Current Knowledge on the Transportation by Road of Cattle, including Unweaned Calves
Abstract
:Simple Summary
Abstract
1. Introduction
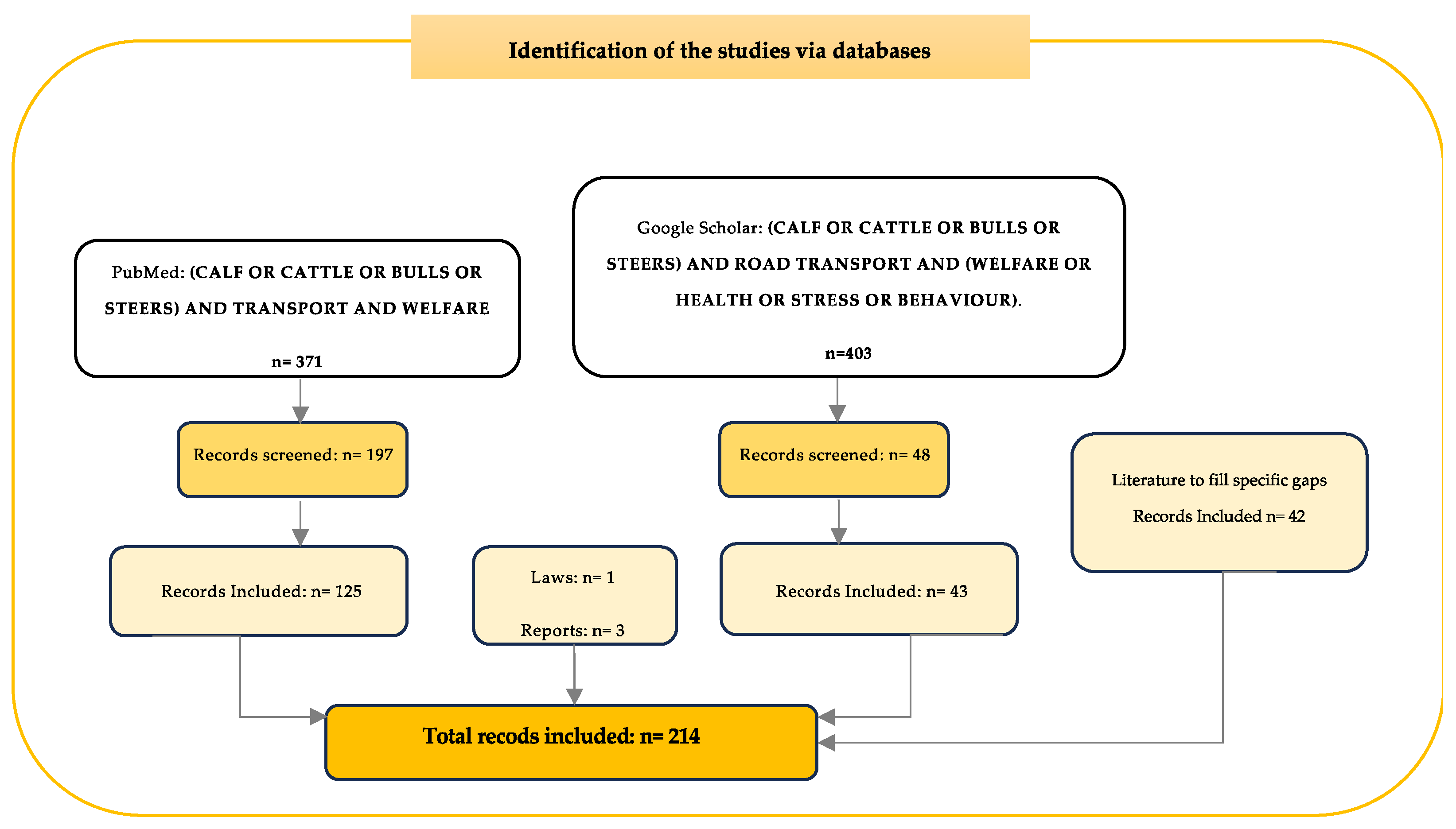
2. Material and Methods
3. Evaluation of Transportation Stress
3.1. Measurements of Stress during Transportation
3.1.1. Metabolic Changes Observed during Transportation Stress
3.1.2. Changes in Performance Variables Observed during Transportation Stress
3.1.3. Immunological and Inflammatory Changes Observed during Transportation Stress
3.1.4. Disease and Transport Stress
3.1.5. Injuries and Transport Stress
3.2. Factors Affecting the Extent of Transportation Stress
3.2.1. Age of the Cattle during Transportation
3.2.2. Type of Cattle
3.2.3. Road Conditions and Driver Variables
3.2.4. Stocking Density
3.2.5. Transport Duration
Rest Periods
3.2.6. Hot and Cold Weather
3.2.7. Fitness for Transport
4. Concerns about Transportation Stress
4.1. Economic Concerns
4.2. Welfare Concern
4.3. Societal Concerns
4.4. Limitations and Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swanson, J.C.; Morrow-Tesch, J. Cattle Transport: Historical, Research, and Future Perspectives. J. Anim. Sci. 2001, 79, E102–E109. [Google Scholar]
- González, L.A.; Schwartzkopf-Genswein, K.S.; Bryan, M.; Silasi, R.; Brown, F. Benchmarking Study of Industry Practices during Commercial Long Haul Transport of Cattle in Alberta, Canada. J. Anim. Sci. 2012, 90, 3606–3617. [Google Scholar] [CrossRef]
- González, L.A.; Schwartzkopf-Genswein, K.S.; Bryan, M.; Silasi, R.; Brown, F. Factors Affecting Body Weight Loss during Commercial Long Haul Transport of Cattle in North America. J. Anim. Sci. 2012, 90, 3630–3639. [Google Scholar] [CrossRef] [PubMed]
- González, L.A.; Schwartzkopf-Genswein, K.S.; Bryan, M.; Silasi, R.; Brown, F. Relationships between Transport Conditions and Welfare Outcomes during Commercial Long Haul Transport of Cattle in North America. J. Anim. Sci. 2012, 90, 3640–3651. [Google Scholar] [CrossRef] [PubMed]
- González, L.A.; Schwartzkopf-Genswein, K.S.; Bryan, M.; Silasi, R.; Brown, F. Space Allowance during Commercial Long Distance Transport of Cattle in North America. J. Anim. Sci. 2012, 90, 3618–3629. [Google Scholar] [CrossRef] [PubMed]
- Schwartzkopf-Genswein, K.S.; Faucitano, L.; Dadgar, S.; Shand, P.; González, L.A.; Crowe, T.G. Road Transport of Cattle, Swine and Poultry in North America and Its Impact on Animal Welfare, Carcass and Meat Quality: A Review. Meat Sci. 2012, 92, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Cernicchiaro, N.; Renter, D.G.; White, B.J.; Babcock, A.H.; Fox, J.T. Associations between Weather Conditions during the First 45 Days after Feedlot Arrival and Daily Respiratory Disease Risks in Autumn-Placed Feeder Cattle in the United States. J. Anim. Sci. 2012, 90, 1328–1337. [Google Scholar] [CrossRef]
- Cernicchiaro, N.; White, B.J.; Renter, D.G.; Babcock, A.H.; Kelly, L.; Slattery, R. Effects of Body Weight Loss during Transit from Sale Barns to Commercial Feedlots on Health and Performance in Feeder Cattle Cohorts Arriving to Feedlots from 2000 to 2008. J. Anim. Sci. 2012, 90, 1940–1947. [Google Scholar] [CrossRef]
- Cernicchiaro, N.; White, B.J.; Renter, D.G.; Babcock, A.H.; Kelly, L.; Slattery, R. Associations between the Distance Traveled from Sale Barns to Commercial Feedlots in the United States and Overall Performance, Risk of Respiratory Disease, and Cumulative Mortality in Feeder Cattle during 1997 to 2009. J. Anim. Sci. 2012, 90, 1929–1939. [Google Scholar] [CrossRef]
- Schwartzkopf-Genswein, K.; Ahola, J.; Edwards-Callaway, L.; Hale, D.; Paterson, J. Symposium Paper: Transportation Issues Affecting Cattle Well-Being and Considerations for the Future. Prof. Anim. Sci. 2016, 32, 707–716. [Google Scholar] [CrossRef]
- Schwartzkopf-Genswein, K.; Grandin, T. Cattle Transport by Road. In Livestock Handling and Transport; CABI: Wallingford, UK, 2014; pp. 143–173. [Google Scholar]
- Minka, N.S.; Ayo, J.O. Effects of Different Road Conditions on Rectal Temperature, Behaviour and Traumatic Injuries during Transportation of Different Crosses of Temperate/Tropical Breeds of Heifers. Anim. Prod. Sci. 2018, 58, 2321–2328. [Google Scholar]
- Caulfield, M.P.; Cambridge, H.; Foster, S.F.; McGreevy, P.D. Heat Stress: A Major Contributor to Poor Animal Welfare Associated with Long-Haul Live Export Voyages. Vet. J. 2014, 199, 223–228. [Google Scholar]
- Zulkifli, I.; Abubakar, A.A.; Sazili, A.Q.; Goh, Y.M.; Imlan, J.C.; Kaka, U.; Sabow, A.B.; Awad, E.A.; Othman, A.H.; Raghazali, R.; et al. The Effects of Sea and Road Transport on Physiological and Electroencephalographic Responses in Brahman Crossbred Heifers. Animals 2019, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Goldhawk, C.; Janzen, E.; González, L.A.; Crowe, T.; Kastelic, J.; Pajor, E.; Schwartzkopf-Genswein, K.S. Trailer Microclimate and Calf Welfare during Fall-Run Transportation of Beef Calves in Alberta. J. Anim. Sci. 2014, 92, 5142–5154. [Google Scholar] [CrossRef]
- Goldhawk, C.; Crowe, T.; Janzen, E.; González, L.A.; Kastelic, J.; Pajor, E.; Schwartzkopf-Genswein, K.S. Trailer Microclimate during Commercial Transportation of Feeder Cattle and Relationship to Indicators of Cattle Welfare. J. Anim. Sci. 2014, 92, 5155–5165. [Google Scholar] [CrossRef]
- Grandin, T. Farm Animal Welfare during Handling, Transport, and Slaughter. J. Am. Vet. Med. Assoc. 1994, 204, 372–377. [Google Scholar] [PubMed]
- Huertas, S.; Gil, A.; Piaggio, J.; van Eerdenburg, F. Transportation of Beef Cattle to Slaughterhouses and How This Relates to Animal Welfare and Carcase Bruising in an Extensive Production System. Anim. Welf. 2010, 19, 281–285. [Google Scholar] [CrossRef]
- Zanardi, E.; De Luca, S.; Alborali, G.L.; Ianieri, A.; Varrà, M.O.; Romeo, C.; Ghidini, S. Relationship between Bruises on Carcasses of Beef Cattle and Transport-Related Factors. Animals 2022, 12, 1997. [Google Scholar] [CrossRef]
