Predicting Disparity between ASF-Managed Areas and Wild Boar Habitats: A Case of South Korea
Abstract
:Simple Summary
Abstract
1. Introduction
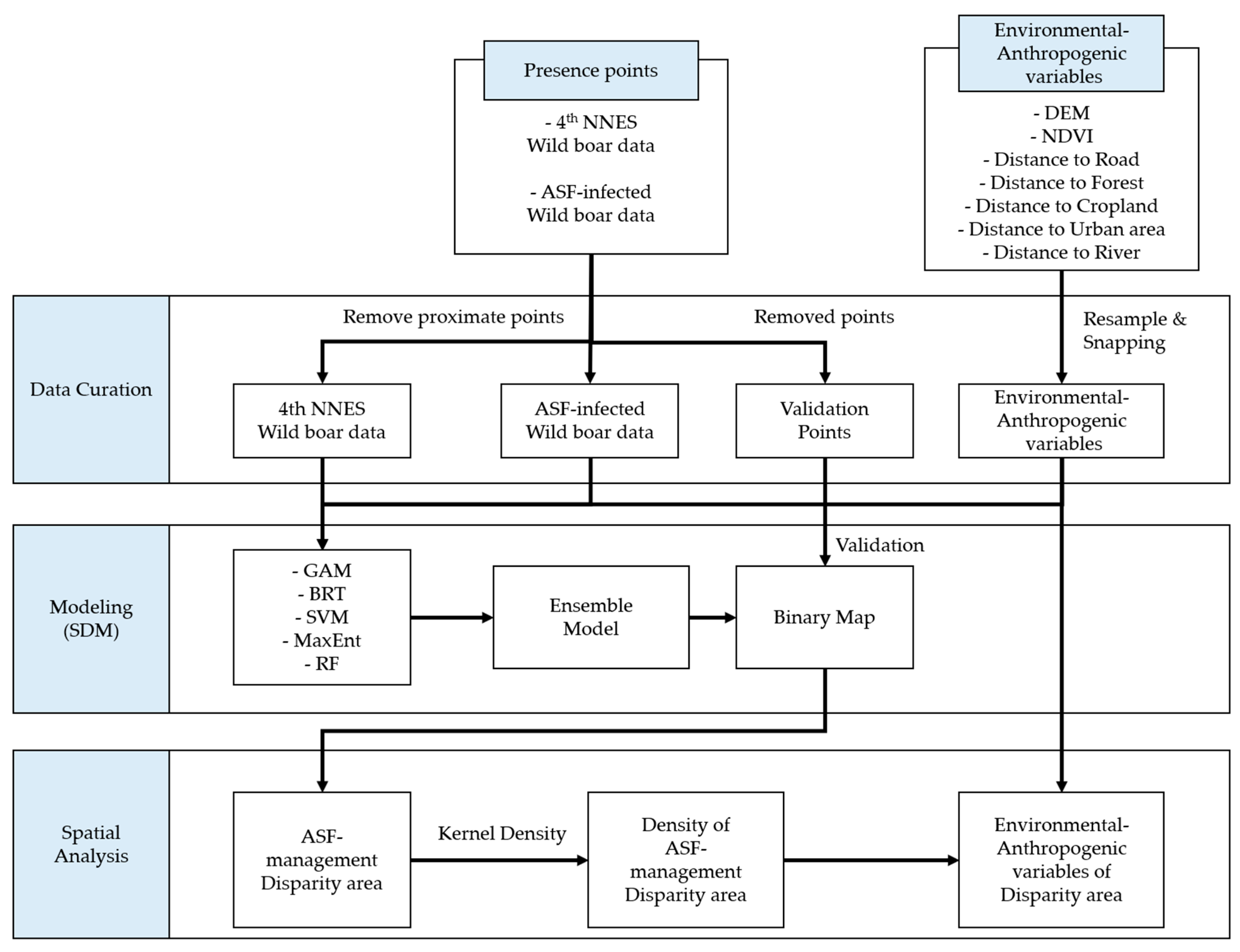
2. Materials and Methods
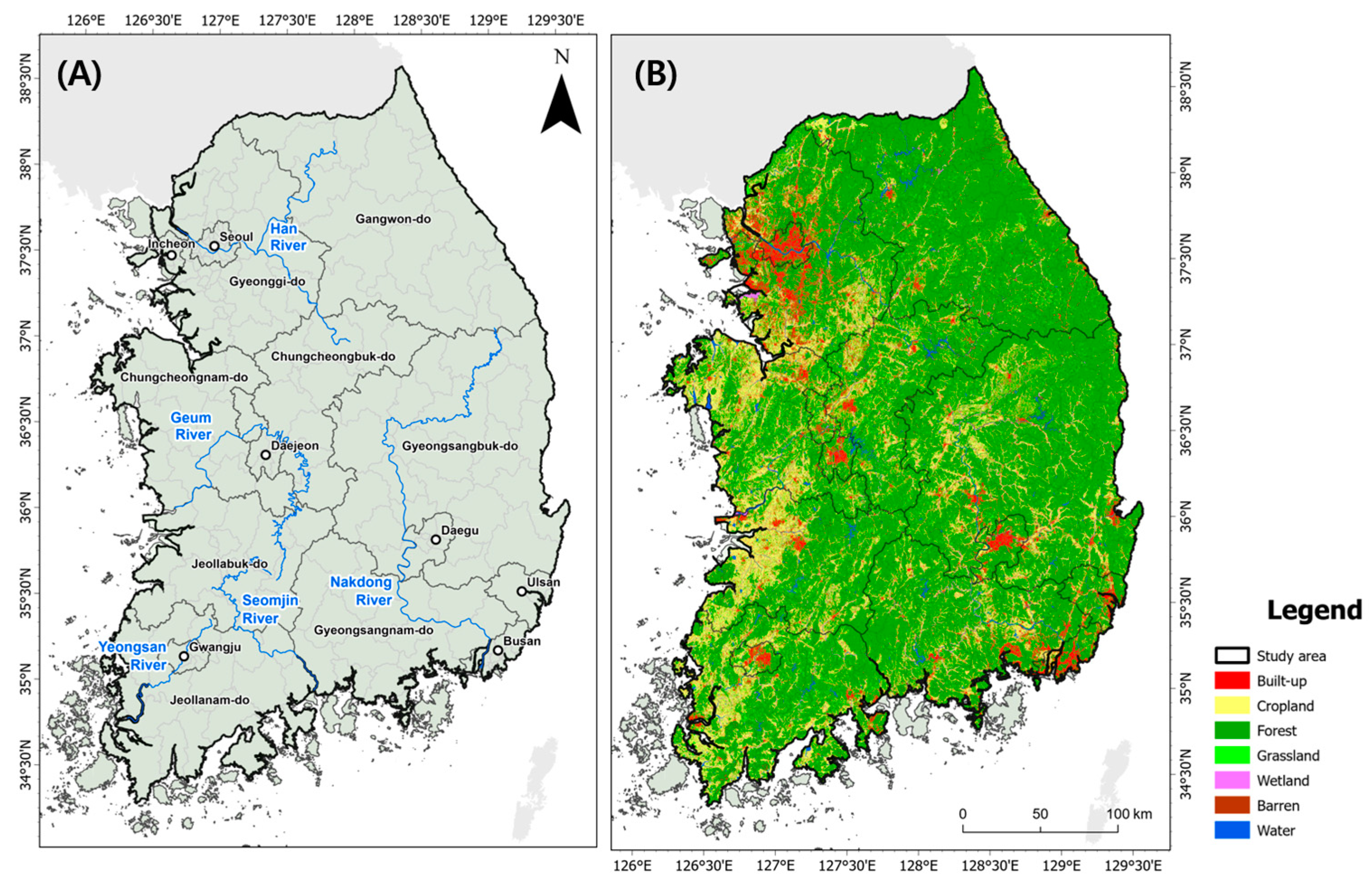
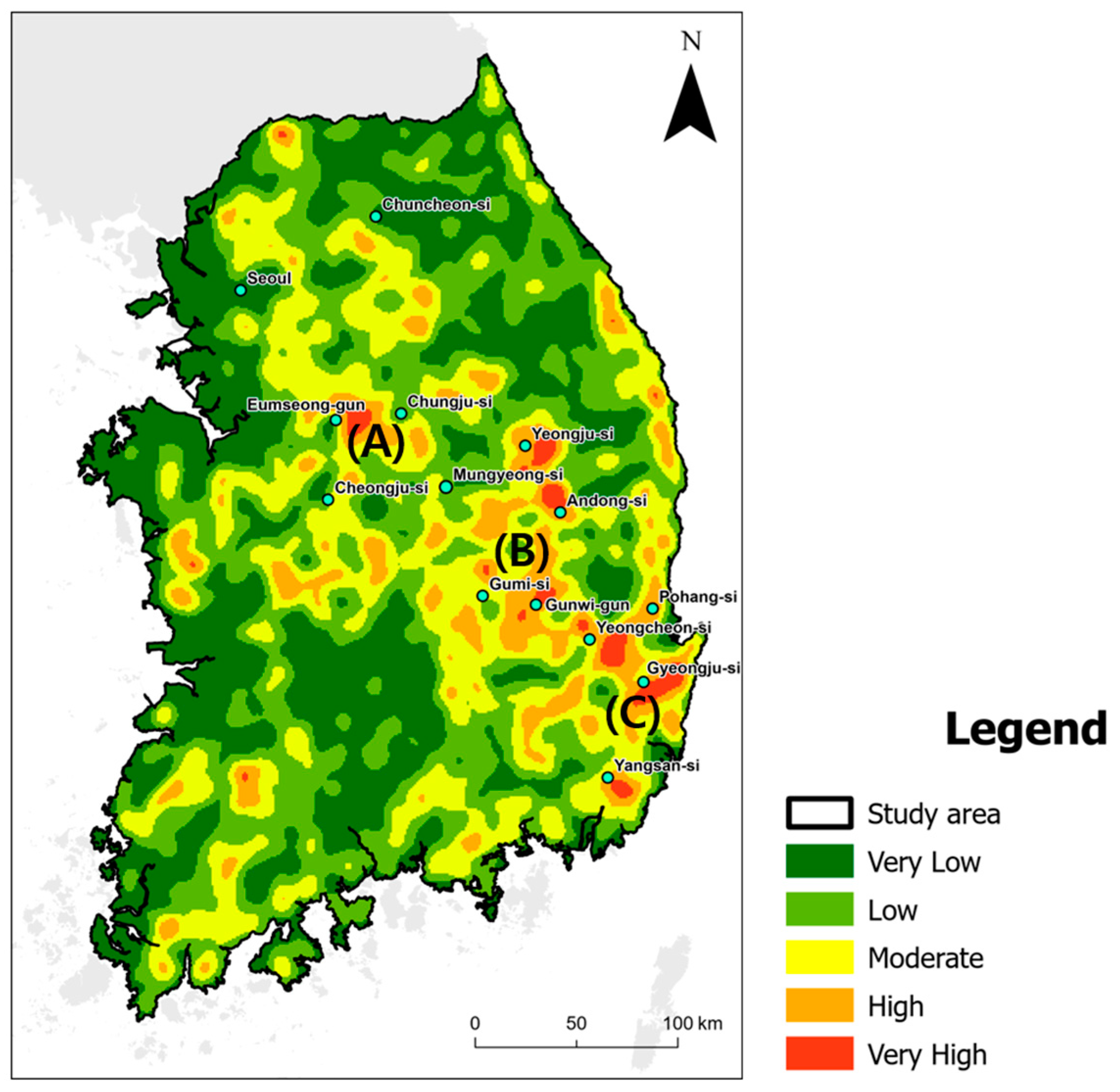
2.1. Study Area
2.2. Evaluating Disparity between Wild Boar Habitats and ASF-Managed Areas
2.2.1. SDMs
- SVM, originally developed by Cortes and Vapnik (1995) [43], is an inductive modeling technique primarily used for data processing. This algorithm determines the maximum margin hyperplane that represents the greatest separation between classes. Therefore, the SVM is placed close to the decision boundary, and it is very useful to avoid overfitting, yielding an excellent generalization performance for solving numerous non-linear regression and time-series problems, and requires only minimal model tuning with a small training dataset.
