Interspecies Retinal Diversity and Optic Nerve Anatomy in Odontocetes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Collection and Storage
2.2. Sampling and Histological Processing
2.2.1. Optic Nerve Cross-Sections
2.2.2. Retinal Cross-Sections
2.3. Immunohistochemistry
2.4. Image Acquisition and Analyses
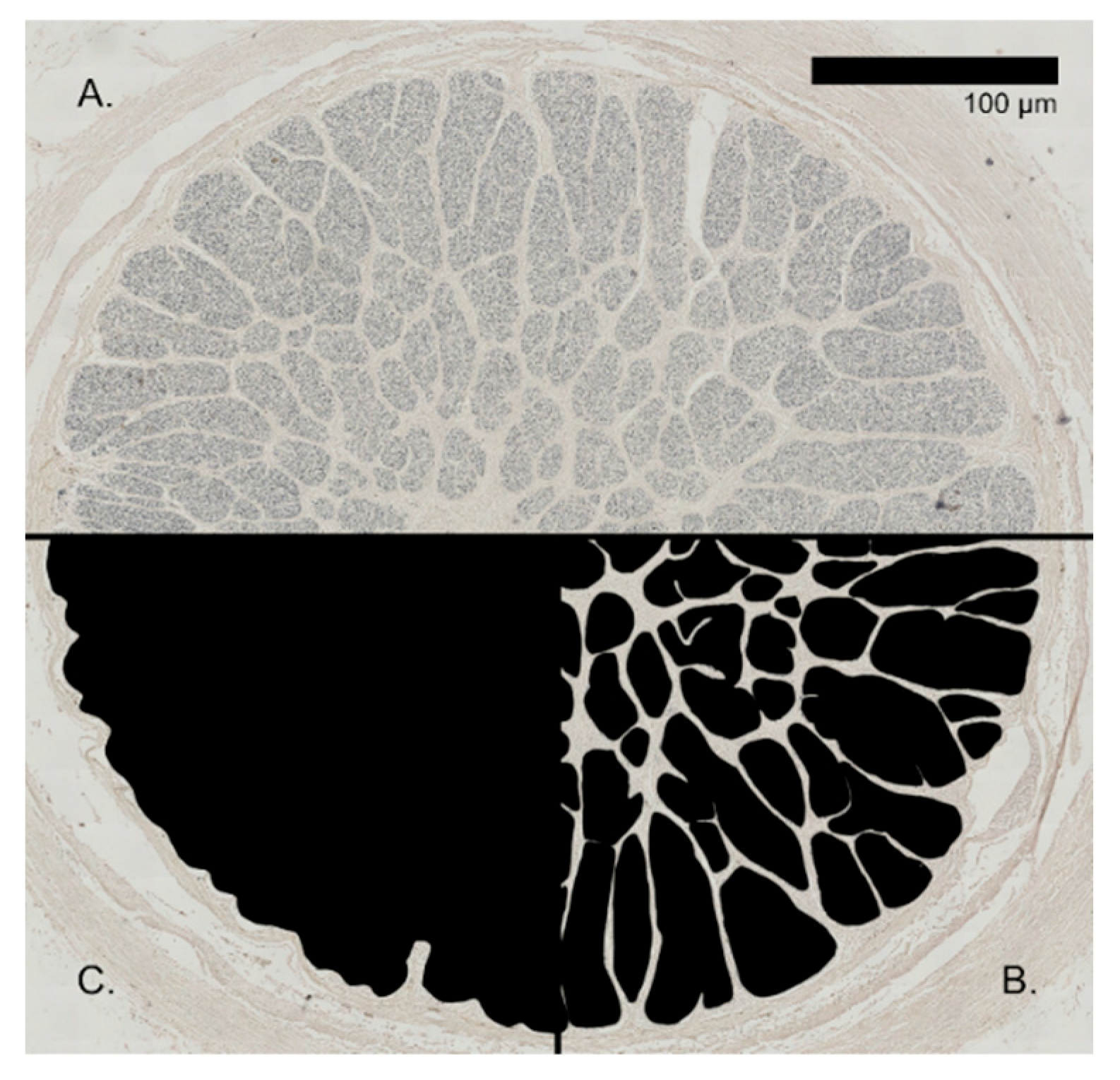
2.4.1. Optic Nerve Cross-Sections
2.4.2. Retinal Cross-Sections
3. Results
3.1. Optic Nerve
3.2. Retina
3.2.1. Retinal Thickness and Ganglion Cell Linear Density
3.2.2. Retinal Ganglion Cell Measurements
3.3. Immunohistochemistry
3.3.1. Neurofilament 200
3.3.2. Calretinin
4. Discussion
4.1. Optic Nerve
4.2. Retina
4.3. Immunohistochemistry
4.4. Limits of the Present Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Land, M.F.; Nilsson, D.E. Animal Eyes, 2nd ed.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Nilsson, D.E. The evolution of eyes and visually guided behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2833–2847. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, D.; Mooney, R.D. Sensation and sensory processing. In Neuroscience, 6th ed.; Purves, D., Augustine, G., Fitzpatrick, D., Hall, W.C., LaMantia, A., Mooney, R., White, L.E., Eds.; Sinauer Associates: New York, NY, USA, 2018; pp. 191–354. [Google Scholar]
- Masland, R.H. The neuronal organization of the retina. Neuron 2012, 76, 266–280. [Google Scholar] [CrossRef]
- Stewart, J.D. Peripheral nerve fascicles: Anatomy and clinical relevance. Muscle Nerve 2003, 28, 525–541. [Google Scholar] [CrossRef]
- Piccolino, M. Cajal and the retina: A 100-year retrospective. Trends Neurosci. 1988, 11, 521–525. [Google Scholar] [CrossRef]
- Lettvin, J.Y.; Maturana, H.R.; McCulloch, W.S.; Pitts, W.H. What the Frog’s Eye Tells the Frog’s Brain. Proc. IRE 1959, 47, 1940–1951. [Google Scholar] [CrossRef]
- Wehner, R. ‘Matched filters’—Neural models of the external world. J. Comp. Physiol. A 1987, 161, 511–531. [Google Scholar] [CrossRef]
- Sanes, J.R.; Masland, R.H. The types of retinal ganglion cells: Current status and implications for neuronal classification. Annu. Rev. Neurosci 2015, 38, 221–246. [Google Scholar] [CrossRef] [PubMed]
- Supin, A.Y.; Popov, V.V.; Mass, A.M. The Sensory Physiology of Aquatic Mammals; Springer: New York, NY, USA, 2001. [Google Scholar]
- Madsen, C.J.; Herman, L.M. Social and ecological correlates of cetacean visian and visual appearance. In Cetacean Behavior: Mechanisms and Functions; Herman, L.M., Ed.; Wiley Interscience: New York, NY, USA, 1980; pp. 101–148. [Google Scholar]