- Hultgren, J.; Segerkvist, K.A.; Berg, C.; Karlsson, A.H.; Öhgren, C.; Algers, B. Preslaughter Stress and Beef Quality in Relation to Slaughter Transport of Cattle. Livest. Sci. 2022, 264, 105073. [Google Scholar] [CrossRef]
- Carrasco-García, A.A.; Pardío-Sedas, V.T.; León-Banda, G.G.; Ahuja-Aguirre, C.; Paredes-Ramos, P.; Hernández-Cruz, B.C.; Murillo, V.V. Effect of Stress during Slaughter on Carcass Characteristics and Meat Quality in Tropical Beef Cattle. Asian-Australas J. Anim. Sci. 2020, 33, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Kenny, F.J.; Tarrant, P. V The Physiological and Behavioural Responses of Crossbred Friesian Steers to Short-Haul Transport by Road. Livest. Prod. Sci. 1987, 17, 63–75. [Google Scholar] [CrossRef]
- Tennessen, T.; Price, M.A.; Berg, R.T. Comparative Responses of Bulls and Steers to Transportation. Can. J. Anim. Sci. 1984, 64, 333–338. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare (AHAW); Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar Schmidt, C.; et al. Welfare of Cattle during Transport. EFSA J. 2022, 20, e07442. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.L.; Dybkjr, L.; Herskin, M.S. Road Transport of Farm Animals: Effects of Journey Duration on Animal Welfare. Animal 2011, 5, 415–427. [Google Scholar] [CrossRef]
- Valadez-Noriega, M.; Estévez-Moreno, L.X.; Rayas-Amor, A.A.; Rubio-Lozano, M.S.; Galindo, F.; Miranda-de la Lama, G.C. Livestock Hauliers’ Attitudes, Knowledge and Current Practices towards Animal Welfare, Occupational Wellbeing and Transport Risk Factors: A Mexican Survey. Prev. Vet. Med. 2018, 160, 76–84. [Google Scholar] [CrossRef]
- Valkova, L.; Vecerek, V.; Voslarova, E.; Kaluza, M.; Takacova, D.; Brscic, M. Animal Welfare during Transport: Comparison of Mortality during Transport from Farm to Slaughter of Different Animal Species and Categories in the Czech Republic. Ital. J. Anim. Sci. 2022, 21, 914–923. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; Blunt, H.; Brigham, T.; Chang, S.; et al. PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.; Hattingh, J.; Ganhao, M. Stress in Cattle Assessed after Handling, after Transport and after Slaughter. Vet. Rec. 1988, 123, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, J.; Reisine, T.D. Stress Hormones: Their Interaction and Regulation. Science 1984, 224, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Tasker, J.G.; Di, S.; Malcher-Lopes, R. Rapid Glucocorticoid Signaling via Membrane-Associated Receptors. Endocrinology 2006, 147, 5549–5556. [Google Scholar] [CrossRef] [PubMed]
- Almeida, P.; Mullineaux, D.; Raphael, W.; Wickens, C.; Zanella, A. Early Detection of Lameness in Heifers with Hairy Heel Warts Using a Pressure Plate. Anim. Welf. 2007, 16, 135–137. [Google Scholar] [CrossRef]
- Blecha, F.; Boyles, S.L.; Riley, J.G. Shipping Suppresses Lymphocyte Blastogenic Responses in Angus and Brahman x Angus Feeder Calves. J. Anim. Sci. 1984, 59, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Earley, B.; O’Riordan, E.G. Effects of Transporting Bulls at Different Space Allowances on Physiological, Haematological and Immunological Responses to a 12-H Journey by Road. Ir. J. Agric. Food Res. 2006, 45, 39–50. [Google Scholar]
- Gupta, S.; Earley, B.; Crowe, M.A. Effect of 12-Hour Road Transportation on Physiological, Immunological and Haematological Parameters in Bulls Housed at Different Space Allowances. Vet. J. 2007, 173, 605–616. [Google Scholar] [CrossRef]
- Yagi, Y.; Shiono, H.; Chikayama, Y.; Ohnuma, A.; Nakamura, I.; Yayou, K.-I. Transport Stress Increases Somatic Cell Counts in Milk, and Enhances the Migration Capacity of Peripheral Blood Neutrophils of Dairy Cows. J. Vet. Med. Sci. 2004, 66, 381–387. [Google Scholar] [CrossRef]
- Marcato, F.; van den Brand, H.; Kemp, B.; van Reenen, K. Evaluating Potential Biomarkers of Health and Performance in Veal Calves. Front. Vet. Sci. 2018, 5, 133. [Google Scholar] [CrossRef] [PubMed]
- Marcato, F.; van den Brand, H.; Kemp, B.; Engel, B.; Wolthuis-Fillerup, M.; van Reenen, K. Transport of Young Veal Calves: Effects of Pre-Transport Diet, Transport Duration and Type of Vehicle on Health, Behavior, Use of Medicines, and Slaughter Characteristics. Front. Vet. Sci. 2020, 7, 576469. [Google Scholar] [CrossRef]
- Marcato, F.; van den Brand, H.; Kemp, B.; Engel, B.; Wolthuis-Fillerup, M.; van Reenen, K. Effects of Pretransport Diet, Transport Duration, and Type of Vehicle on Physiological Status of Young Veal Calves. J. Dairy Sci. 2020, 103, 3505–3520. [Google Scholar] [CrossRef]
- Marcato, F.; van den Brand, H.; Kemp, B.; Engel, B.; Schnabel, S.K.; Hoorweg, F.A.; Wolthuis-Fillerup, M.; van Reenen, K. Effects of Transport Age and Calf and Maternal Characteristics on Health and Performance of Veal Calves. J. Dairy Sci. 2022, 105, 1452–1468. [Google Scholar] [CrossRef]
- Marcato, F.; van den Brand, H.; Jansen, C.A.; Rutten, V.P.M.G.; Kemp, B.; Engel, B.; Wolthuis-Fillerup, M.; van Reenen, K. Effects of Pre-Transport Diet, Transport Duration and Transport Condition on Immune Cell Subsets, Haptoglobin, Cortisol and Bilirubin in Young Veal Calves. PLoS ONE 2021, 16, e0246959. [Google Scholar] [CrossRef]
- Lay, D.C.; Friend, T.H.; Randel, R.D.; Bowers, C.L.; Grissom, K.K.; Jenkins, O.C. Behavioral and Physiological Effects of Freeze or Hot-Iron Branding on Crossbred Cattle. J. Anim. Sci. 1992, 70, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Lauder, J.K.; Marti, S.; Schwartzkopf-Genswein, K.S.; Jelinski, M.D.; Janzen, E.D. Measuring Behavioral and Physiological Responses to Pain Mitigation for Ovariectomy in Bos Taurus Yearling Beef Heifers. J. Anim. Sci. 2020, 98, skz386. [Google Scholar] [CrossRef] [PubMed]