- The BRT model has been one of the most widely used approaches over the past two decades [44]. It is adept at handling nonlinearity, selecting predictor variables, and quantifying the relative importance of predictors for ecological questions. BRT outperformed regression-based models in analyzing complex species core-habitat relationships [44,45,46].
- GAM, originally developed by Hastie and Tibshirani (2017) [47], is a general linear model in which the linear response variable depends linearly on unknown smooth functions using predictor variables. It was used to predict relative abundance based on the species occurrence dataset. GAM is to concentrate on constructing one “best model”, which has a specialty than other SDMs. GAM is also used to predict species habitats in a variety of ecosystems and occasionally to detect specific ecological events.
- RF, originally developed by Ho (1995) [48], is method for classification and regression that is conducted by constructing multiple decision trees during model training. In classification (or prediction), the results are created using the average prediction from the individual tree returns. This method can handle both linear and nonlinear relationships and prevent overfitting in the training set. However, this method is difficult to interpret because of its complexity. Additionally, it is computationally intensive for large datasets.
- MaxEnt, originally developed by Phillips et al. (2006) [49], is a well-known SDM technique. This is called maximum entropy modeling and is based on the density estimation principle of Jaynes (1957) [50,51]. Based on the variables (topographic, climatic, anthropogenic, etc.) and occurrence locations, the model can distribute a probability that represents the suitability of the conditions for the target species. MaxEnt was implemented in a presence-only analysis; thus, it did not rely on the confirmed absence data.
