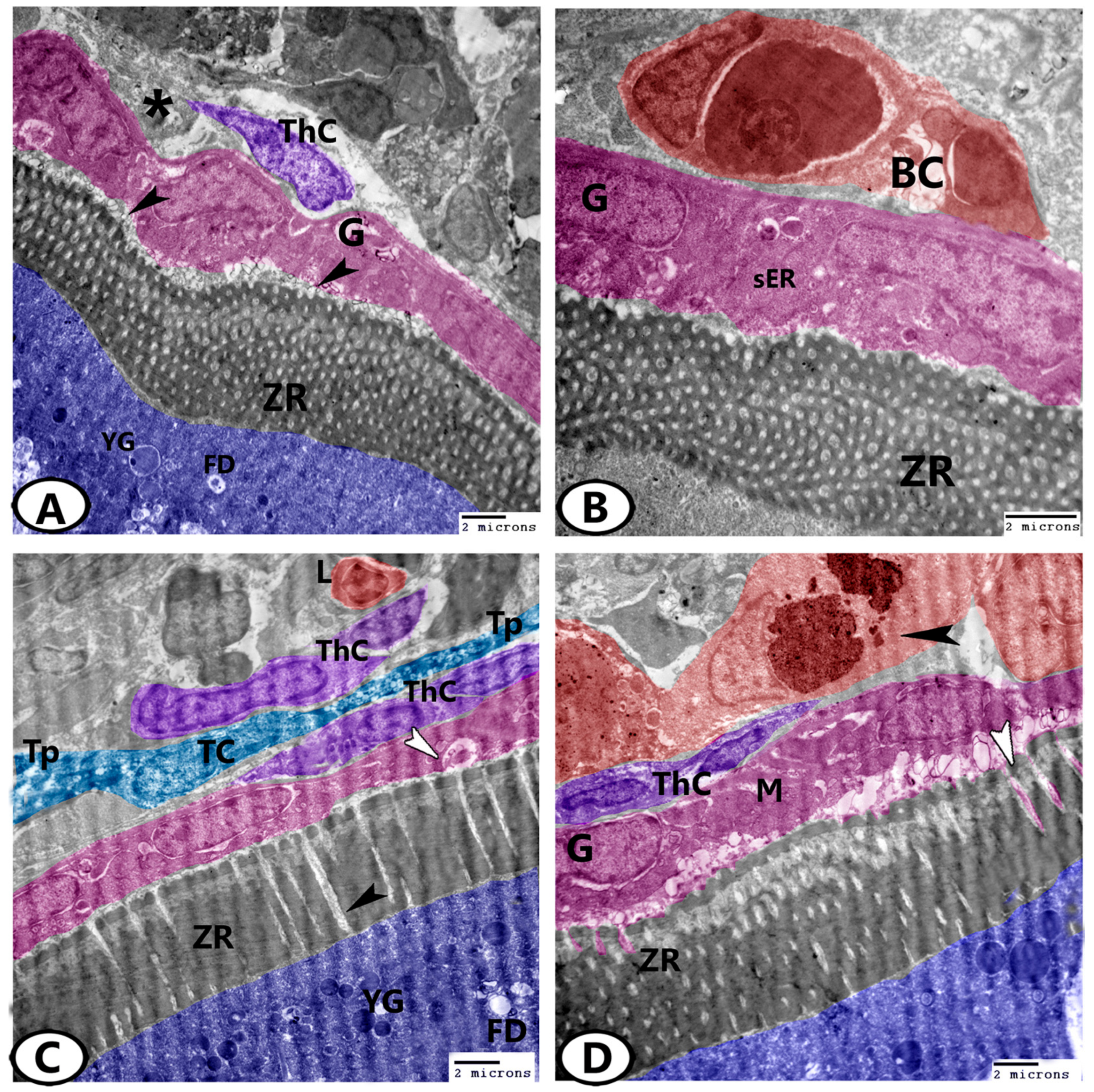
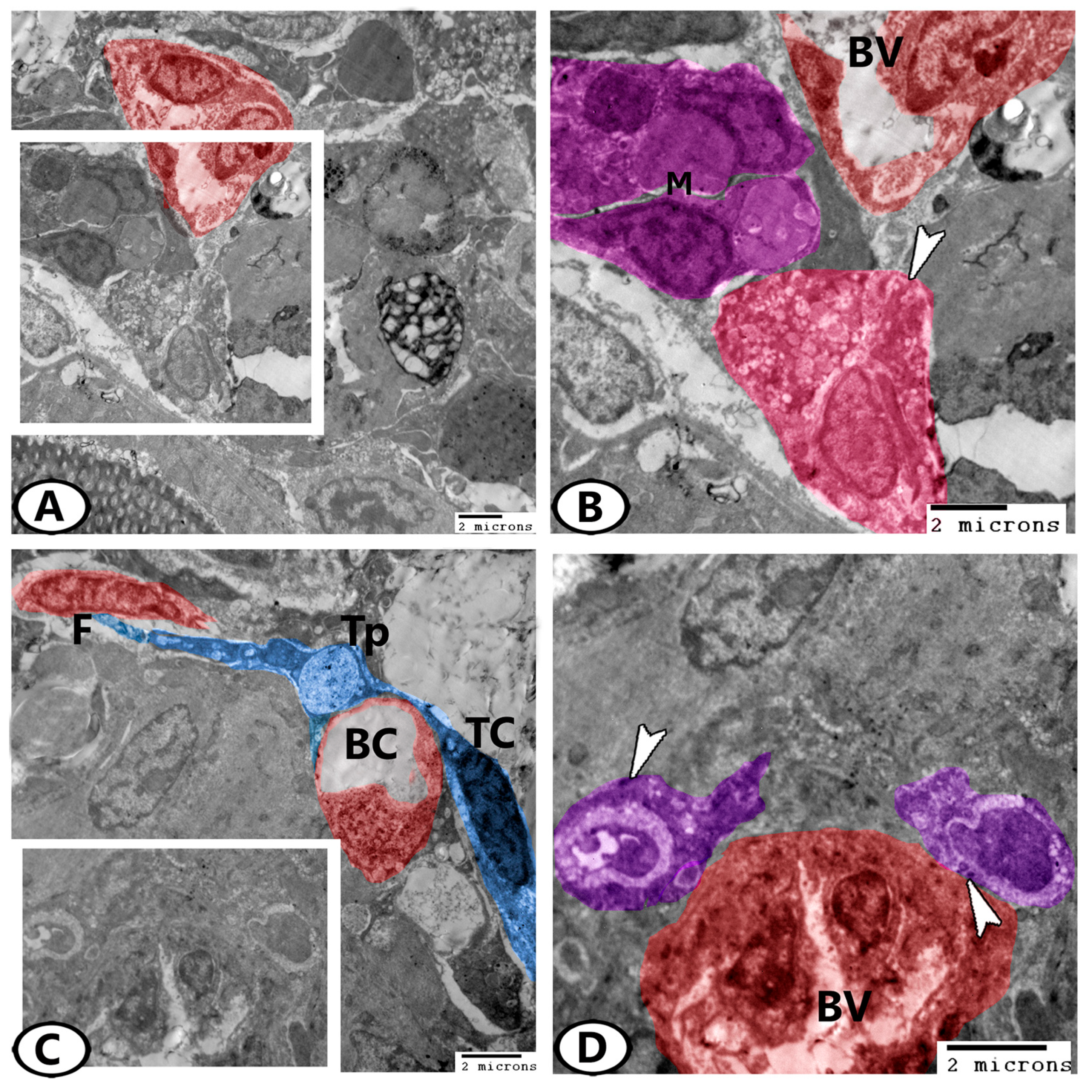
3.3. Transmission Electron Microscopy (TEM)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selman, K.; Wallace, R.A.; Sarka, A.; Qi, X. Stages of oocyte development in the zebrafish, Brachydanio rerio. J. Morphol. 1993, 218, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ge, W. Zebrafish as a model for studying ovarian development: Recent advances from targeted gene knockout studies. Mol. Cell. Endocrinol. 2020, 507, 110778. [Google Scholar] [CrossRef] [PubMed]
- Clelland, E.; Peng, C. Endocrine/paracrine control of zebrafish ovarian development. Mol. Cell. Endocrinol. 2009, 312, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Elkouby, Y.M.; Mullins, M.C. Coordination of cellular differentiation, polarity, mitosis and meiosis–new findings from early vertebrate oogenesis. Dev. Biol. 2017, 430, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhu, Z. Designing future farmed fishes using genome editing. Sci. China Life Sci. 2019, 62, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C. The husbandry of zebrafish (Danio rerio): A review. Aquaculture 2007, 269, 1–20. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Spence, R.; Gerlach, G.; Lawrence, C.; Smith, C. The behaviour and ecology of the zebrafish, Danio rerio. Biol. Rev. 2008, 83, 13–34. [Google Scholar] [CrossRef]
- Nasiadka, A.; Clark, M.D. Zebrafish breeding in the laboratory environment. ILAR J. 2012, 53, 161–168. [Google Scholar] [CrossRef]
- Wafer, L.N.; Jensen, V.B.; Whitney, J.C.; Gomez, T.H.; Flores, R.; Goodwin, B.S. Effects of environmental enrichment on the fertility and fecundity of zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 291–294. [Google Scholar]
- Santos, E.M.; Workman, V.L.; Paull, G.C.; Filby, A.L.; Van Look, K.J.W.; Kille, P.; Tyler, C.R. Molecular basis of sex and reproductive status in breeding zebrafish. Physiol. Genom. 2007, 30, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Klüver, N.; Kondo, M.; Herpin, A.; Mitani, H.; Schartl, M. Divergent expression patterns of Sox9 duplicates in teleosts indicate a lineage specific subfunctionalization. Dev. Genes Evol. 2005, 215, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.F.-L.; Pai, C.-I.; Wyatt, M.; Yan, Y.-L.; Postlethwait, J.; Chung, B.-C. Two sox9 genes on duplicated zebrafish chromosomes: Expression of similar transcription activators in distinct sites. Dev. Biol. 2001, 231, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Østbye, T.K.; Galloway, T.F.; Nielsen, C.; Gabestad, I.; Bardal, T.; Andersen, Ø. The two myostatin genes of Atlantic salmon (Salmo salar) are expressed in a variety of tissues. Eur. J. Biochem. 2001, 268, 5249–5257. [Google Scholar] [CrossRef] [PubMed]