- Marti, S.; Velarde, A.; de la Torre, J.L.; Bach, A.; Aris, A.; Serrano, A.; Manteca, X.; Devant, M. Effects of Ring Castration with Local Anesthesia and Analgesia in Holstein Calves at 3 Months of Age on Welfare Indicators. J. Anim. Sci. 2010, 88, 2789–2796. [Google Scholar] [CrossRef]
- Meléndez, D.M.; Marti, S.; Pajor, E.A.; Moya, D.; Heuston, C.E.M.; Gellatly, D.; Janzen, E.D.; Schwartzkopf-Genswein, K.S. Effect of Band and Knife Castration of Beef Calves on Welfare Indicators of Pain at Three Relevant Industry Ages: I. Acute Pain. J. Anim. Sci. 2017, 95, 4352–4366. [Google Scholar] [CrossRef] [PubMed]
- Meléndez, D.M.; Marti, S.; Pajor, E.A.; Sidhu, P.K.; Gellatly, D.; Moya, D.; Janzen, E.D.; Coetzee, J.F.; Schwartzkopf-Genswein, K.S. Effect of Meloxicam and Lidocaine Administered Alone or in Combination on Indicators of Pain and Distress during and after Knife Castration in Weaned Beef Calves. PLoS ONE 2018, 13, e0207289. [Google Scholar] [CrossRef]
- Pang, W.Y.; Earley, B.; Sweeney, T.; Crowe, M.A. Effect of Carprofen Administration during Banding or Burdizzo Castration of Bulls on Plasma Cortisol, in Vitro Interferon-γ Production, Acute-Phase Proteins, Feed Intake, and Growth. J. Anim. Sci. 2006, 84, 351–359. [Google Scholar] [CrossRef]
- Hickey, M.C.; Drennan, M.; Earley, B. The Effect of Abrupt Weaning of Suckler Calves on the Plasma Concentrations of Cortisol, Catecholamines, Leukocytes, Acute-Phase Proteins and in Vitro Interferon-Gamma Production. J. Anim. Sci. 2003, 81, 2847–2855. [Google Scholar] [CrossRef]
- Gupta, S.; Earley, B.; Ting, S.T.L.; Crowe, M.A. Effect of Repeated Regrouping and Relocation on the Physiological, Immunological, and Hematological Variables and Performance of Steers. J. Anim. Sci. 2005, 83, 1948–1958. [Google Scholar] [CrossRef]
- Grandin, T. Assessment of Stress during Handling and Transport. J. Anim. Sci. 1997, 75, 249. [Google Scholar] [CrossRef]
- Griffin, J.F.T. Stress and Immunity: A Unifying Concept. Vet. Immunol. Immunopathol. 1989, 20, 263–312. [Google Scholar] [CrossRef]
- Arthington, J.D.; Eicher, S.D.; Kunkle, W.E.; Martin, F.G. Effect of Transportation and Commingling on the Acute-Phase Protein Response, Growth, and Feed Intake of Newly Weaned Beef Calves. J. Anim. Sci. 2003, 81, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Crookshank, H.R.; Elissalde, M.H.; White, R.G.; Clanton, D.C.; Smalley, H.E. Effect of Transportation and Handling of Calves upon Blood Serum Composition. J. Anim. Sci. 1979, 48, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.E.; Ewbank, R. The Effect of Road Transportation on the Blood Constituents and Behaviour of Calves. I. Six Months Old. Br. Vet. J. 1983, 139, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Takahashi, H.; Matsumoto, H. The Effects of Road Transportation on Peripheral Blood Lymphocyte Subpopulations, Lymphocyte Blastogenesis and Neutrophil Function in Calves. Br. Vet. J. 1987, 143, 166–174. [Google Scholar] [CrossRef]
- Tarrant, P.J.V.; Kenny, F.J.; Harrington, D.; Murphy, M.H. Long Distance Transportation of Steers to Slaughter: Effect of Stocking Density on Physiology, Behaviour and Carcass Quality. Livest. Prod. Sci. 1992, 30, 223–238. [Google Scholar] [CrossRef]
- Knowles, G.; Warriss, P.D.; Brown, S.N.; Edwards, J.E. Effects on Cattle of Transportation by Road for up to 31 Hours. Vet. Rec. 1999, 145, 575–582. [Google Scholar] [CrossRef]
- Warriss, P.D.; Brown, S.N.; Knowles, T.G.; Kestin, S.C.; Edwards, J.E.; Dolan, S.K.; Phillips, A.J. Effects on Cattle of Transport by Road for up to 15 Hours. Vet. Rec. 1995, 136, 319–323. [Google Scholar] [CrossRef]
- Grigor, P.N.; Cockram, M.S.; Steele, W.B.; Le Sueur, C.J.; Forsyth, R.E.; Guthrie, J.A.; Johnson, A.K.; Sandilands, V.; Reid, H.W.; Sinclair, C.; et al. Effects of Space Allowance during Transport and Duration of Midjourney Lairage Period on the Physiological, Behavioural and Immunological Responses of Young Calves during and after Transport. Anim. Sci. 2001, 73, 341–360. [Google Scholar] [CrossRef]
- Fell, L.R.; Shutt, D.A. Adrenocortical Response of Calves to Transport Stress as Measured by Salivary Cortisol. Can. J. Anim. Sci. 1986, 66, 637–641. [Google Scholar] [CrossRef]
- Kent, J.E.; Ewbank, R. The Effect of Road Transportation on the Blood Constituents and Behaviour of Calves. II. One to Three Weeks Old. Br. Vet. J. 1986, 142, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Odore, R.; Badino, P.; Re, G.; Barbero, R.; Cuniberti, B.; D’Angelo, A.; Girardi, C.; Fraccaro, E.; Tarantola, M. Effects of Housing and Short-Term Transportation on Hormone and Lymphocyte Receptor Concentrations in Beef Cattle. Res. Vet. Sci. 2011, 90, 341–345. [Google Scholar] [CrossRef]
- Agnes, F.; Sartorelli, P.; Abdi, B.H.; Locatelli, A. Effect of Transport Loading or Noise on Blood Biochemical Variables in Calves. Am. J. Vet. Res. 1990, 51, 1679–1681. [Google Scholar] [PubMed]
- Higuchi, H.; Katoh, N.; Miyamoto, T.; Uchida, E.; Yuasa, A.; Takahashi, K. Dexamethasone-Induced Haptoglobin Release by Calf Liver Parenchymal Cells. Am. J. Vet. Res. 1994, 55, 1080–1085. [Google Scholar]
- Dixit, V.D.; Marahrens, M.; Parvizi, N. Transport Stress Modulates Adrenocorticotropin Secretion from Peripheral Bovine Lymphocytes. J. Anim. Sci. 2001, 79, 729–734. [Google Scholar] [CrossRef]
- Eldridge, G.A.; Winfield, C.G.; Cahill, D.J. Responses of Cattle to Different Space Allowances, Pen Sizes and Road Conditions during Transport. Aust. J. Exp. Agric. 1988, 28, 155–159. [Google Scholar] [CrossRef]
- Jongman, E.C.; Butler, K.L. The Effect of Age, Stocking Density and Flooring during Transport on Welfare of Young Dairy Calves in Australia. Animals 2014, 4, 184–189. [Google Scholar] [CrossRef]
- Trunkfield, H.R.; Broom, D.M. The Welfare of Calves during Handling and Transport. Appl. Anim. Behav. Sci. 1990, 28, 135–152. [Google Scholar] [CrossRef]