- West, J.A.; Sivak, J.F.; Murphy, C.J.; Kovacs, K.M. A comparative study of the anatomy of the iris and ciliary body in aquatic mammals. Can. J. Zool. 1991, 69, 2594–2607. [Google Scholar] [CrossRef]
- Meshida, K.; Lin, S.; Domning, D.P.; Reidenberg, J.S.; Wang, P.; Gilland, E. Cetacean Orbital Muscles: Anatomy and Function of the Circular Layers. Anat. Rec. 2020, 303, 1792–1811. [Google Scholar] [CrossRef]
- Meredith, R.W.; Gatesy, J.; Emerling, C.A.; York, V.M.; Springer, M.S. Rod monochromacy and the coevolution of cetacean retinal opsins. PLoS Genet. 2013, 9, e1003432. [Google Scholar] [CrossRef]
- Peichl, L.; Behrmann, G.; Kröger, R.H.H. For whales and seals the ocean is not blue: A visual pigment loss in marine mammals. Eur. J. Neurosci. 2001, 13, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Peichl, L. Diversity of mammalian photoreceptor properties: Adaptations to habitat and lifestyle? Anat. Rec. 2005, 287, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, B.; Huggenberger, S.; Oelschläger, H. Head and Senses. In Anatomy of Dolphins: Insights into Body Structure and Function; Academic Press: Cambridge, MA, USA, 2016; pp. 133–196. [Google Scholar]
- Young, N.M.; Hope, G.M.; Dawson, W.W.; Jenkins, R.L. The Tapetum Fibrosum in the Eyes of Two Small Whales. Mar. Mammal Sci. 1988, 4, 281–290. [Google Scholar] [CrossRef]
- Mass, A.M.; Supin, A.Y. Ganglion Cell Topography and Retinal Resolution in the Bottlenose Dolphin Tursiops truncatus. Brain Behav. Evol. 1995, 45, 257–265. [Google Scholar] [CrossRef]
- Mass, A.M.; Supin, A.Y. Ganglion Cell Topography and Retinal Resolution in an Irrawaddy Dolphin (Orcaella brevirostris). Aquat. Mamm. 2022, 48, 68–74. [Google Scholar] [CrossRef]
- Mass, A.M.; Supin, A.Y.; Abramov, A.V.; Mukhametov, L.M.; Rozanova, E.I. Ocular anatomy, ganglion cell distribution and retinal resolution of a killer whale (Orcinus orca). Brain Behav. Evol. 2013, 81, 1–11. [Google Scholar] [CrossRef]
- Mengual, R.; García, M.; Segovia, Y.; Pertusa, J.F. Ocular morphology, topography of ganglion cell distribution and visual resolution of the pilot whale (Globicephala melas). Zoomorphology 2015, 134, 339–349. [Google Scholar] [CrossRef]
- Curcio, C.A.; Allen, K.A. Topography of ganglion cells in human retina. J. Comp. Neurol. 1990, 300, 5–25. [Google Scholar] [CrossRef]
- Hebel, R.; Holländer, H. Size and distribution of ganglion cells in the bovine retina. Vis. Res. 1979, 19, 667–673. [Google Scholar] [CrossRef]
