Reaction to Novel Objects and Fecal Glucocorticoid Metabolite Levels in Two Species of Nocturnal Geckos
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Housing
2.2. Experimental Design
2.2.1. Fecal Corticosterone Metabolite Levels
2.2.2. Novel Object Test
2.3. Data Analysis
2.3.1. Behavioral Measures
2.3.2. FCM Score
2.3.3. Correlation and Principal Component Analyses
3. Results
3.1. Equivalence of Novel Object Tests
3.2. Individual and Group Response to All Novel Objects
3.3. Classification of Individual Behavioral Responses
- Group B1 included animals that did not approach or manipulate the object, nor did they display exploratory behavior.
- Group B2 included animals with a significant exploratory behavior that took time to detect and approach the object, but they did not manipulate it.
- Group B3 included animals with a significant exploratory behavior, initially attracted by the object although they needed time to manipulate it.
- Group B4 included animals attracted to the object from the beginning of the experiment that “frequently” manipulated it or stayed close to it.
3.4. Corticosterone Metabolite Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Zanten, T.C.; Simpson, S.C. Managing the health of captive groups of reptiles and amphibians. Vet. Clin. N. Am. Exot. Anim. Pract. 2021, 24, 609–645. [Google Scholar] [CrossRef]
- Auliya, M.; Altherr, S.; Ariano-Sanchez, D.; Baard, E.H.; Brown, C.; Brown, R.M.; Ziegler, T. Trade in live reptiles, its impact on wild populations, and the role of the European market. Biol. Conserv. 2016, 204, 103–119. [Google Scholar] [CrossRef]
- Marshall, B.M.; Strine, C.; Hughes, A.C. Thousands of reptile species threatened by under-regulated global trade. Nat. Commun. 2020, 11, 4738. [Google Scholar] [CrossRef]
- Binding, S.; Farmer, H.; Krusin, L.; Cronin, K. Status of animal welfare research in zoos and aquariums: Where are we, where to next? J. Zoo. Aquar. Res. 2020, 8, 166–174. [Google Scholar] [CrossRef]
- Burghardt, G.M. Environmental enrichment and cognitive complexity in reptiles and amphibians: Concepts, review, and implications for captive populations. Appl. Anim. Behav. Sci. 2013, 147, 286–298. [Google Scholar] [CrossRef]
- Melfi, V.A. There are big gaps in our knowledge, and thus approach, to zoo animal welfare: A case for evidence-based zoo animal management. Zoo Biol. 2009, 28, 574–588. [Google Scholar] [CrossRef]
- Schilliger, L.; Vergneau-Grosset, C.; Desmarchelier, M.R. Clinical Reptile Behavior. Vet. Clin. N. Am. Exot. Anim. Pract. 2021, 24, 175–195. [Google Scholar] [CrossRef]
- Warwick, C.; Jessop, M.; Arena, P.; Pliny, A.; Nicholas, E.; Lambiris, A. Future of keeping pet reptiles and amphibians: Animal welfare and public health perspective. Vet. Rec. 2017, 181, 454–455. [Google Scholar] [CrossRef]
- Whittaker, A.L.; Golder-Dewar, B.; Triggs, J.L.; Sherwen, S.L.; McLelland, D.J. Identification of animal-based welfare indicators in captive reptiles: A delphi consultation survey. Animals 2021, 11, 2010. [Google Scholar] [CrossRef]
- Benn, A.L.; McLelland, D.J.; Whittaker, A.L. A review of welfare assessment methods in reptiles, and preliminary application of the welfare quality® protocol to the pygmy blue-tongue skink, Tiliqua adelaidensis, using animal-based measures. Animals 2019, 9, 27. [Google Scholar] [CrossRef]
- Silvestre, A.M. How to assess stress in reptiles. J. Exot. Pet Med. 2014, 23, 240–243. [Google Scholar] [CrossRef]
- Carbajal, A.; Tallo-Parra, O.; Monclús, L.; Aresté, M.; Fernández-Bellon, H.; Almagro, V.; Lopez-Bejar, M. Corticosterone measurement in Komodo dragon shed skin. Herpetol. J. 2018, 28, 110–116. [Google Scholar]