- Friend, T.H. Behavioral Aspects of Stress. J. Dairy Sci. 1991, 74, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.G.; Brown, S.N.; Edwards, J.E.; Phillips, A.J.; Warriss, P.D. Effect on Young Calves of a One-Hour Feeding Stop during a 19-Hour Road Journey. Vet. Rec. 1999, 144, 687–692. [Google Scholar] [CrossRef]
- Pisoni, L.; Devant, M.; Blanch, M.; Pastor, J.J.; Marti, S. Simulation of Feed Restriction and Fasting: Effects on Animal Recovery and Gastrointestinal Permeability in Unweaned Angus-Holstein Calves. J. Dairy Sci. 2022, 105, 2572–2586. [Google Scholar] [CrossRef]
- Pisoni, L.; Devant, M.; Bassols, A.M.; Saco, Y.; Pato, R.; Pujols, J.; Marti, S. The Effects of Colostrum Consumption and Feed Restriction during Marketing and Transportation in the Recovery of Dairy Beef Male Holstein Calves. J. Dairy Sci. 2023, in press. [CrossRef]
- Campbell, N.; Reece, J.; Mitchell, L. Biology, 5th ed.; Reece, J., Ed.; Always Learning (Pearson); Benjamin Cummings: San Francisco, CA, USA, 1999; ISBN 9781486007042. [Google Scholar]
- Earley, B.; Murray, M.; Prendiville, D.J. Effect of Road Transport for up to 24 Hours Followed by Twenty-Four Hour Recovery on Live Weight and Physiological Responses of Bulls. BMC Vet. Res. 2010, 6, 38. [Google Scholar] [CrossRef]
- Earley, B.; Murray, M.; Prendiville, D.J.; Pintado, B.; Borque, C.; Canali, E. The Effect of Transport by Road and Sea on Physiology, Immunity and Behaviour of Beef Cattle. Res. Vet. Sci. 2012, 92, 531–541. [Google Scholar] [CrossRef]
- Tarrant, P.V. Animal Behaviour and Environment in the Dark-Cutting Condition in Beef—A Review. Ir. J. Food Sci. Technol. 1989, 13, 1–21. [Google Scholar]
- Seideman, S.C.; Cross, H.R.; Smith, G.C.; Durland, P.R. Factors Associated with Fresh Meat Color: A Review. J. Food Qual. 1984, 6, 211–237. [Google Scholar] [CrossRef]
- McVeigh, J.M.; Tarrant, P.V. Effect of Propranolol on Muscle Glycogen Metabolism during Social Regrouping of Young Bulls. J. Anim. Sci. 1983, 56, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Rojo, A.; Mota-Rojas, D.; García-Galicia, I.; Ramírez-Bribiesca, E.; Olmos-Hernández, A.; Guerrero-Legarreta, I. Dark Cutting in Large Ruminants: Effect of Management and Environmental Factors. Agro Productividad. 2020, 13, 93–98. [Google Scholar] [CrossRef]
- Tarrant, P.V.; Sherington, J. An Investigation of Ultimate PH in the Muscles of Commercial Beef Carcasses. Meat Sci. 1980, 4, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Price, M.A.; Makarechian, M.; Tennessen, T.; Mathison, G.W. The effects of zeranol on the feedlot performance of beef bulls. Can. J. Anim. Sci. 1983, 63, 803–809. [Google Scholar] [CrossRef]
- Hedrick, H.B. Preventive Treatments during the Pre-Slaughter Period. In The Problem of Dark-Cutting in Beef; Springer Netherlands: Dordrecht, The Netherlands, 1981; pp. 213–230. [Google Scholar]
- Ishizaki, H.; Hanafusa, Y.; Kariya, Y. Influence of Truck-Transportation on the Function of Bronchoalveolar Lavage Fluid Cells in Cattle. Vet. Immunol. Immunopathol. 2005, 105, 67–74. [Google Scholar] [CrossRef]
- Mackenzie, A.M.; Drennan, M.; Rowan, T.G.; Dixon, J.B.; Carter, S.D. Effect of Transportation and Weaning on Humoral Immune Responses of Calves. Res. Vet. Sci. 1997, 63, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Riondato, F.; D’Angelo, A.; Miniscalco, B.; Bellino, C.; Guglielmino, R. Effects of Road Transportation on Lymphocyte Subsets in Calves. Vet. J. 2008, 175, 364–368. [Google Scholar] [CrossRef]
- Baumann, H.; Gauldie, J. The Acute Phase Response. Immunol. Today 1994, 15, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Horadagoda, N.U.; Knox, K.M.G.; Gibbs, H.A.; Reid, S.W.J.; Horadagoda, A.; Edwards, S.E.R.; Eckersall, P.D. Acute Phase Proteins in Cattle: Discrimination between Acute and Chronic Inflammation. Vet. Rec. 1999, 144, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Murata, H.; Miyamoto, T. Bovine Haptoglobin as a Possible Immunomodulator in the Sera of Transported Calves. Br. Vet. J. 1993, 149, 277–283. [Google Scholar] [CrossRef]
- Miller, J.K.; Brzezinska-Slebodzinska, E.; Madsen, F.C. Oxidative Stress, Antioxidants, and Animal Function. J. Dairy Sci. 1993, 76, 2812–2823. [Google Scholar] [CrossRef]
- Chirase, N.K.; Greene, L.W.; Purdy, C.W.; Loan, R.W.; Auvermann, B.W.; Parker, D.B.; Walborg, E.F., Jr.; Stevenson, D.E.; Yong, X.; Klaunig, J.E. Effect of Transport Stress on Respiratory Disease, Serum Antioxidant Status, and Serum Concentrations of Lipid Peroxidation Biomarkers in Beef Cattle. Am. J. Vet. Res. 2004, 65, 860–864. [Google Scholar] [CrossRef]
- Earley, B.; Buckham Sporer, K.; Gupta, S. Invited Review: Relationship between Cattle Transport, Immunity and Respiratory Disease. In Proceedings of the Animal; Cambridge University Press: Cambridge, UK, 2017; Volume 11, pp. 486–492. [Google Scholar]
- Mormede, P.; Soissons, J.; Bluthe, R.M.; Raoult, J.; Legarff, G.; Levieux, D.; Dantzer, R. Effect of Transportation on Blood Serum Composition, Disease Incidence, and Production Traits in Young Calves. Influence of the Journey Duration. Ann. Rech. Vet. 1982, 13, 369–384. [Google Scholar]
- Irwin, M.R.; McConnell, S.; Coleman, J.D.; Wilcox, G.E. Bovine Respiratory Disease Complex: A Comparison of Potential Predisposing and Etiologic Factors in Australia and the United States. J. Am. Vet. Med. Assoc. 1979, 175, 1095–1099. [Google Scholar]
- Irwin, M.R.; Melendy, D.R.; Amoss, M.S.; Hutcheson, D.P. Roles of Predisposing Factors and Gonadal Hormones in the Buller Syndrome of Feedlot Steers. J. Am. Vet. Med. Assoc. 1979, 174, 367–370. [Google Scholar]
- Kelley, K.W.; Osborne, C.A.; Evermann, J.F.; Parish, S.M.; Hinrichs, D.J. Whole Blood Leukocyte vs. Separated Mononuclear Cell Blastogenesis in Calves: Time-Dependent Changes after Shipping. Can. J. Comp. Med. 1981, 45, 249–258. [Google Scholar]