- Coimbra, J.P.; Bertelsen, M.F.; Manger, P.R. Retinal ganglion cell topography and spatial resolving power in the river hippopotamus (Hippopotamus amphibius). J. Comp. Neurol. 2017, 525, 2499–2513. [Google Scholar] [CrossRef]
- Murayama, T.; Somiya, H. Distribution of Ganglion Cells and Object Localizing Ability in the Retina of Three Cetaceans. Fish. Sci. 1998, 64, 27–30. [Google Scholar] [CrossRef]
- Mazzatenta, A.; Caleo, M.; Baldaccini, N.E.; Maffei, L. A comparative morphometric analysis of the optic nerve in two cetacean species, the striped dolphin (Stenella coeruleoalba) and fin whale (Balaenoptera physalus). Vis. Neurosci. 2001, 18, 319–325. [Google Scholar] [CrossRef]
- Gao, G.; Zhou, K. Fiber analysis of the optic and cochlear nerves of small cetaceans. In Marine Mammal Sensory Systems; Thomas, J.A., Kastelein, R.A., Supin, A.Y., Eds.; Springer: Boston, MA, USA, 1992; pp. 39–52. [Google Scholar]
- Dawson, W.W.; Ulshafer, R.J.; Hawthorne, M.N.; Jenkins, R.L. Contents of the optic nerve of a small cetacean. Aquat. Mamm. 1983, 10, 45–56. [Google Scholar]
- Dral, A.D.G. Some quantitative aspects of the retina of Tursiops truncatus. Aquat. Mamm. 1975, 2, 28–31. [Google Scholar]
- Jonas, J.B.; Muller-Bergh, J.A.; Shclotzer-Schrehardt, U.M.; Naumann, G.O.H. Histomorphometry of the human optic nerve. Investig. Ophthalmol. Vis. Sci. 1990, 31, 736–744. [Google Scholar]
- Kunii, N.; Goto, N.; Matsumoto, K.; Kawamura, N. Morphometric of the Human Optic Nerve Fiber with Various Other Human Nerve Fibers. Neurol. Med.-Chir. 1999, 39, 922–927. [Google Scholar] [CrossRef]
- Ninomiya, H.; Imamura, E.; Inomata, T. Comparative anatomy of the ophthalmic rete and its relationship to ocular blood flow in three species of marine mammal. Vet. Ophthalmol. 2014, 17, 100–105. [Google Scholar] [CrossRef]
- Baden, T.; Euler, T.; Berens, P. Understanding the retinal basis of vision across species. Nat. Rev. Neurosci. 2020, 21, 5–20. [Google Scholar] [CrossRef]
- Ijsseldijk, L.L.; Brownlow, A.C.; Mazzariol, S. Best practice on cetacean post mortem investigation and tissue sampling. In Proceedings of the 25th Meeting of the Advisory Committee, Stralsund, Germany, 17–19 September 2019. [Google Scholar]
- Alfred, Y.K.N.; Stone, J. The Optic Nerve of the Cat: Appearance and Loss of Axons during Normal Development. Dev. Brain Res. 1982, 5, 263–271. [Google Scholar] [CrossRef]
- Dolman, C.L.; McCormick, A.Q.; Drance, S.M. Aging of the Optic Nerve. Arch. Opthalmol. 1980, 98, 2053–2058. [Google Scholar] [CrossRef]