- Racine, H.; Guthrie, K.S.; Hill, T.; Loughman, Z. Impact of Indigestible Materials on the Efficiency of Fecal Corticosterone Immunoassay Testing in Pituophis Species. Animals 2022, 12, 1410. [Google Scholar] [CrossRef]
- Martín, J.; Barja, I.; Rodríguez-Ruiz, G.; Recio, P.; Cuervo, J.J. Hidden but potentially stressed: A non-invasive technique to quantify fecal glucocorticoid levels in a fossorial amphisbaenian reptile. Animals 2023, 13, 109. [Google Scholar] [CrossRef]
- Moszuti, S.A.; Wilkinson, A.; Burman, O.H. Response to novelty as an indicator of reptile welfare. Appl. Anim. Behav. Sci. 2017, 193, 98–103. [Google Scholar] [CrossRef]
- Takola, E.; Krause, E.T.; Müller, C.; Schielzeth, H. Novelty at second glance: A critical appraisal of the novel object paradigm based on meta-analysis. Anim. Behav. 2021, 180, 123–142. [Google Scholar] [CrossRef]
- Hall, B.A.; Melfi, V.; Burns, A.; McGill, D.M.; Doyle, R.E. Curious creatures: A multi-taxa investigation of responses to novelty 660 in a zoo environment. PeerJ 2018, 6, e4454. [Google Scholar] [CrossRef]
- Forkman, B.; Boissy, A.; Meunier-Salaün, M.-C.; Canali, E.; Jonest, R. A critical review of fear tests used on cattle, pigs, sheep, poultry and horses. Physiol. Behav. 2007, 92, 340–374. [Google Scholar] [CrossRef]
- Šimková, O.; Frýdlová, P.; Žampachová, B.; Frynta, D.; Landová, E. Development of behavioural profile in the Northern common boa (Boa imperator): Repeatable independent traits or personality? PLoS ONE 2017, 12, e0177911. [Google Scholar] [CrossRef]
- Horváth, G.; Martin, J.; López, P.; Garamszegi, L.Z.; Bertók, P.; Herczeg, G. Blood parasite infection intensity covaries with risk-taking personality in male carpetan rock lizards (Iberolacerta cyreni). Ethology 2016, 122, 355–363. [Google Scholar] [CrossRef]
- Horváth, G.; Rodríguez-Ruiz, G.; Martín, J.; López, P.; Herczeg, G. Maternal diet affects juvenile Carpetan rock lizard performance and personality. Nat. Ecol. Evol. 2019, 9, 14476–14488. [Google Scholar] [CrossRef]
- Horváth, G.; Jiménez-Robles, O.; Martín, J.; López, P.; De la Riva, I.; Herczeg, G. Linking behavioral thermoregulation, boldness, and individual state in male Carpetan rock lizards. Nat. Ecol. Evol. 2020, 10, 10230–10241. [Google Scholar] [CrossRef]
- Ibáñez, A.; Martín, J.; Gazzola, A.; Pellitteri-Rosa, D. Freshwater turtles reveal personality traits in their antipredatory behaviour. Behav. Process. 2018, 157, 142–147. [Google Scholar] [CrossRef]
- Michelangeli, M.; Payne, E.; Spiegel, O.; Sinn, D.L.; Leu, S.T.; Gardner, M.G.; Sih, A. Personality, spatiotemporal ecological variation and resident/explorer movement syndromes in the sleepy lizard. J. Anim. Ecol. 2022, 91, 210–223. [Google Scholar] [CrossRef]
- Barrett, L.P.; Anthony, K.L.; Eliades, S.J.; Siler, C.D.; Lock, B.; Snyder, R.J. Personality assessment of headstart Texas horned lizards (Phrynosoma cornutum) in human care prior to release. Appl. Anim. Behav. Sci. 2022, 254, 105690. [Google Scholar] [CrossRef]
- Mell, H.; Josserand, R.; Decencière, B.; Artacho, P.; Meylan, S.; Le Galliard, J.F. Do personalities co-vary with metabolic expenditure and glucocorticoid stress response in adult lizards? Behav. Ecol. Sociobiol. 2016, 70, 951–961. [Google Scholar] [CrossRef]
- Learmonth, M.J. The matter of non-avian reptile sentience, and why it “matters” to them: A conceptual, ethical and scientific review. Animals 2020, 10, 901. [Google Scholar] [CrossRef]
- Michelangeli, M.; Melki-Wegner, B.; Laskowski, K.; Wong, B.B.; Chapple, D.G. Impacts of caudal autotomy on personality. Anim. Behav. 2020, 162, 67–78. [Google Scholar] [CrossRef]
- Talavera, J.B.; Carriere, A.; Swierk, L.; Putman, B.J. Tail autotomy is associated with boldness in male but not female water anoles. Behav. Ecol. Sociobiol. 2021, 75, 1–10. [Google Scholar] [CrossRef]
