Intrahepatic Cholangiocarcinoma Identified in a Zoo-Housed Sandhill Crane (Grus canadensis): An Anatomopathological and Metagenomic Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. History
2.2. Sample Collection and High-Throughput Sequencing
2.3. Bioinformatic Analysis
3. Results
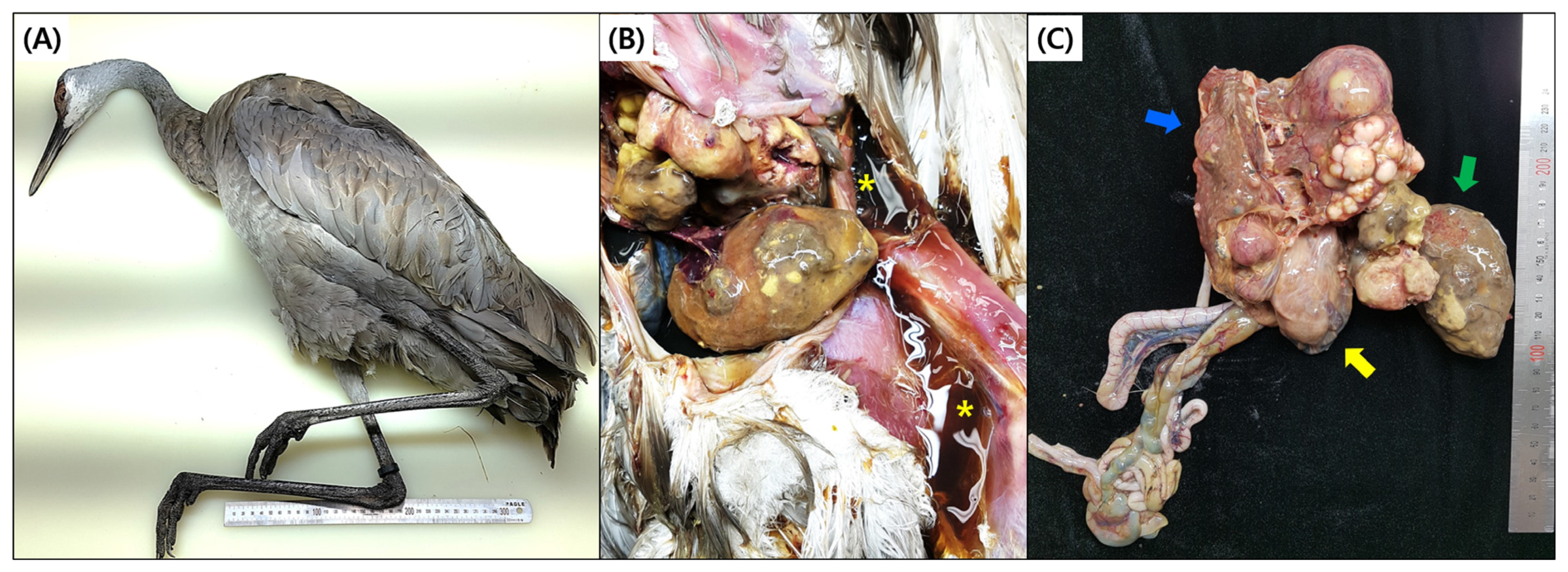
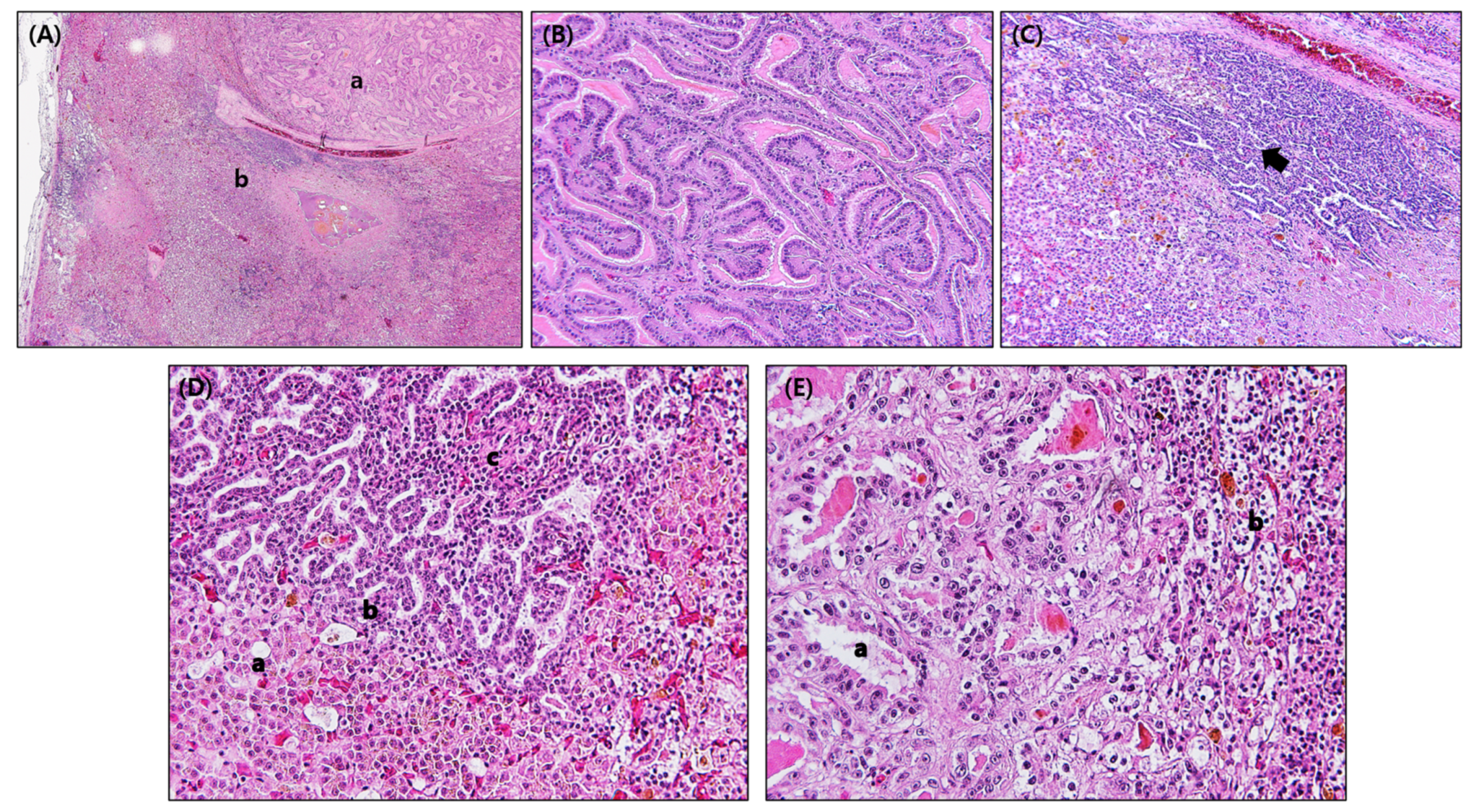
3.1. Gross Findings and Histopathology
3.2. Virus Identification
3.3. Metagenomics Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kuruvilla, S.; Del Tin, J.; Pennington, M.; Heron, T. Sandhill Crane (Antigone canadensis)–Ecological Profile. Available online: https://humanwildlifeecology.wordpress.com/2017/02/05/sandhill-crane-ecological-profile (accessed on 5 February 2017).
- Barzen, J.; Ballinger, K. Sandhill and Whooping Cranes; Wildlife Damage Maneagement Technical Series; National Wildlife Research Center: Ft. Collines, CO, USA, 2017; p. 16. [Google Scholar]
- Krapu, G.; Ivey, G.; Barzen, J. Species review: Sandhill crane (Grus canadensis). In Crane Conservation Strategy; International Crane Foundation: Baraboo, WI, USA, 2019; pp. 42–44. [Google Scholar]
- Thomas, N.J.; Hunter, D.B.; Atkinson, C.T. Infectious Diseases of Wild Birds; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Candelora, K.L.; Spalding, M.G.; Sellers, H.S. Survey for antibodies to infectious bursal disease virus serotype 2 in wild Turkeys and Sandhill cranes of Florida, USA. J. Wildl. Dis. 2010, 46, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Olsen, G.H.; Carpenter, J.W.; Gee, G.F.; Thomas, N.J.; Dein, F.J. Mycotoxin-induced disease in captive whooping cranes (Grus americana) and sandhill cranes (Grus canadensis). J. Zoo Wildl. Med. 1995, 26, 569–576. [Google Scholar]
- Simpson, C.F.; Forrester, D.J.; Nesbitt, S.A. AVIAN POX IN FLORIDA SANDHILL CRANES 1. J. Wildl. Dis. 1975, 11, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Novilla, M.N.; Carpenter, J.W.; Spraker, T.R.; Jeffers, T.K. Parental Development of Eimerian Coccidia in Sandhill and Whopping Cranes 1. J. Protozool. 1981, 28, 248–255. [Google Scholar] [CrossRef]
- Hawkins, S.; Garner, M.M.; Hartup, B.K. Neoplasia in captive cranes. J. Zoo Wildl. Med. 2021, 52, 689–697. [Google Scholar] [CrossRef]
- Allen, J.L.; Martin, H.D.; Crowley, A.M. Metastatic cholangiocarcinoma in a Florida sandhill crane. J. Am. Vet. Med. Assoc. 1985, 187, 1215. [Google Scholar]