- Visser, F.; Miller, P.J.O.; Antunes, R.N.; Oudejans, M.G.; Mackenzie, M.L.; Aoki, K.; Lam, F.P.A.; Kvadsheim, P.H.; Huisman, J.; Tyack, P.L. The social context of individual foraging behaviour in long-finned pilot whales (Globicephala melas). Behaviour 2014, 151, 1453–1477. [Google Scholar] [CrossRef]
- Baird, R.W. False Killer Whale: Pseudorca crassidens. In Encyclopedia of Marine Mammals, 2nd ed.; Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 405–406. [Google Scholar]
- Hartman, K.L.; Visser, F.; Hendriks, A.J.E. Social structure of Risso’s dolphins (Grampus griseus) at the Azores: A stratified community based on highly associated social units. Can. J. Zool. 2008, 86, 294–306. [Google Scholar] [CrossRef]
- Occelli, L.M.; Pasmanter, N.; Ayoub, E.E.; Petersen-Jones, S.M. Changes in retinal layer thickness with maturation in the dog: An in vivo spectral domain—Optical coherence tomography imaging study. BMC Vet. Res. 2020, 16, 225. [Google Scholar] [CrossRef] [PubMed]
- Mass, A.M.; Supin, A.Y. Ganglion Cell Topography and Retinal Resolution in the Bottlenose Dolphin Tursiops truncatus at an Early Stage of Postnatal Development. Biol. Bull. 2021, 47, 665–673. [Google Scholar] [CrossRef]
- Dawson, W.W.; Hawthorne, M.N.; Jenkins, R.L.; Goldston, R.T. Giant neural systems in the inner retina and optic nerve of small whales. J. Comp. Neurol. 1982, 205, 1–7. [Google Scholar] [CrossRef]
- Finnie, J.; Manavis, J.; Chidlow, G.; Casson, R.; Blumbergs, P. Temporal Sequence of Post-Mortem Autolysis in the Mouse Retina. J. Comp. Pathol. 2021, 187, 17–26. [Google Scholar] [CrossRef]
- Straznicky, C.; Vickers, J.C.; Gábriel, R.; Costa, M. A neurofilament protein antibody selectively labels a large ganglion cell type in the human retina. Brain Res. 1992, 582, 123–128. [Google Scholar] [CrossRef]
- Lee, E.S.; Lee, J.Y.; Jeon, C.J. Types and density of calretinin-containing retinal ganglion cells in mouse. Neurosci. Res. 2010, 66, 141–150. [Google Scholar] [CrossRef]
- Nag, T.C.; Wadhwa, S. Developmental expression of calretinin immunoreactivity in the human retina and a comparison with two other EF-hand calcium binding proteins. Neuroscience 1999, 91, 41–50. [Google Scholar] [CrossRef]
- Haverkamp, S.; Albert, L.; Balaji, V.; Nemec, P.; Dedek, K. Expression of cell markers and transcription factors in the avian retina compared with that in the marmoset retina. J. Comp. Neurol. 2021, 529, 3171–3193. [Google Scholar] [CrossRef]
- Kovács-Öller, T.; Szarka, G.; Ganczer, A.; Tengolics, A.; Balogh, B.; Volgyi, B. Expression of Ca2+-Binding Buffer Proteins in the Human and Mouse Retinal Neurons. Int. J. Mol. Sci. 2019, 20, 2229. [Google Scholar] [CrossRef] [PubMed]