- Buckham Sporer, K.R.; Weber, P.S.D.; Burton, J.L.; Earley, B.; Crowe, M.A. Transportation of Young Beef Bulls Alters Circulating Physiological Parameters that May Be Effective Biomarkers of Stress. J. Anim. Sci. 2008, 86, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Sporer, K.R.B.; Xiao, L.; Tempelman, R.J.; Burton, J.L.; Earley, B.; Crowe, M.A. Transportation Stress Alters the Circulating Steroid Environment and Neutrophil Gene Expression in Beef Bulls. Vet. Immunol. Immunopathol. 2008, 121, 300–320. [Google Scholar] [CrossRef]
- Grandin, T. Solving Livestock Handling Problems Solving Livestock Handling Problems. Vet. Med. 1994, 89, 989–998. [Google Scholar]
- Wigham, E.E.; Butterworth, A.; Wotton, S. Assessing Cattle Welfare at Slaughter—Why Is It Important and What Challenges Are Faced? Meat Sci. 2018, 145, 171–177. [Google Scholar] [CrossRef]
- Strappini, A.C.; Metz, J.H.M.; Gallo, C.B.; Kemp, B. Origin and Assessment of Bruises in Beef Cattle at Slaughter. Animal 2009, 3, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Strappini, A.C.; Metz, J.H.M.; Gallo, C.; Frankena, K.; Vargas, R.; de Freslon, I.; Kemp, B. Bruises in Culled Cows: When, Where and How Are They Inflicted? Animal 2013, 7, 485–491. [Google Scholar] [CrossRef]
- Losada-Espinosa, N.; Estévez-Moreno, L.; Bautista-Fernández, M.; Losada, H.; María, G.; Miranda-de la Lama, G. Integrative Surveillance of Cattle Welfare at the Abattoir Level: Risk Factors Associated with Liver Condemnation, Severe Hoof Disorders, Carcase Bruising and High Muscle PH. Anim. Welf. 2021, 30, 393–407. [Google Scholar] [CrossRef]
- Tarrant, V.; Grandin, T. Cattle Transport. In Livestock Handling and Transport; CABI: Wallingford, UK, 2000; pp. 151–173. [Google Scholar]
- Kenny, F.J.; Tarrant, P.V. The Reaction of Young Bulls to Short-Haul Road Transport. Appl. Anim. Behav. Sci. 1987, 17, 209–227. [Google Scholar] [CrossRef]
- Scientific Committee on Animal Health and Animal Welfare (SCAHAW). The Welfare of Animals during Transport (Details for Horses, Pigs, Sheep and Cattle); European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Tarrant, P.V.; Kenny, F.J.; Harrington, D. The Effect of Stocking Density during 4 Hour Transport to Slaughter on Behaviour, Blood Constituents and Carcass Bruising in Friesian Steers. Meat Sci. 1988, 24, 209–222. [Google Scholar] [CrossRef]
- Garcia, J.A.B.; ZambardaVaz, R.; Vaz, F.N.; Restle, J.; Mendonça, F.S. Pre-Slaughter Factors Associated with Severe Bruising in Different Primary Commercial Cuts of Bovine Carcasses. Revista Ciência Agronômica 2019, 50, 681–690. [Google Scholar] [CrossRef]
- Mendonça, F.S.; Vaz, R.Z.; Vaz, F.N.; Leal, W.S.; Silveira, I.D.B.; Restle, J.; Boligon, A.A.; Cardoso, F.F. Causes of Bruising in Carcasses of Beef Cattle during Farm, Transport, and Slaughterhouse Handling in Brazil. Anim. Sci. J. 2019, 90, 288–296. [Google Scholar] [CrossRef]
- Anderson, B.; Horder, J.C. The Australian Carcass Bruise Scoring System [Visual Appraisal of Damage]. Qld. Agric. J. 1979, 105, 281–287. [Google Scholar]
- Alam, M.R.; Gregory, N.G.; Jabbar, M.A.; Uddin, M.S.; Kibria, A.S.M.G.; Silva-Fletcher, A. Skin Injuries Identified in Cattle and Water Buffaloes at Livestock Markets in Bangladesh. Vet. Rec. 2010, 167, 415–419. [Google Scholar] [CrossRef]
- José-Pérez, N.; Mota-Rojas, D.; Ghezzi, M.; Rosmini, M.; Medina, P.M.; Bertoni, A.; Rodríguez-González, D.; Domínguez-Oliva, A.; Legarreta, I.G. Effects of Transport on Water Buffaloes (Bubalus Bubalis): Factors Associated with the Frequency of Skin Injuries and Meat Quality. J. Anim. Behav. Biometeorol. 2022, 10, 1–9. [Google Scholar] [CrossRef]
- Staples, G.E.; Haugse, C.N. Losses in Young Calves after Transportation. Br. Vet. J. 1974, 130, 374–379. [Google Scholar] [CrossRef]
- Chase, C.C.L. Acceptable Young Calf Vaccination Strategies—What, When, and How? Vet. Clin. N. Am. Food Anim. Pract. 2022, 38, 17–37. [Google Scholar] [CrossRef]
- Morein, B.; Abusugra, I.; Blomqvist, G. Immunity in Neonates. Vet. Immunol. Immunopathol. 2002, 87, 207–213. [Google Scholar] [CrossRef]
- Chase, C.C.L.; Hurley, D.J.; Reber, A.J. Neonatal Immune Development in the Calf and Its Impact on Vaccine Response. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 87–104. [Google Scholar] [CrossRef]
- Chase, C.C.L. Enteric Immunity. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cave, J.G.; Callinan, A.P.L.; Woonton, W.K. Mortalities in Bobby Calves Associated with Long Distance Transport. Aust. Vet. J. 2005, 83, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.W.; Jordaan, P. Pre-Slaughter Mortality and Post-Slaughter Wastage in Bobby Veal Calves at a Slaughter Premises in New Zealand. N. Z. Vet. J. 2013, 61, 127–132. [Google Scholar] [CrossRef]
- Seppä-Lassila, L.; Oksanen, J.; Herva, T.; Dorbek-Kolin, E.; Kosunen, H.; Parviainen, L.; Soveri, T.; Orro, T. Associations between Group Sizes, Serum Protein Levels, Calf Morbidity and Growth in Dairy-Beef Calves in a Finnish Calf Rearing Unit. Prev. Vet. Med. 2018, 161, 100–108. [Google Scholar] [CrossRef]
- Velarde, A.; Teixeira, D.; Devant, M.; Martí, S. Research for ANIT Committee—Particular Welfare Needs in Animal Transport: Unweaned Animals and Pregnant Females; EPRS: Brussels, Belgium, 2021. [Google Scholar]
- Edwards, T.A. Control Methods for Bovine Respiratory Disease for Feedlot Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 273–284. [Google Scholar] [CrossRef]
- USDA Part II: Baseline Reference of Feed Lot Health and Health Management, 1999. USDA: APHIS: VS, CEAH, National Animal Health Monitoring System 2000, #N335.1000. Available online: https://www.aphis.usda.gov/animal_health/nahms/feedlot/downloads/feedlot99/Feedlot99_dr_PartII.pdf (accessed on 2 September 2022).