- McDonald, R.P.; Vickaryous, M.K. Evidence for neurogenesis in the medial cortex of the leopard gecko, Eublepharis macularius. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- McLean, K.E.; Vickaryous, M.K. A novel amniote model of epimorphic regeneration: The leopard gecko, Eublepharis macularius. BMC Dev. Biol. 2011, 11, 1–25. [Google Scholar] [CrossRef]
- Glimm, T.; Kiskowski, M.; Moreno, N.; Chiari, Y. Capturing and analyzing pattern diversity: An example using the melanistic spotted patterns of leopard geckos. PeerJ 2021, 9, e11829. [Google Scholar] [CrossRef]
- Bashaw, M.J.; Gibson, M.D.; Schowe, D.M.; Kucher, A.S. Does enrichment improve reptile welfare? Leopard geckos (Eublepharis macularius) respond to five types of environmental enrichment. Appl. Anim. Behav. Sci. 2016, 184, 150–160. [Google Scholar] [CrossRef]
- Zieliński, D. The Effect of Enrichment on Leopard Geckos (Eublepharis macularius) Housed in Two Different Maintenance Systems (Rack System vs. Terrarium). Animals 2023, 13, 1111. [Google Scholar] [CrossRef]
- Kundey, S.M.; Phillips, M. Recognition of novelty in leopard geckos (Eublepharis macularius) and tiger salamanders (Ambystoma tigrinum). Behav. Process. 2021, 184, 104320. [Google Scholar] [CrossRef]
- Katlein, N.; Ray, M.; Wilkinson, A.; Claude, J.; Kiskowski, M.; Wang, B.; Glaberman, S.; Chiari, Y. Does colour impact responses to images in geckos? J. Zool. 2022, 317, 138–146. [Google Scholar] [CrossRef]
- Nordberg, E.; Denny, R.; Schwarzkopf, L. Testing measures of boldness and exploratory activity in native versus invasive species: Geckos as a model system. Anim. Behav. 2021, 177, 215–222. [Google Scholar] [CrossRef]
- Szabo, B.; Ringler, E. Fear of the new? Geckos hesitate to attack novel prey, feed near objects and enter a novel space. Anim. Cogn. 2023, 26, 537–549. [Google Scholar] [CrossRef]
- Landová, E.; Musilová, V.; Polák, J.; Sedláčková, K.; Frynta, D. Antipredatory reaction of the leopard gecko Eublepharis macularius to snake predators. Curr. Zool. 2016, 62, 439–450. [Google Scholar] [CrossRef]
- Lance, V.A.; Grumbles, J.S.; Rostal, D.C. Sex differences in plasma corticosterone in desert tortoises, Gopherus agassizii, during the reproductive cycle. J. Exp. Zool. 2001, 289, 285–289. [Google Scholar] [CrossRef]
- Langkilde, T.; Shine, R. How much stress do researchers inflict on their study animals? A case study using a scincid lizard, Eulamprus heatwolei. J. Exp. Biol. 2006, 209, 1035–1043. [Google Scholar] [CrossRef]
- Fernandez-Lazaro, G.; Latorre, R.; Alonso-García, E.; Núñez, I.B. Nonhuman primate welfare: Can there be a relationship between personality, lateralization and physiological indicators? Behav. Process. 2019, 166, 103897. [Google Scholar] [CrossRef]
- Fuller, G.; Margulis, S.W.; Santymire, R. The effectiveness of indigestible markers for identifying individual animal feces and their prevalence of use in North American zoos. Zoo. Biol. 2011, 30, 379–398. [Google Scholar] [CrossRef]
- Barja, I.; Escribano-Ávila, G.; Lara-Romero, C.; Virgós, E.; Benito, J.; Rafart, E. Non-invasive monitoring of adrenocortical activity in European badgers (Meles meles) and effects of sample collection and storage on faecal cortisol metabolite concentrations. Anim. Biol. 2012, 62, 419–432. [Google Scholar] [CrossRef]
- Washburn, B.E.; Millspaugh, J.J. Effects of simulated environmental conditions on glucocorticoid metabolite measurements in white-tailed deer feces. Gen. Comp. Endocrinol. 2002, 127, 217–222. [Google Scholar] [CrossRef]