- Ruzafa, N.; Pereiro, X.; Vecino, E. Immunohistochemical Characterisation of the Whale Retina. Front Neuroanat. 2022, 16, 813369. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G.; Hinrichsen, C.F.; Weller, W.L.; Haight, J.R. How thick should a retina be? A comparative study of mammalian species with and without intraretinal vasculature. Vis. Res. 1991, 31, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Lundström, Y.; Lundström, P.; Popova, S.N.; Lindblom, R.P.F.; Alafuzoff, I. Detection of Canges in Immunohistochemical Stains Caused by Postmortem Delay and Fixation Time. Appl. Immunohistochem. Mol. Morphol. 2019, 27, 238–245. [Google Scholar] [CrossRef]





| Species | Peak RGC Density (Cells/mm2) | RGC Diameter Range (in µm) | Mean Fibre Density of ON (Axons/mm2) | Axon Diameter Range (in µm) | Data from |
|---|---|---|---|---|---|
| Bos taurus | 4300–6300 | 3.5–35 | [24] | ||
| Globicephala melas | 241–294 | 10–75 | [22] | ||
| Grampus griseus | No data available | ||||
| Hippopotamus amphibius | 1800 | [25] | |||
| Pseudorca crassidens | 475 | [26] | |||
| Stenella coeruleoalba | 24,100 | 0.5–15.2 | [27] | ||
| Tursiops truncatus | 460–826 | 9–60 | 19,053–77,000 | 0.27–25.6 | [19,28,29,30] |
| Ziphius cavirostris | No data available | ||||
| Species | Common Name | Sex | Age | Cause of Death | Use | PMI/DCC |
|---|---|---|---|---|---|---|
| Artiodactyls | ||||||
| Bos taurus | Bovine | Unknown | Adult | Commercial slaughter | R + O | 4 h |
| Hippopotamus amphibius | Common hippopotamus | M | 46 | Cardiac arrest | ON | <24 h |
| Odontocetes | ||||||
| Globicephala melas | Long-finned pilot whale | F | Newborn | Unknown | R + O | DCC 1 |
| Grampus griseus | Risso’s dolphin | F | Adult | Septic shock | R + O | DCC 1 |
| Pseudorca crassidens | False killer whale | F | Newborn | Unknown | R + O | DCC 2 |
| Stenella coeruleoalba | Striped dolphin | M | Juvenile | Caudal fin lesion | R + O | DCC 1 |
| Tursiops truncatus | Common bottlenose dolphin | F | Adult | Unknown | R + O | DCC 1 |
| Ziphius cavirostris | Cuvier’s beaked whale | M | Juvenile | Unknown | ON | DCC 2 |
| Antibody | Production Method | Product Details | Dilution |
|---|---|---|---|
| Anti-CR | Produced by immunisation of mice with recombinant human calretinin-22 k (identical with calretinin up to Arg178) | Swant, mouse monoclonal, Cat# 6B3, Lot n° 010,399, RRID: AB_10000320 | 1:500 |