- Smith, D.R. Risk Factors for Bovine Respiratory Disease in Beef Cattle. Anim. Health Res. Rev. 2020, 21, 149–152. [Google Scholar] [CrossRef]
- Phillips, W.A.; Juniewicz, P.E.; Zavy, M.T.; Von Tungeln, D.L. The Effects of the Stress of Weaning and Transit on Performance and Metabolic Profile of Beef Calves of Different Genotypes. Can. J. Anim. Sci. 1987, 67, 991–999. [Google Scholar] [CrossRef]
- Tarrant, P.V. Transportation of Cattle by Road. Appl. Anim. Behav. Sci. 1990, 28, 153–170. [Google Scholar] [CrossRef]
- Knowles, G. A Review of the Road Transport of Cattle. Vet. Rec. 1999, 144, 197–201. [Google Scholar] [CrossRef]
- Weeks, C. A Review of Welfare in Cattle, Sheep and Pig Lairages, with Emphasis on Stocking Rates, Ventilation and Noise. Anim. Welf. 2008, 17, 275–284. [Google Scholar] [CrossRef]
- Todd, S.E.; Mellor, D.J.; Stafford, K.J.; Gregory, N.G.; Bruce, R.A.; Ward, R.N. Effects of Food Withdrawal and Transport on 5- to 10-Day-Old Calves. Res. Vet. Sci. 2000, 68, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Uetake, K.; Tanaka, T.; Sato, S. Effects of Haul Distance and Stocking Density on Young Suckling Calves Transported in Japan. Anim. Sci. J. 2011, 82, 587–590. [Google Scholar] [CrossRef]
- Fisher, A.D.; Stevens, B.H.; Conley, M.J.; Jongman, E.C.; Lauber, M.C.; Hides, S.J.; Anderson, G.A.; Duganzich, D.M.; Mansell, P.D. The Effects of Direct and Indirect Road Transport Consignment in Combination with Feed Withdrawal in Young Dairy Calves. J. Dairy Res. 2014, 81, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 1/2005 of the European Council of 22 December 2004 on the Protection of Animals during Transport and Related Operations and Amending Directives 64/432/EEC and 93/119/EC and Regulation (EC) No 1255/9. Available online: https://eur-lex.europa.eu/eli/reg/2005/1/oj (accessed on 2 September 2022).
- Petherick, J.C.; Phillips, C.J.C. Space Allowances for Confined Livestock and Their Determination from Allometric Principles. Appl. Anim. Behav. Sci. 2009, 117, 1–12. [Google Scholar] [CrossRef]
- Keane, M.P.; McGee, M.; O’Riordan, E.G.; Kelly, A.K.; Earley, B. Performance and Welfare of Steers Housed on Concrete Slatted Floors at Fixed and Dynamic (Allometric Based) Space Allowances. J. Anim. Sci. 2018, 96, 880–889. [Google Scholar] [CrossRef]
- Roadknight, N.; Mansell, P.; Jongman, E.; Courtman, N.; Fisher, A. Invited Review: The Welfare of Young Calves Transported by Road. J. Dairy Sci. 2021, 104, 6343–6357. [Google Scholar] [CrossRef]
- Boulton, A.C.; Kells, N.J.; Cogger, N.; Johnson, C.B.; O’Connor, C.; Webster, J.; Palmer, A.; Beausoleil, N.J. Risk Factors for Bobby Calf Mortality across the New Zealand Dairy Supply Chain. Prev. Vet. Med. 2020, 174, 104836. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.L.; Smith, J.L.; Smith, D.L.; Swanson, D.L.; Drake, T.R.; Wolfgang, D.R.; Wheeler, E.F. Characteristics of Veal Calves upon Arrival, at 28 and 84 Days, and at End of the Production Cycle. J. Dairy Sci. 2000, 83, 843–854. [Google Scholar] [CrossRef]
- Meléndez, D.M.; Marti, S.; Haley, D.B.; Schwinghamer, T.D.; Schwartzkopf-Genswein, K.S. Effect of Transport and Rest Stop Duration on the Welfare of Conditioned Cattle Transported by Road. PLoS ONE 2020, 15, e0228492. [Google Scholar] [CrossRef]
- Meléndez, D.M.; Marti, S.; Haley, D.B.; Schwinghamer, T.D.; Schwartzkopf-Genswein, K.S. Effects of Conditioning, Source, and Rest on Indicators of Stress in Beef Cattle Transported by Road. PLoS ONE 2021, 16, e0244854. [Google Scholar] [CrossRef]
- Cooke, R.F.; Guarnieri Filho, T.A.; Cappellozza, B.I.; Bohnert, D.W. Rest Stops during Road Transport: Impacts on Performance and Acute-Phase Protein Responses of Feeder Cattle. J. Anim. Sci. 2013, 91, 5448–5454. [Google Scholar] [CrossRef]
- Marti, S.; Wilde, R.E.; Moya, D.; Heuston, C.E.M.; Brown, F.; Schwartzkopf-Genswein, K.S. Effect of Rest Stop Duration during Long-Distance Transport on Welfare Indicators in Recently Weaned Beef Calves. J. Anim. Sci. 2017, 95, 636–644. [Google Scholar] [CrossRef]
- Ross, M.; Widowsk, T.; Haley, D. The Effects of Feeding Space on the Behavioural Responses of Cattle during Rest Periods Offered as Part of Long-Distance Transportation. Anim. Welf. 2016, 25, 217–225. [Google Scholar] [CrossRef]
- Olivares Guzman, P. The Effect of Environmental and Animal Characteristics on Cattle Behaviour at Rest-Stops During Long-Distance Transportation; University of Guelph: Guelph, ON, Canada, 2023. [Google Scholar]
- Guzman, P.O.; Marti, S.; Pearl, D.L.; Schwartzkopf-Genswein, K.S.; Melendez, D.; Haley, D.B. PSXIII-1 Does Providing Bedding Change the Latency and Duration of Cattle Lying Behavior During Long-Distance Transport Rest Stops? J. Anim. Sci. 2022, 100, 206–207. [Google Scholar] [CrossRef]
- Guzman, P.O.; Pearl, D.L.; Schwartzkopf-Genswein, K.S.; Widowski, T.M.; Meléndez Suárez, D.M.; Marti, S.; Haley, D.B. 6 Does Bedding Influence Lying Behaviour of Cattle Unloaded for Rest During Long-Distance Transportation? J. Anim. Sci. 2021, 99, 2. [Google Scholar] [CrossRef]
- Uddin, M.S.; Schwartzkopf-Genswein, K.S.; Waldner, M.; Meléndez, D.M.; Niu, Y.D.; Alexander, T.W. Auction Market Placement and a Rest Stop during Transportation Affect the Respiratory Bacterial Microbiota of Beef Cattle. Front. Microbiol. 2023, 14, 1192763. [Google Scholar] [CrossRef] [PubMed]
- Theurer, M.E.; White, B.J.; Anderson, D.E.; Miesner, M.D.; Mosier, D.A.; Coetzee, J.F.; Amrine, D.E. Effect of Transportation during Periods of High Ambient Temperature on Physiologic and Behavioral Indices of Beef Heifers. Am. J. Vet. Res. 2013, 74, 481–490. [Google Scholar] [CrossRef]
- de Castro Júnior, S.L.; Silva, I.J.O.d. The Specific Enthalpy of Air as an Indicator of Heat Stress in Livestock Animals. Int. J. Biometeorol. 2021, 65, 149–161. [Google Scholar] [CrossRef]
- McDowell, R.E.; Lee, D.H.K.; Fohrman, M.H. The Measurement of Water Evaporation from Limited Areas of a Normal Body Surface. J. Anim. Sci. 1954, 13, 405–416. [Google Scholar] [CrossRef]
- Bianca, W.; Hales, J.R. Sweating, Panting and Body Temperatures of Newborn and One-Year-Old Calves at High Environmental Temperatures. Br. Vet. J. 1970, 126, 45–53. [Google Scholar] [CrossRef]
- Spain, J.N.; Spiers, D.E. Effects of Supplemental Shade on Thermoregulatory Response of Calves to Heat Challenge in a Hutch Environment. J. Dairy Sci. 1996, 79, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.L.; Drackley, J.K. The Development, Nutrition, and Management of the Young Calf; Iowa State University Press: Ames, IA, USA, 1998; ISBN 0813829801. [Google Scholar]
- Ganaie, A.H.; Ghasura, R.S.; Mir, N.A.; Bumla, N.A.; Sankar, G.; Wani, S.A. Biochemica and Physiological Changes during Thermal Stress in Bovines. J. Vet. Sci. Technol. 2013, 4, 423–430. [Google Scholar] [CrossRef]