- Williams, S.M.; Reece, R.L.; Hafner, S. Other Tumors. In Diseases of Poultry, 14th ed.; Swayne, D.E., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; p. 637. [Google Scholar]
- Silva, R.F.; Fadly, A.M.; Taylor, S.P. Development of a polymerase chain reaction to differentiate avian leukosis virus (ALV) subgroups: Detection of an ALV contaminant in commercial Marek’s disease vaccines. Avian Dis. 2007, 51, 663–667. [Google Scholar] [CrossRef]
- Yamamoto, K.R.; Alberts, B.M.; Benzinger, R.; Lawhorne, L.; Treiber, G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology 1970, 40, 734–744. [Google Scholar] [CrossRef]
- Veronese, F.M.; Mero, A. The impact of PEGylation on biological therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef]
- Froussard, P. A random-PCR method (rPCR) to construct whole cDNA library from low amounts of RNA. Nucleic Acids Res. 1992, 20, 2900. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Nurk, S.; Meleshko, D.; Korobeynikov, A.; Pevzner, P.A. metaSPAdes: A new versatile metagenomic assembler. Genome Res. 2017, 27, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Freeman, K.; Hahn, K.; Jones, M.; Petersen, M.; Toal, R. Unusual presentation of an Amazon parrot (Amazona species) with hepatocellular carcinoma. Avian Pathol. 1999, 28, 203–206. [Google Scholar] [CrossRef]
- Van Wettere, A.; Degernes, L.; Barnes, H.J. Combined hepatocellular-cholangiocarcinoma in a lesser flamingo (Phoenicopterus minor). Avian Pathol. 2010, 39, 275–278. [Google Scholar] [CrossRef]
- Wadsworth, P.; Majeed, S.K.; Brancker, W.M.; Jones, D. Some hepatic neoplasms in non-domesticated birds. Avian Pathol. 1978, 7, 551–555. [Google Scholar] [CrossRef]
- Ling, Y.; Guo, Y.; Yang, L. Pathological observations of hepatic tumours in ducks. Avian Pathol. 1993, 22, 131–140. [Google Scholar] [CrossRef]
- Chung, T.; Park, Y.N. Up-to-date pathologic classification and molecular characteristics of intrahepatic cholangiocarcinoma. Front. Med. 2022, 9, 857140. [Google Scholar] [CrossRef]
- Nakanuma, Y.; Klimstra, D.S.; Komuta, M.; Zen, Y. Intrahepatic cholangiocarcinoma. In WHO Classification of Tumours. Digestive System Tumours, 5th ed.; The WHO Classification of Tumours Editorial Board, Ed.; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Komuta, M.; Govaere, O.; Vandecaveye, V.; Akiba, J.; Van Steenbergen, W.; Verslype, C.; Laleman, W.; Pirenne, J.; Aerts, R.; Yano, H. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology 2012, 55, 1876–1888. [Google Scholar] [CrossRef]