- Elliott, B.; Renshaw, D.; Getting, S.; Mackenzie, R. The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol. 2012, 205, 324–340. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Soh, T.; Fujihara, N.; Hattori, M.A. Function of TGF-β2 in the growth of chicken primordial germ cells and germinal ridge stroma cells during embryonic development. J. Exp. Zool. Part A Comp. Exp. Biol. 2004, 301, 290–296. [Google Scholar] [CrossRef]
- Singleman, C.; Holtzman, N.G. Growth and maturation in the zebrafish, Danio rerio: A staging tool for teaching and research. Zebrafish 2014, 11, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Tsang, B.; Gerlai, R. Breeding and larviculture of zebrafish (Danio rerio). In Laboratory Fish in Biomedical Research; Elsevier: Amsterdam, The Netherlands, 2022; pp. 63–80. [Google Scholar]
- Bancroft, J.; Gamble, M. Theory and Practice of Histological Techniques, 5th ed.; Churchill Livingstone Pub: London, UK, 2002; Volume 172, pp. 593–620. [Google Scholar]
- Karnovsky, M. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 137A. [Google Scholar]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208. [Google Scholar] [CrossRef]
- Mokhtar, D.M. Fish Histology: From Cells to Organs; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Koç, N.D.; Aytekin, Y.; Yüce, R. Ovary maturatıon stages and histological investigation of ovary of the Zebrafish (Danio rerio). Braz. Arch. Biol. Technol. 2008, 51, 513–522. [Google Scholar]
- Hara, A.; Hiramatsu, N.; Fujita, T. Vitellogenesis and choriogenesis in fishes. Fish. Sci. 2016, 82, 187–202. [Google Scholar] [CrossRef]
- Williams, V.N.; Reading, B.J.; Hiramatsu, N.; Amano, H.; Glassbrook, N.; Hara, A.; Sullivan, C.V. Multiple vitellogenins and product yolk proteins in striped bass, Morone saxatilis: Molecular characterization and processing during oocyte growth and maturation. Fish Physiol. Biochem. 2014, 40, 395–415. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, N.; Todo, T.; Sullivan, C.V.; Schilling, J.; Reading, B.J.; Matsubara, T.; Ryu, Y.-W.; Mizuta, H.; Luo, W.; Nishimiya, O.; et al. Ovarian yolk formation in fishes: Molecular mechanisms underlying formation of lipid droplets and vitellogenin-derived yolk proteins. Gen. Comp. Endocrinol. 