- Brown-Brandl, T.M. Understanding Heat Stress in Beef Cattle. Revista Brasileira Zootecnia 2018, 47, e20160414. [Google Scholar] [CrossRef]
- Sarnighausen, V.C.R. Estimation of Thermal Comfort Indexes for Production Animals Using Multiple Linear Regression Models. J. Anim. Behav. Biometeorol. 2019, 7, 73–77. [Google Scholar] [CrossRef]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of Heat Stress on Animal Physiology, Metabolism, and Meat Quality: A Review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef]
- Carabaño, M.J.; Ramón, M.; Menéndez-Buxadera, A.; Molina, A.; Díaz, C. Selecting for Heat Tolerance. Anim. Front. 2019, 9, 62–68. [Google Scholar] [CrossRef]
- Becker, C.A.; Collier, R.J.; Stone, A.E. Invited Review: Physiological and Behavioral Effects of Heat Stress in Dairy Cows. J. Dairy Sci. 2020, 103, 6751–6770. [Google Scholar] [CrossRef]
- Sammad, A.; Wang, Y.J.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional Physiology and Biochemistry of Dairy Cattle under the Influence of Heat Stress: Consequences and Opportunities. Animals 2020, 10, 793. [Google Scholar] [CrossRef]
- Mader, T.L.; Davis, M.S.; Brown-Brandl, T. Environmental Factors Influencing Heat Stress in Feedlot Cattle. J. Anim. Sci. 2006, 84, 712–719. [Google Scholar] [CrossRef]
- Gaughan, J.; SM, H.; Hahn, G.; Mader, T.; Eigenberg, R.A. Respiration Rate—Is It a Good Measure of Heat Stress in Cattle? Asian-Australas J. Anim. Sci. 2000, 13, 329–332. [Google Scholar]
- Gaughan, J.B.; Mader, T.L.; Holt, S.M.; Sullivan, M.L.; Hahn, G.L. Assessing the Heat Tolerance of 17 Beef Cattle Genotypes. Int. J. Biometeorol. 2010, 54, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Norris, R.T.; Richards, R.B.; Creeper, J.H.; Jubb, T.F.; Madin, B.; Kerr, J. Cattle Deaths during Sea Transport from Australia. Aust. Vet. J. 2003, 81, 156–161. [Google Scholar] [CrossRef]
- Godyń, D.; Herbut, P.; Angrecka, S. Measurements of Peripheral and Deep Body Temperature in Cattle—A Review. J. Therm. Biol. 2019, 79, 42–49. [Google Scholar] [CrossRef]
- Brito, L.F.; Oliveira, H.R.; McConn, B.R.; Schinckel, A.P.; Arrazola, A.; Marchant-Forde, J.N.; Johnson, J.S. Large-Scale Phenotyping of Livestock Welfare in Commercial Production Systems: A New Frontier in Animal Breeding. Front. Genet. 2020, 11, 793. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Banhazi, T.; Perano, K.; Ghahramani, A.; Bowtell, L.; Wang, C.; Li, B. A Review of Measuring, Assessing and Mitigating Heat Stress in Dairy Cattle. Biosyst. Eng. 2020, 199, 4–26. [Google Scholar] [CrossRef]
- Michell, A.R. Textbook of Veterinary Physiology: [3rd Edn, 2002] Edited by J.G. Cunningham, Saunders, Philadelphia. Vet. J. 2003, 165, 90–91. [Google Scholar] [CrossRef]
- Ammer, S.; Lambertz, C.; Gauly, M. Is Reticular Temperature a Useful Indicator of Heat Stress in Dairy Cattle? J. Dairy Sci. 2016, 99, 10067–10076. [Google Scholar] [CrossRef]
- Stewart, M.; Webster, J.R.; Verkerk, G.A.; Schaefer, A.L.; Colyn, J.J.; Stafford, K.J. Non-Invasive Measurement of Stress in Dairy Cows Using Infrared Thermography. Physiol. Behav. 2007, 92, 520–525. [Google Scholar] [CrossRef]
- McManus, C.; Tanure, C.B.; Peripolli, V.; Seixas, L.; Fischer, V.; Gabbi, A.M.; Menegassi, S.R.O.; Stumpf, M.T.; Kolling, G.J.; Dias, E.; et al. Infrared Thermography in Animal Production: An Overview. Comput. Electron. Agric. 2016, 123, 10–16. [Google Scholar] [CrossRef]
- Lei, M.C.; Félix, L.; Cardoso, R.; Monteiro, S.M.; Silva, S.; Venâncio, C. Non-Invasive Biomarkers in Saliva and Eye Infrared Thermography to Assess the Stress Response of Calves during Transport. Animals 2023, 13, 2311. [Google Scholar] [CrossRef]
- Rodríguez-González, D.; Guerrero Legarreta, I.; Cruz-Monterrosa, R.G.; Napolitano, F.; Titto, C.G.; Abd El-Aziz, A.H.; Hernández-Avalos, I.; Casas-Alvarado, A.; Domínguez-Oliva, A.; Mota-Rojas, D. Assessment of Thermal Changes in Water Buffalo Mobilized from the Paddock and Transported by Short Journeys. Front. Vet. Sci. 2023, 10, 1184577. [Google Scholar] [CrossRef] [PubMed]
- Idris, M.; Uddin, J.; Sullivan, M.; McNeill, D.M.; Phillips, C.J.C. Non-Invasive Physiological Indicators of Heat Stress in Cattle. Animals 2021, 11, 71. [Google Scholar] [CrossRef]
- Dahl-Pedersen, K.; Herskin, M.S.; Houe, H.; Thomsen, P.T. A Descriptive Study of the Clinical Condition of Cull Dairy Cows before Transport to Slaughter. Livest. Sci. 2018, 218, 108–113. [Google Scholar] [CrossRef]
- Cockram, M.S. Fitness of Animals for Transport to Slaughter. Can. Vet. J. 2019, 60, 423–429. [Google Scholar] [PubMed]
- Phillips, W.A.; Juniewicz, P.E.; Zavy, M.T.; Von Tungeln, D.L. The Effect of the Stress of Weaning and Transport on White Blood Cell Patterns and Fibrinogen Concentration of Beef Calves of Different Genotypes. Can. J. Anim. Sci. 1989, 69, 333–340. [Google Scholar] [CrossRef]
- Fraser, A.F.; Broom, D.M. Farm Animal Behaviour and Welfare, 3rd ed.; CAB International: Wallingford, UK, 1997; ISBN 0851991602. [Google Scholar]
- Gregory, N.G.; Benson, T.; Mason, C.W. Cattle Handling and Welfare Standards in Livestock Markets in the UK. J. Agric. Sci. 2009, 147, 345–354. [Google Scholar] [CrossRef]
- Bravo, V.; Gallo, C.; Acosta-Jamett, G. Effects of Short Transport and Prolonged Fasting in Beef Calves. Animals 2018, 8, 170. [Google Scholar] [CrossRef]
- Albright, J.L.; Stouffer, D.K.; Kenyon, N.J. Behaviour of Veal Calves in Individual Stalls and Group Pens; EAAP Publication (Netherlands): Wageningen, The Netherlands, 1991. [Google Scholar]
- Lensink, B.J.; Fernandez, X.; Cozzi, G.; Florand, L.; Veissier, I. The Influence of Farmers’ Behavior on Calves’ Reactions to Transport and Quality of Veal Meat. J. Anim. Sci. 2001, 79, 642. [Google Scholar] [CrossRef]
- Griffin, D. Economic Impact Associated with Respiratory Disease in Beef Cattle. Vet. Clin. N. Am. Food Anim. Pract. 1997, 13, 367–377. [Google Scholar] [CrossRef]
- Speer, N.C.; Slack, G.; Troyer, E. Economic Factors Associated with Livestock Transportation. J. Anim. Sci. 2001, 79, E166. [Google Scholar] [CrossRef]
- Lekeux, P. Bovine Respiratory Disease Complex. Bov. Pract. 1995, 29, 71–75. [Google Scholar] [CrossRef]
- Lynch, E.; Earley, B.; McGee, M.; Doyle, S. Effect of Post-Weaning Management Practices on Physiological and Immunological Responses of Weaned Beef Calves. Ir. J. Agric. Food Res. 2011, 6, 39. [Google Scholar] [CrossRef]
- USDA Feedlot 2011 Part IV: Health and Health Management on U.S. Feedlots with a Capacity of 1000 or More Head. Available online: https://www.aphis.usda.gov/animal_health/nahms/feedlot/downloads/feedlot2011/Feed11_dr_PartIV_1.pdf (accessed on 2 September 2022).