- Theise, N.D.; Saxena, R.; Portmann, B.C.; Thung, S.N.; Yee, H.; Chiriboga, L.; Kumar, A.; Crawford, J.M. The canals of Hering and hepatic stem cells in humans. Hepatology 1999, 30, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Haber, P.K.; Sia, D.J. Cell of origin in biliary tract cancers and clinical implications. JHEP Rep. 2021, 3, 100226. [Google Scholar] [CrossRef] [PubMed]
- Di Bisceglie, A.M. Hepatitis B and hepatocellular carcinoma. Hepatology 2009, 49, S56–S60. [Google Scholar] [CrossRef]
- Balogh, J.; Victor, I.I.I.D.; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Yokosuka, O.; Imazeki, F.; Tagawa, M.; Omata, M. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: A prospective study of 251 patients. Hepatology 1995, 21, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Emeli, T.O.; Serrao, R. Delayed perihepatic abscess caused by Cutibacterium acnes following right partial hepatectomy. BMJ Case Rep. 2022, 15, e247279. [Google Scholar] [CrossRef] [PubMed]
- Yasutomi, E.; Ueda, Y.; Asaji, N.; Yamamoto, A.; Yoshida, R.; Hatazawa, Y.; Hayashi, H.; Shiomi, Y.; Yano, Y.; Kodama, Y. Liver abscess caused by Cutibacterium namnetense after transarterial chemoembolization for hepatocellular carcinoma. Clin. J. Gastroenterol. 2021, 14, 246–250. [Google Scholar] [CrossRef]
- Grat, M.; Wronka, K.M.; Krasnodebski, M.; Lewandowski, Z.; Kosinska, I.; Grat, K.; Stypulkowski, J.; Rejowski, S.; Wasilewicz, M.; Galecka, M.; et al. Profile of gut microbiota associated with the presence of hepatocellular cancer in patients with liver cirrhosis. Transpl. Proc. 2016, 48, 1687–1691. [Google Scholar] [CrossRef]
- Cheong, H.S.; Tan, S.P.; Wong, D.M.K.; Koo, W.L.Y.; Wong, S.H.; Tan, N.S. The blood microbiome and health: Current evidence, controversies and challenges. Int. J. Mol. Sci. 2023, 24, 5633. [Google Scholar] [CrossRef]
- Khalyfa, A.A.; Punatar, S.; Yarbrough, A. Hepatocellular carcinoma: Understanding the inflammatory implications of the microbiome. Int. J. Mol. Sci. 2022, 23, 8164. [Google Scholar] [CrossRef]