2015, 221, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Elkouby, Y.M.; Mullins, M.C. Methods for the analysis of early oogenesis in Zebrafish. Dev. Biol. 2017, 430, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Corriero, A.; Zupa, R.; Mylonas, C.C.; Passantino, L. Atresia of ovarian follicles in fishes, and implications and uses in aquaculture and fisheries. J. Fish Dis. 2021, 44, 1271–1291. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M.; Hussein, M.M. Microanalysis of fish ovarian follicular atresia: A possible synergic action of somatic and immune cells. Microsc. Microanal. 2020, 26, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, B.D.; Weber, G.M.; Sullivan, C.V.; Levine, M.A. Isolation and characterization of myostatin complementary deoxyribonucleic acid clones from two commercially important fish: Oreochromis mossambicus and Morone chrysops. Endocrinology 2001, 142, 1412–1418. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Sayed, R.K.; Zaccone, G.; Alesci, A.; Hussein, M.M. The potential role of the pseudobranch of molly fish (Poecilia sphenops) in immunity and cell regeneration. Sci. Rep. 2023, 13, 8665. [Google Scholar] [CrossRef]
- Hussein, M.M.; Sayed, R.K.A.; Mokhtar, D.M. Structural and immunohistochemical analysis of the cellular compositions of the liver of molly fish (Poecilia sphenops), focusing on its immune role. Zool. Lett. 2023, 9, 1. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Sayed, R.K.A.; Zaccone, G.; Albano, M.; Hussein, M.T. Ependymal and Neural Stem Cells of Adult Molly Fish (Poecilia sphenops, Valenciennes, 1846) Brain: Histomorphometry, Immunohistochemical, and Ultrastructural Studies. Cells 2022, 11, 2659. [Google Scholar] [CrossRef]
- Radaelli, G.; Rowlerson, A.; Mascarello, F.; Patruno, M.; Funkenstein, B. Myostatin precursor is present in several tissues in teleost fish: A comparative immunolocalization study. Cell Tissue Res. 2003, 311, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.B.; Goetz, F.W. Differential skeletal muscle expression of myostatin across teleost species, and the isolation of multiple myostatin isoforms. Febs Lett. 2001, 491, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Cheewasopit, W.; Laird, M.; Glister, C.; Knight, P.G. Myostatin is expressed in bovine ovarian follicles and modulates granulosal and thecal steroidogenesis. Reproduction 2018, 156, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fang, L.; Cong, L.; Chung, J.P.W.; Li, T.C.; Chan, D.Y.L. Myostatin: A multifunctional role in human female reproduction and fertility—A short review. Reprod. Biol. Endocrinol. 