- Casas, F.; Benítez-López, A.; Tarjuelo, R.; Barja, I.; Viñuela, J.; García, J.T.; García, J.T.; Mougeot, F. Changes in behaviour and faecal glucocorticoid levels in response to increased human activities during weekends in the pin-tailed sandgrouse. Sci. Nat. 2016, 103, 91. [Google Scholar] [CrossRef]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Sovrano, V.A.; Quaresmini, C.; Stancher, G. Tortoises in front of mirrors: Brain asymmetries and lateralized behaviours in the tortoise (Testudo hermanni). Behav. Brain Res. 2018, 352, 183–186. [Google Scholar] [CrossRef]
- Helfman, G.S. Threat-sensitive predator avoidance in damselfish trumpetfish interactions. Behav. Ecol. Sociobiol. 1989, 24, 47–58. [Google Scholar] [CrossRef]
- Sih, A.; Bolnick, D.I.; Luttbeg, B.; Orrock, J.L.; Peacor, S.D.; Pintor, L.M.; Vonesh, J.R. Predator-prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 2010, 119, 610–621. [Google Scholar] [CrossRef]
- Cornelis, J.; Nordberg, E.J.; Schwarzkopf, L. Antipredator behaviour of invasive geckos in response to chemical cues from snakes. Ethology 2019, 125, 57–63. [Google Scholar] [CrossRef]
- Lloyd, R.; Alford, R.A.; Schwarzkopf, L. Chemical discrimination among predators by lizards: Responses of three skink species to the odours of high and low-threat varanid predators. Austral. Ecol. 2009, 34, 50–54. [Google Scholar] [CrossRef]
- Amo, L.; López, P.; Martín, J. Can wall lizards combine chemical and visual cues to discriminate predatory from non-predatory snakes inside refuges? Ethology 2006, 112, 478–484. [Google Scholar] [CrossRef]
- Cisterne, A.; Vanderduys, E.P.; Pike, D.A.; Schwarzkopf, L. Wary invaders and clever natives: Sympatric house geckos show disparate responses to predator scent. Behav. Ecol. 2014, 25, 604–611. [Google Scholar] [CrossRef]
- Highcock, L.; Carter, A.J. Intraindividual variability of boldness is repeatable across contexts in a wild lizard. PLoS ONE 2014, 9, e95179. [Google Scholar] [CrossRef]
- Roth, T.C.; Rosier, M.; Krochmal, A.R.; Clark, L. A multi-trait, field-based examination of personality in a semi-aquatic turtle. Ethology 2020, 126, 851–857. [Google Scholar] [CrossRef]
- Waters, R.M.; Bowers, B.B.; Burghardt, G.M. Personality and Individuality in Reptile Behavior. In Personality in Nonhuman Animals; Vonk, J., Weiss, A., Kuczaj, S., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Reale, D.; Reader, S.M.; Sol, D.; Mcdougall, P.T.; Dingemanse, N.J. Integrating animal temperament within ecology and evolution. Biol. Rev. 2007, 82, 291–318. [Google Scholar] [CrossRef]
- Carere, C.; Caramaschi, D.; Fawcett, T.W. Covariation between personalities and individual differences in coping with stress: Converging evidence and hypotheses. Curr. Zool. 2010, 56, 728–741. [Google Scholar] [CrossRef]
- Cockrem, J.F. Stress, corticosterone responses and avian personalities. J. Ornithol. 2007, 148, 169–178. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Korte, S.M.; De Boer, S.F.; Van Der Vegt, B.J.; Van Reenen, C.G.; Hopster, H.; De Jong, I.C.; Ruis, M.A.W.; Blokhuis, H.J. Coping styles in animals: Current status in behavior and stress- physiology. Neurosci. Biobehav. Rev. 1999, 23, 925–935. [Google Scholar] [CrossRef]
- Bateman, P.W.; Fleming, P.A. To cut a long tail short: A review of lizard caudal autotomy studies carried out over the last 20 years. J. Zool. 2009, 277, 1–14. [Google Scholar] [CrossRef]
- Medger, K.; Verburgt, L.; Bateman, P.W. The influence of tail autotomy on the escape response of the Cape dwarf gecko, Lygodactylus capensis. Ethology 2008, 114, 42–52. [Google Scholar] [CrossRef]
- Daniels, C.B. Running: An escape strategy enhanced by autotomy. Herpetologica 1983, 39, 162–165. Available online: https://www.jstor.org/stable/3892556 (accessed on 20 October 2023).