- Antonio, N.; Bonnelykke-Behrndtz, M.L.; Ward, L.C.; Collin, J.; Christensen, I.J.; Steiniche, T.; Schmidt, H.; Feng, Y.; Martin, P. The wound inflammatory response exacerbates growth of preneoplastic cells and progression to cancer. EMBO J. 2015, 34, 2219–2236. [Google Scholar] [CrossRef] [PubMed]
| Intrahepatic Cholangiocarcinoma Sample | SPF Chicken Liver (Control) | |||
|---|---|---|---|---|
| No. of Reads | % Trimmed Reads (% Bacteria) | No. of Reads | % Trimmed Reads (% Bacteria) | |
| Raw Reads | 13,851,588 | - | 14,527,223 | - |
| Trimmed Reads | 13,067,715 | - | 13,914,506 | - |
| Host Reads | 8,461,396 | 64.8 | 13,385,586 | 96.2 |
| Eukaryota | 238,614 | 1.8 | 5169 | <0.1 |
| Bacteria | 2,194,087 | 16.8 | 10,648 | 0.1 |
| Cutibacterium acnes | 388,437 | (17.7) | - | - |
| Escherichia coli | 288,422 | (13.1) | - | - |
| Shigella flexneri | 195,335 | (8.9) | - | - |
| Chlamydia abortus | 140,030 | (6.4) | - | - |
| Hyphomonadaceae bacterium | - | - | 1844 | (17.3) |
| Firmicutes | - | - | 1700 | (16.0) |
| Others | 1,181,863 | (53.9) | 7184 | (67.5) |
| Virus | 4216 | <0.1 | 668 | <0.1 |
| Phage (Siphoviridae) | 4154 | 23 | ||
| Equine infectious anemia virus | 58 | - | ||
| Fowl aviadenovirus E | 4 | - | ||
| Chicken chapparvovirus | - | 645 | ||
| Others | 24,546 | 0.2 | 487 | <0.1 |
| Unclassified | 2,144,856 | 16.4 | 511,948 | 3.7 |
| Total | 4,606,319 | 100 | 528,920 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-R.; Kim, H.-S.; Kwon, Y.-K. Intrahepatic Cholangiocarcinoma Identified in a Zoo-Housed Sandhill Crane (Grus canadensis): An Anatomopathological and Metagenomic Study. Animals 2023, 13, 3469. https://doi.org/10.3390/ani13223469
Kim H-R, Kim H-S, Kwon Y-K. Intrahepatic Cholangiocarcinoma Identified in a Zoo-Housed Sandhill Crane (Grus canadensis): An Anatomopathological and Metagenomic Study. Animals. 2023; 13(22):3469. https://doi.org/10.3390/ani13223469
Chicago/Turabian StyleKim, Hye-Ryoung, Hyeon-Su Kim, and Yong-Kuk Kwon. 2023. "Intrahepatic Cholangiocarcinoma Identified in a Zoo-Housed Sandhill Crane (Grus canadensis): An Anatomopathological and Metagenomic Study" Animals 13, no. 22: 3469. https://doi.org/10.3390/ani13223469
APA StyleKim, H.-R., Kim, H.-S., & Kwon, Y.-K. (2023). Intrahepatic Cholangiocarcinoma Identified in a Zoo-Housed Sandhill Crane (Grus canadensis): An Anatomopathological and Metagenomic Study. Animals, 13(22), 3469. https://doi.org/10.3390/ani13223469