2022, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Sato, F.; Aramaki, S.; Soh, T.; Yamauchi, N.; Hattori, M.-A. Ubiquitous expression of myostatin in chicken embryonic tissues: Its high expression in testis and ovary. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, W. Communications of Oocyte-Granulosa Cells in the Chum Salmon Ovary Detected by Transmission Electron Microscopy: (oocyte/granulosa cell/intercellular junction/salmonid ovary/teleost). Dev. Growth Differ. 1985, 27, 553–561. [Google Scholar] [CrossRef]
- Harlow, C.R.; Hillier, S.G. Connective tissue growth factor in the ovarian paracrine system. Mol. Cell. Endocrinol. 2002, 187, 23–27. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, L.; Guo, Y.; Yu, H.; Cheng, H.; Huang, X.; Tiersch, T.R.; Berta, P. Similar gene structure of two Sox9a genes and their expression patterns during gonadal differentiation in a teleost fish, rice field eel (Monopterus albus). Mol. Reprod. Dev. Inc. Gamete Res. 2003, 66, 211–217. [Google Scholar] [CrossRef]
- Yokoi, H.; Kobayashi, T.; Tanaka, M.; Nagahama, Y.; Wakamatsu, Y.; Takeda, H.; Araki, K.; Morohashi, K.-I.; Ozato, K. Sox9 in a teleost fish, medaka (Oryzias latipes): Evidence for diversified function of Sox9 in gonad differentiation. Mol. Reprod. Dev. Inc. Gamete Res. 2002, 63, 5–16. [Google Scholar] [CrossRef]
- Du, Q.-Y.; Wang, F.-Y.; Hua, H.-Y.; Chang, Z.-J. Cloning and study of adult-tissue-specific expression of Sox9 in Cyprinus carpio. J. Genet. 2007, 86, 85–91. [Google Scholar] [CrossRef]
- Bhat, I.A.; Rather, M.A.; Saha, R.; Pathakota, G.-B.; Pavan-Kumar, A.; Sharma, R. Expression analysis of Sox9 genes during annual reproductive cycles in gonads and after nanodelivery of LHRH in Clarias batrachus. Res. Vet. Sci. 2016, 106, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Raghuveer, K.; Senthilkumaran, B. Isolation of sox9 duplicates in catfish: Localization, differential expression pattern during gonadal development and recrudescence, and hCG-induced up-regulation of sox9 in testicular slices. Reproduction 2010, 140, 477. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yuan, H.; Sun, D.; Liang, B.; Zhang, S. Sequence and expression of three members of the Sox gene in Amur sturgeon (Acipenser schrenckii). J. Appl. Ichthyol. 2006, 22, 77–81. [Google Scholar] [CrossRef]