- Lu, H.L.; Ding, G.H.; Ding, P.; Ji, X.A. Tail autotomy plays no important role in influencing locomotor performance and anti-predator behavior in a cursorial gecko. Ethology 2010, 116, 627–634. [Google Scholar] [CrossRef]
- Mason, G.J.; Latham, N. Can’t stop, won’t stop: Is stereotypy a reliable animal welfare indicator? Anim. Welfare 2004, 13, S57–S69. [Google Scholar] [CrossRef]
- Carazo, P.; Noble, D.W.; Chandrasoma, D.; Whiting, M.J. Sex and boldness explain individual differences in spatial learning in a lizard. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133275. [Google Scholar] [CrossRef]
- Strickland, K.; Gardiner, R.; Schultz, A.J.; Frere, C.H. The social life of eastern water dragons: Sex differences, spatial overlap and genetic relatedness. Anim. Behav. 2014, 97, 53–61. [Google Scholar] [CrossRef]
- Harrison, L.M.; Noble, D.W.; Jennions, M.D. A meta-analysis of sex differences in animal personality: No evidence for the greater male variability hypothesis. Biol. Rev. 2022, 97, 679–707. [Google Scholar] [CrossRef]
- Kalliokoski, O.; Timm, J.A.; Ibsen, I.B.; Hau, J.; Frederiksen, A.M.B.; Bertelsen, M.F. Fecal glucocorticoid response to environmental stressors in green iguanas (Iguana iguana). Gen. Comp. Endocrinol. 2012, 177, 93–97. [Google Scholar] [CrossRef]
- Dupoué, A.; Brischoux, F.; Lourdais, O.; Angelier, F. Influence of temperature on the corticosterone stress–response: An experiment in the children’s python (Antaresia childreni). Gen. Comp. Endocrinol. 2013, 193, 178–184. [Google Scholar] [CrossRef]