- Thompson, P.N.; Stone, A.; Schultheiss, W.A. Use of Treatment Records and Lung Lesion Scoring to Estimate the Effect of Respiratory Disease on Growth during Early and Late Finishing Periods in South African Feedlot Cattle. J. Anim. Sci. 2006, 84, 488–498. [Google Scholar] [CrossRef]
- Blakebrough-Hall, C.; McMeniman, J.P.; González, L.A. An Evaluation of the Economic Effects of Bovine Respiratory Disease on Animal Performance, Carcass Traits, and Economic Outcomes in Feedlot Cattle Defined Using Four BRD Diagnosis Methods. J. Anim. Sci. 2020, 98, skaa005. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.J.; Duganzich, D.M. A Note on Effect of Starvation on the Bovine Alimentary Tract and Its Contents. Anim. Prod. 1980, 31, 111–113. [Google Scholar] [CrossRef]
- Broom, D.M. Indicators of Poor Welfare. Br. Vet. J. 1986, 142, 524–526. [Google Scholar] [CrossRef]
- Broom, D.M. A History of Animal Welfare Science. Acta Biotheor. 2011, 59, 121–137. [Google Scholar] [CrossRef]
- Duncan, I.J.H.; Hild, S.; Schweitzer, L. Animal Welfare: From Science to Law. In Animal Welfare: A Brief History; La Fondation Droit Animal, Éthique et Sciences (LFDA): Paris, France, 2019; pp. 13–19. ISBN 978-2-9512167-5-4. [Google Scholar]
- Fraser, D.; Weary, D.M.; Pajor, E.A.; Milligan, B.N. A Scientific Conception of Animal Welfare That Reflects Ethical Concerns. Anim. Welf. 1997, 6, 187–205. [Google Scholar] [CrossRef]
- Mellor, D. Updating Animal Welfare Thinking: Moving beyond the “Five Freedoms” towards “A Life Worth Living”. Animals 2016, 6, 21. [Google Scholar] [CrossRef]
- Webster, J. Animal Welfare: Freedoms, Dominions and “A Life Worth Living”. Animals 2016, 6, 35. [Google Scholar] [CrossRef]
- Broom, D.M. Welfare Assessment and Welfare Problem Areas during Handling and Transport. In Livestock Handling and Transport; Grandin, T., Ed.; CABI: Wallingford, UK, 2000; pp. 43–61. [Google Scholar]
- Broom, D.M.; Johnson, K.G. Stress and Animal Welfare: Key Issues in the Biology of Humans and Other Animals, 2nd ed.; Springer: Berlin, Germany, 1999; ISBN 3030321525. [Google Scholar]
- Serra, M.; Patricia, C.; Wolkers, B.; Urbinati, E.C. Physiological Indicators of Animal Welfare. Revista Brasileira Zoociências 2018, 19, 70–96. [Google Scholar] [CrossRef]
- Grandin, T. Handling Methods and Facilities to Reduce Stress on Cattle. Vet. Clin. N. Am. Food Anim. Pract. 1998, 14, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Bachelard, N. Animal Transport as Regulated in Europe: A Work in Progress as Viewed by an NGO. Anim. Front. 2022, 12, 16–24. [Google Scholar] [CrossRef]
- Lundmark, F.; Berg, C.; Röcklinsberg, H. Private Animal Welfare Standards—Opportunities and Risks. Animals 2018, 8, 4. [Google Scholar] [CrossRef]
- Alonso, M.E.; González-Montaña, J.R.; Lomillos, J.M. Consumers’ Concerns and Perceptions of Farm Animal Welfare. Animals 2020, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, D.; Napolitano, F.; Legarreta, I.G.; Mora-Medina, P.; Ghezzi, M.; José-Pérez, N.; Domínguez-Oliva, A.; Mota-Rojas, D. Critical Aspects of Legislation and Their Impact on the Welfare of Water Buffaloes during Transport. J. Anim. Behav. Biometeorol. 2022, 10, 1–13. [Google Scholar] [CrossRef]
- Coleman, G. Public Animal Welfare Discussions and Outlooks in Australia. Anim. Front. 2018, 8, 14–19. [Google Scholar] [CrossRef]
- Hampton, J.O.; Jones, B.; McGreevy, P.D. Social License and Animal Welfare: Developments from the Past Decade in Australia. Animals 2020, 10, 2237. [Google Scholar] [CrossRef]
- Vigors, B.; Sandøe, P.; Lawrence, A.B. Positive Welfare in Science and Society: Differences, Similarities and Synergies. Front. Anim. Sci. 2021, 2, 738193. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar Schmidt, C.; Michel, V.; et al. Welfare of Equidae during Transport. EFSA J. 2022, 20, e07444. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar Schmidt, C.; Michel, V.; et al. Welfare of Small Ruminants during Transport. EFSA J. 2022, 20, e07404. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar Schmidt, C.; Michel, V.; et al. Welfare of Pigs during Transport. EFSA J. 2022, 20, e07445. [Google Scholar] [CrossRef] [PubMed]
- Auge, M.-L. EPRS|European Parliamentary Research Service. 2018, pp. 1–12. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2018/630345/EPRS_BRI(2018)630345_EN.pdf (accessed on 4 May 2023).
- Dahl-Pedersen, K. Danish Cattle Farmers’ Experience With Fitness for Transport—A Questionnaire Survey. Front. Vet. Sci. 2022, 9, 797149. [Google Scholar] [CrossRef] [PubMed]
- Marquou, S.; Blouin, L.; Djakite, H.; Laplante, R.; Buczinski, S. Health Parameters and Their Association with Price in Young Calves Sold at Auction for Veal Operations in Québec, Canada. J. Dairy Sci. 2019, 102, 6454–6465. [Google Scholar] [CrossRef]



| Category | Weight, kg | Area m2/calf 1 | Area m2/calf, k = 0.02 | Area m2/calf, k = 0.027 | Area m2/calf, k = 0.033 |
|---|---|---|---|---|---|
| Small calves | 50 | 0.30–0.40 | 0.27 | 0.37 | 0.45 |
| Medium calves | 110 | 0.40–0.70 | 0.46 | 0.62 | 0.76 |
| Heavy calves | 200 | 0.70–0.95 | 0.68 | 0.92 | 1.13 |
| Medium size cattle | 325 | 0.95–1.30 | 0.95 | 1.28 | 1.56 |
| Heavy cattle | 550 | 1.30–1.60 | 1.34 | 1.81 | 2.22 |
| Very heavy cattle | >700 | >1.60 | >1.58 | >2.13 | >2.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buckham-Sporer, K.; Earley, B.; Marti, S. Current Knowledge on the Transportation by Road of Cattle, including Unweaned Calves. Animals 2023, 13, 3393. https://doi.org/10.3390/ani13213393
Buckham-Sporer K, Earley B, Marti S. Current Knowledge on the Transportation by Road of Cattle, including Unweaned Calves. Animals. 2023; 13(21):3393. https://doi.org/10.3390/ani13213393
Chicago/Turabian StyleBuckham-Sporer, Kelly, Bernadette Earley, and Sonia Marti. 2023. "Current Knowledge on the Transportation by Road of Cattle, including Unweaned Calves" Animals 13, no. 21: 3393. https://doi.org/10.3390/ani13213393
APA StyleBuckham-Sporer, K., Earley, B., & Marti, S. (2023). Current Knowledge on the Transportation by Road of Cattle, including Unweaned Calves. Animals, 13(21), 3393. https://doi.org/10.3390/ani13213393