- Berbejillo, J.; Martinez-Bengochea, A.; Bedó, G.; Vizziano-Cantonnet, D. Expression of dmrt1 and sox9 during gonadal development in the Siberian sturgeon (Acipenser baerii). Fish Physiol. Biochem. 2013, 39, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Burcea, A.; Popa, G.O.; Maereanu, M.; Dudu, A.; Georgescu, S.E.; Costache, M. Expression characterization of six genes possibly involved in gonad development for stellate sturgeon individuals (Acipenser stellatus, Pallas 1771). Int. J. Genom. 2018, 2018, 7835637. [Google Scholar] [CrossRef] [PubMed]
- Salvaggio, A.; Marino, F.; Albano, M.; Pecoraro, R.; Camiolo, G.; Tibullo, D.; Bramanti, V.; Lombardo, B.M.; Saccone, S.; Mazzei, V.; et al. Toxic effects of zinc chloride on the bone development in Danio rerio (Hamilton, 1822). Front. Physiol. 2016, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M. Characterization of the fish ovarian stroma during the spawning season: Cytochemical, immunohistochemical and ultrastructural studies. Fish Shellfish. Immunol. 2019, 94, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, T.S.; Viadanna, R.R.; Quagio-Grassiotto, I. Presence, localization and morphology of TELOCYTES in developmental gonads of fishes. J. Morphol. 2019, 280, 654–665. [Google Scholar] [CrossRef]
- Pellegrini, M.-S.F.; Popescu, L.M. Telocytes. Biomol. Concepts 2011, 2, 481–489. [Google Scholar] [CrossRef]
- Hodgkinson, J.W.; Grayfer, L.; Belosevic, M. Biology of bony fish macrophages. Biology 2015, 4, 881–906. [Google Scholar] [CrossRef]
- Bassity, E.; Clark, T.G. Functional identification of dendritic cells in the teleost model, rainbow trout (Oncorhynchus mykiss). PLoS ONE 2012, 7, e33196. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamedien, D.; Mokhtar, D.M.; Abdellah, N.; Awad, M.; Albano, M.; Sayed, R.K.A. Ovary of Zebrafish during Spawning Season: Ultrastructure and Immunohistochemical Profiles of Sox9 and Myostatin. Animals 2023, 13, 3362. https://doi.org/10.3390/ani13213362
Mohamedien D, Mokhtar DM, Abdellah N, Awad M, Albano M, Sayed RKA. Ovary of Zebrafish during Spawning Season: Ultrastructure and Immunohistochemical Profiles of Sox9 and Myostatin. Animals. 2023; 13(21):3362. https://doi.org/10.3390/ani13213362
Chicago/Turabian StyleMohamedien, Dalia, Doaa M. Mokhtar, Nada Abdellah, Mahmoud Awad, Marco Albano, and Ramy K. A. Sayed. 2023. "Ovary of Zebrafish during Spawning Season: Ultrastructure and Immunohistochemical Profiles of Sox9 and Myostatin" Animals 13, no. 21: 3362. https://doi.org/10.3390/ani13213362