| Anti-N200 | Produced by fusion of mouse myeloma cells and splenocytes from a mouse immunised with neurofilament 200 from porcine spinal cord | Sigma-Aldrich, mouse monoclonal, Cat# N5389, Lot n° 050M4779, RRID: AB_260781 | 1:500 |
| Anti-mouse | Horse anti-mouse antibodies directed at both heavy and light chain | Vector Laboratories, Cat# BA-2000, RRID: AB_2313581 | 1:400 |
| Species | Total Nerve Surface Area (in mm2) | Total Fascicular Surface Area (in mm2) | Amount of Nerve Bundles | Percentage of Fascicular Surface to Total |
|---|---|---|---|---|
| Bos taurus (left eye) | 3.973 | 3.138 | 329 | 78.98 |
| Bos taurus (right eye) | 3.796 | 3.059 | 393 | 80.58 |
| Hippopotamus amphibius | 2.174 | 1.777 | 92 | 81.74 |
| Globicephala melas | 1.591 | 1.195 | 54 | 75.11 |
| Grampus griseus | 3.241 | 2.586 | 167 | 79.79 |
| Pseudorca crassidens | 2.527 | 1.916 | 108 | 75.82 |
| Stenella coeruleoalba | 2.014 | 1.937 | 1 | 96.18 |
| Tursiops truncatus | 2.986 | 2.795 | 4 | 93.60 |
| Ziphius cavirostris | 3.450 | 2.959 | 49 | 85.77 |
| Species | Mean Thickness (in µm) | Thickness Range (in µm) | Total RGCs | Mean LD (per mm) | Range LD (per mm) | N200+ Ratio | CR+ Ratio |
|---|---|---|---|---|---|---|---|
| Bos taurus (left eye) | 183.5 | 183.5–270.1 | 563 | 15 | 3–61 | 49.41 | 11.16 |
| Bos taurus (right eye) | 263.8 | 180.6–387.4 | 402 | 20 | 10–53 | 53.66 | 10.57 |
| Globicephala melas | 204.9 | 83.7–293.7 | 158 | 4 | 1–9 | 69.59 | 0 |
| Grampus griseus | 140.2 | 58.1–345.1 | 171 | 5 | 2–9 | 51.66 | 0 |
| Pseudorca crassidens | 228.7 | 93.7–358.3 | 231 | 6 | 1–12 | 31.42 | 0 |
| Stenella coeruleoalba | 160.2 | 55.7–247.8 | 87 | 4 | 1–9 | 65.69 | 0 |
| Tursiops truncatus | 192.5 | 81.3–277.7 | 103 | 6 | 0–12 | 79.81 | 9.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Boeck, M.W.E.; Cozzi, B.; Graïc, J.-M. Interspecies Retinal Diversity and Optic Nerve Anatomy in Odontocetes. Animals 2023, 13, 3430. https://doi.org/10.3390/ani13213430
De Boeck MWE, Cozzi B, Graïc J-M. Interspecies Retinal Diversity and Optic Nerve Anatomy in Odontocetes. Animals. 2023; 13(21):3430. https://doi.org/10.3390/ani13213430
Chicago/Turabian StyleDe Boeck, Michiel W. E., Bruno Cozzi, and Jean-Marie Graïc. 2023. "Interspecies Retinal Diversity and Optic Nerve Anatomy in Odontocetes" Animals 13, no. 21: 3430. https://doi.org/10.3390/ani13213430
APA StyleDe Boeck, M. W. E., Cozzi, B., & Graïc, J.-M. (2023). Interspecies Retinal Diversity and Optic Nerve Anatomy in Odontocetes. Animals, 13(21), 3430. https://doi.org/10.3390/ani13213430