- Gangloff, E.J.; Sparkman, A.M.; Holden, K.G.; Corwin, C.J.; Topf, M.; Bronikowski, A.M. Geographic variation and within-individual correlations of physiological stress markers in a widespread reptile, the common garter snake (Thamnophis sirtalis). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2017, 205, 68–76. [Google Scholar] [CrossRef]
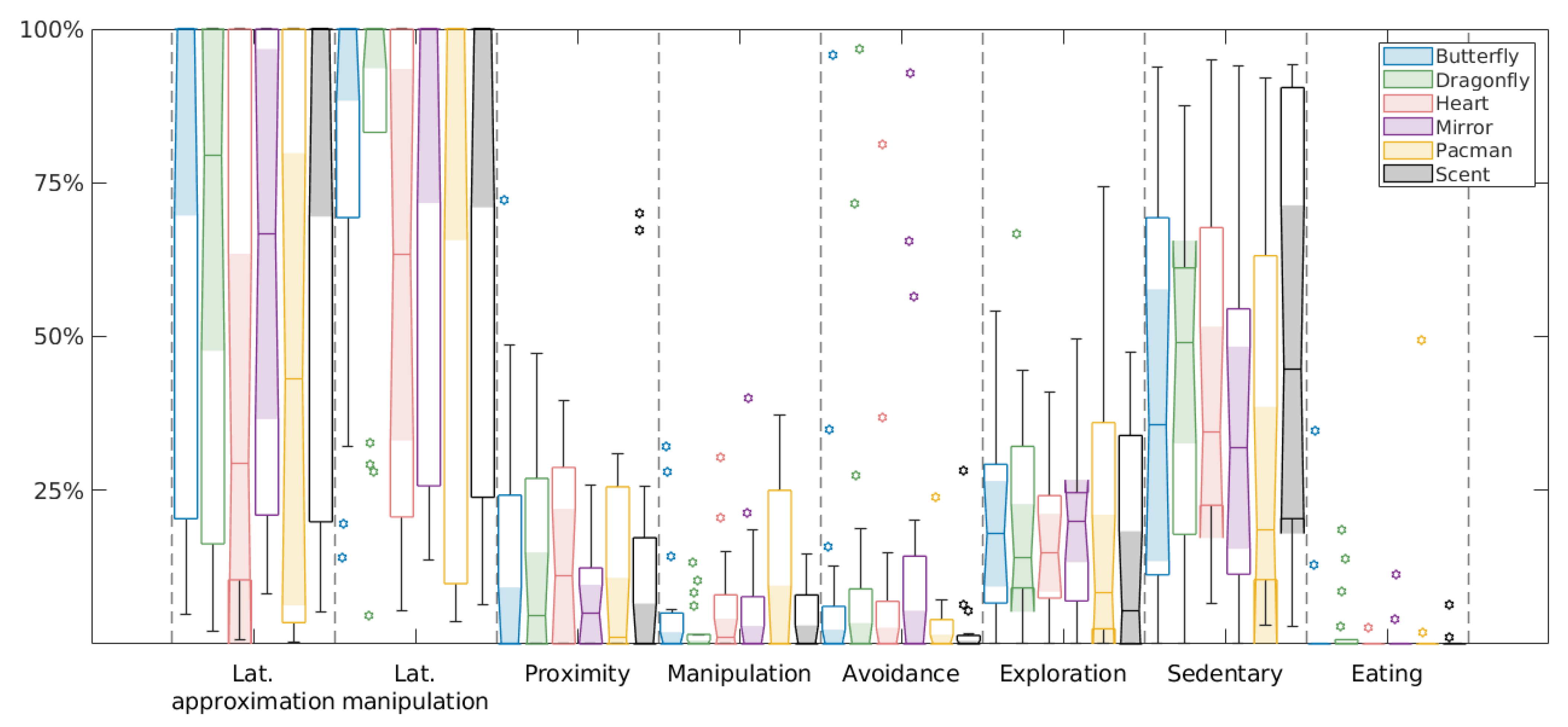
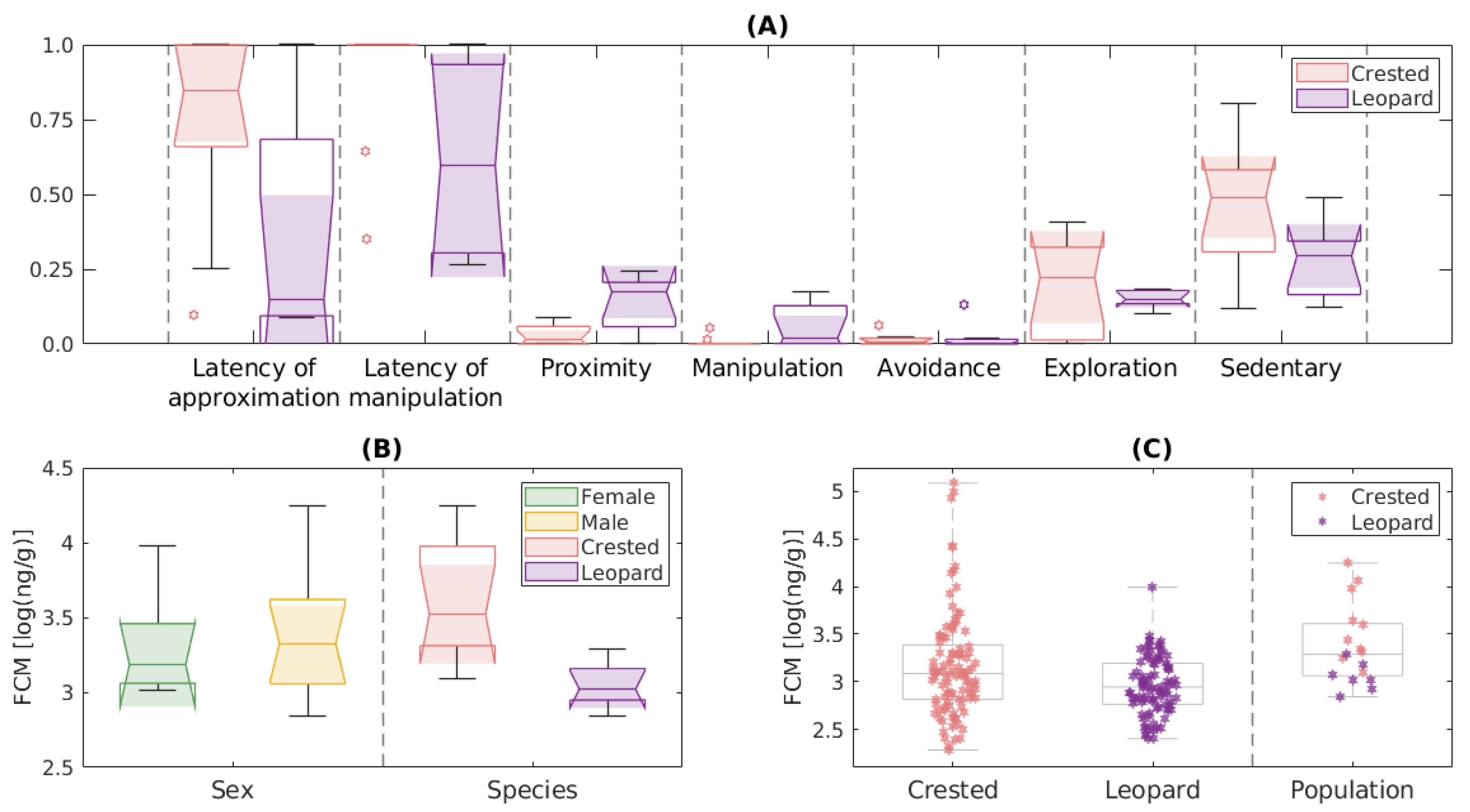

| Subject | Species | Sex a | FCM b (ng/g) | Behavioral Group |
|---|---|---|---|---|
| C1 | Correlophus ciliatus | M | 17,666.4 | B3 |
| C2 | Correlophus ciliatus | M | 1239.1 | B1 |
| C3 | Correlophus ciliatus | M | 11,631.1 | B1 |
| C4 | Correlophus ciliatus | M | 4357.9 | B1 |
| C5 | Correlophus ciliatus | M | 2046.7 | B2 |
| C6 | Correlophus ciliatus | F | 9464.1 | B1 |
| C7 | Correlophus ciliatus | M | 2758.1 | B2 |
| C8 | Correlophus ciliatus | M | 3996.7 | B2 |
| C9 | Correlophus ciliatus | M | 1791.1 | B4 |
| C10 | Correlophus ciliatus | M | 2189.9 | B2 |
| L1 | Eublepharis macularius | F | 1931.5 | B1 |
| L2 | Eublepharis macularius | M | 843.7 | B4 |
| L3 | Eublepharis macularius | F | 1189.1 | B2 |
| L4 | Eublepharis macularius | M | 692.0 | B4 |
| L5 | Eublepharis macularius | M | 1047.8 | B4 |
| L6 | Eublepharis macularius | F | 1030.6 | B2 |
| L7 | Eublepharis macularius | F | 1530.7 | B4 |
| Behavior | Definition |
|---|---|
| Exploration | Moving around enclosure or climbing vertical surfaces at slow speed in no fixed pattern away from the novel object. It can include tongue flicking. |
| Sedentary behavior | Lying or standing with eyes open or closed, not engaged in any active behavior. |
| Proximity | Being within a 2 cm range of the object with any part of the body (at least 50% of it), but the attention is not necessary directed to the object. It can be exploring or sedentary. |
| Manipulation | Stepping on, climbing, biting, or licking the object but actively paying attention at it. Since geckos can have periods of inactivity followed by quick movements, ten seconds was selected as the cut-off time, as in previous studies, to ensure that the lack of movement was not just a momentary stop [36]. Therefore, if the gecko did not move for more than ten seconds it was considered as Proximity. |
| Avoidance | Moving or jumping rapidly away from a stimulus and or hiding with face not visible to observer. |
| Eating | Licking the mix of chopped fruits from the feeder or drinking water. |
| Abnormal Repetitive Behavior (ARB) | Moving around the enclosure or climbing walls on a set path (at least two repetitions required) or repeatedly attempting to climb out of the cage, scratch or burrow into the glass for more than 20 s. |
| Other | To scratch the sand or try to bury the novel object. |
| Latency of Manipulation | Proximity | Manipulation | Exploration | Sedentary | Avoidance | |
|---|---|---|---|---|---|---|
| Latency of approximation | 0.83 (p < 0.001) | −0.90 (p < 0.001) | −0.77 (p < 0.001) | −0.43 (p = 0.083) | 0.77 (p < 0.001) | −0.12 (p = 0.642) |
| Latency of manipulation | −0.85 (p < 0.001) | −0.98 (p < 0.001) | −0.25 (p = 0.320) | 0.76 (p < 0.001) | −0.01 (p = 0.977) | |
| Proximity | 0.85 (p < 0.001) | 0.33 (p = 0.197) | −0.80 (p < 0.001) | 0.12 (p = 0.635) | ||
| Manipulation | 0.25 (p = 0.326) | −0.80 (p < 0.001) | 0.03 (p = 0.923) | |||
| Exploration | −0.53 (p = 0.028) | 0.32 (p = 0.213) | ||||
| Sedentary | −0.24 (p = 0.350) |
| Latency of Approximation | Latency of Manipulation | Proximity | Manipulation | Exploration | Sedentary | Avoidance | Eating | ARB | Other | Variability | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef 1 | 0.6766 | 0.5499 | −0.1618 | −0.1113 | −0.1636 | 0.4153 | 0.0400 | 0.0174 | −0.0095 | −0.0058 | 83.0% |
| Coef 2 | 0.0931 | 0.2005 | −0.2258 | −0.1001 | 0.8509 | −0.1595 | −0.3731 | −0.0353 | 0.0100 | −0.0089 | 8.7% |
| Latency of Approximation | Latency of Manipulation | Exploration | |
|---|---|---|---|
| B1 | 1.00 | 1.00 | 0.01 |
| B2 | 0.76 | 1.00 | 0.22 |
| B3 | 0.25 | 0.65 | 0.34 |
| B4 | 0.10 | 0.33 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Lázaro, G.; Latorre, R.; Fontanillas Pérez, J.C.; Barja, I. Reaction to Novel Objects and Fecal Glucocorticoid Metabolite Levels in Two Species of Nocturnal Geckos. Animals 2023, 13, 3384. https://doi.org/10.3390/ani13213384
Fernández-Lázaro G, Latorre R, Fontanillas Pérez JC, Barja I. Reaction to Novel Objects and Fecal Glucocorticoid Metabolite Levels in Two Species of Nocturnal Geckos. Animals. 2023; 13(21):3384. https://doi.org/10.3390/ani13213384
Chicago/Turabian StyleFernández-Lázaro, Gloria, Roberto Latorre, Juan Carlos Fontanillas Pérez, and Isabel Barja. 2023. "Reaction to Novel Objects and Fecal Glucocorticoid Metabolite Levels in Two Species of Nocturnal Geckos" Animals 13, no. 21: 3384. https://doi.org/10.3390/ani13213384
APA StyleFernández-Lázaro, G., Latorre, R., Fontanillas Pérez, J. C., & Barja, I. (2023). Reaction to Novel Objects and Fecal Glucocorticoid Metabolite Levels in Two Species of Nocturnal Geckos. Animals, 13(21), 3384. https://doi.org/10.3390/ani13213384