2.2.2. Data Curation
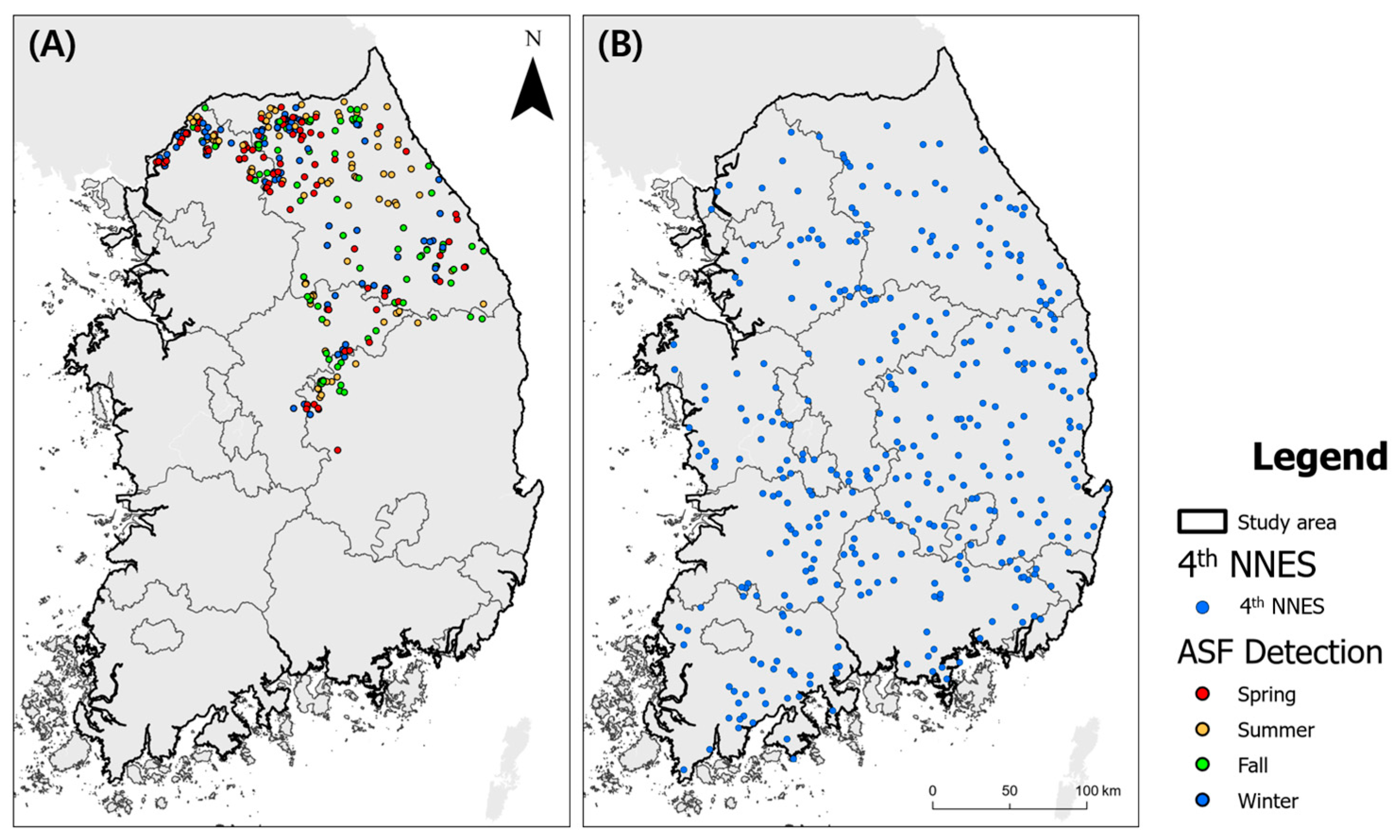
Point Datasets
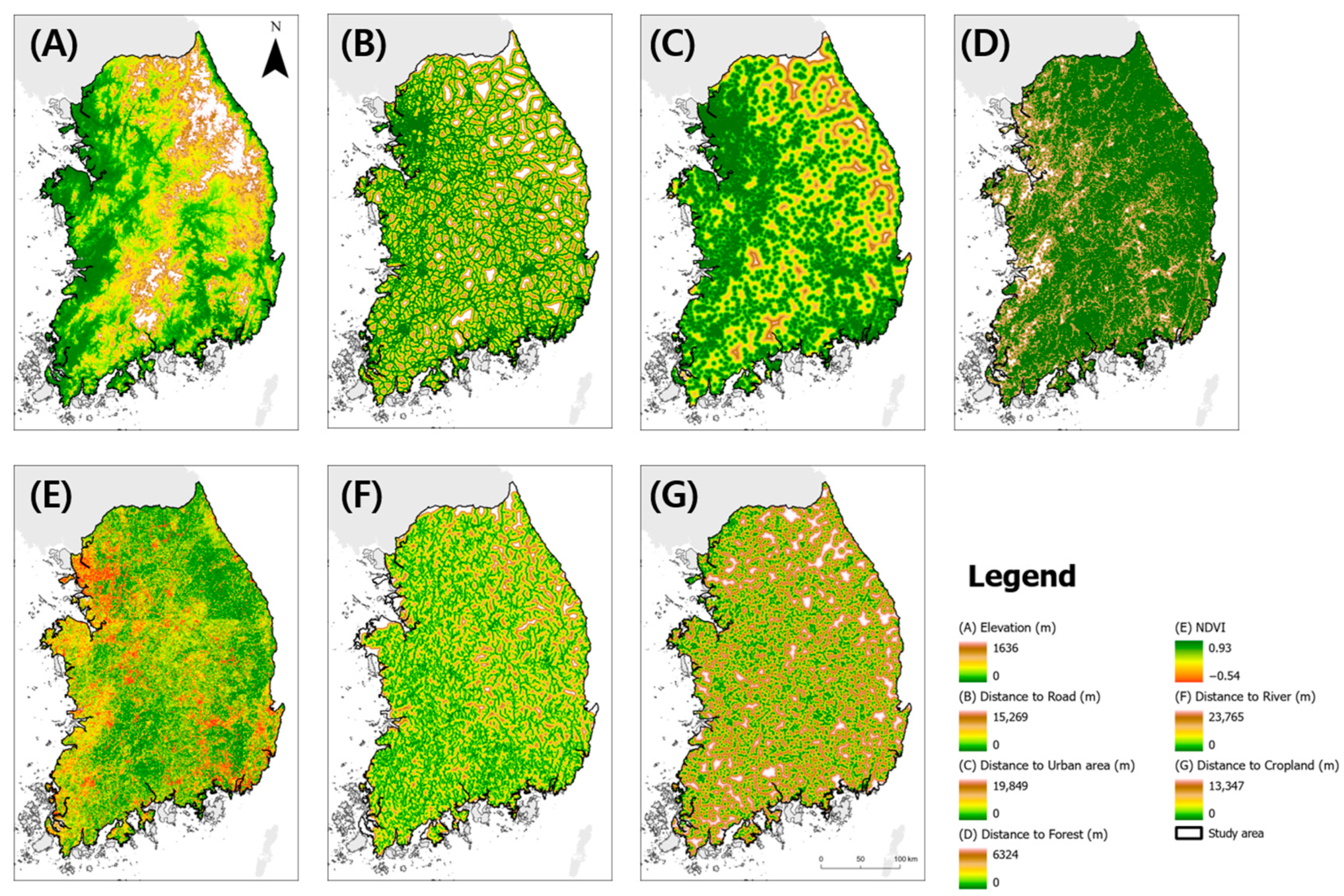
Environmental Variables
- Elevation: To ensure uniformity, we resampled the elevation data to a 1 km resolution using the Resample tool in ArcGIS pro 3.1.0. These elevation data were sourced from a 30 m resolution Digital Elevation Model (DEM) provided by the National Geographic Information Institute.
- Road Network: Road network data were sourced from Geofabrik OpenStreetMap, which encompasses major roads classified as primary, secondary, and trunk roads. These data aid in understanding the accessibility and connectivity of landscapes.
- River Network: The river network was derived from a river network map provided by the Korean Ministry of Environment, which offered insights into the proximity and distribution of water bodies.
- Normalized Difference Vegetation Index (NDVI): Using the Google Earth Engine, we obtained the available Sentinel-2 surface reflectance imagery from 2020 to 2022 and created a composite image. We calculated the NDVI using imagery captured from June to September, coinciding with peak dietary intensity. The NDVI values were subsequently averaged to indicate the vegetation health and density during this critical period.
- Using the Euclidean distance tool in ArcGIS Pro 3.1.0, we calculated the distance of each raster cell to the nearest forest, urban area, road, cropland, and river using the ESA WorldCoverV200. These variables provide valuable insights into the proximity of the key environmental features in each cell.
2.2.3. Ensemble SDM Simulation
The Suitability of Wild Boar Habitats and ASF-Managed Areas
Estimation of Wild Boar Habitats and ASF-Managed Areas
2.2.4. Evaluating Disparity Areas
3. Results
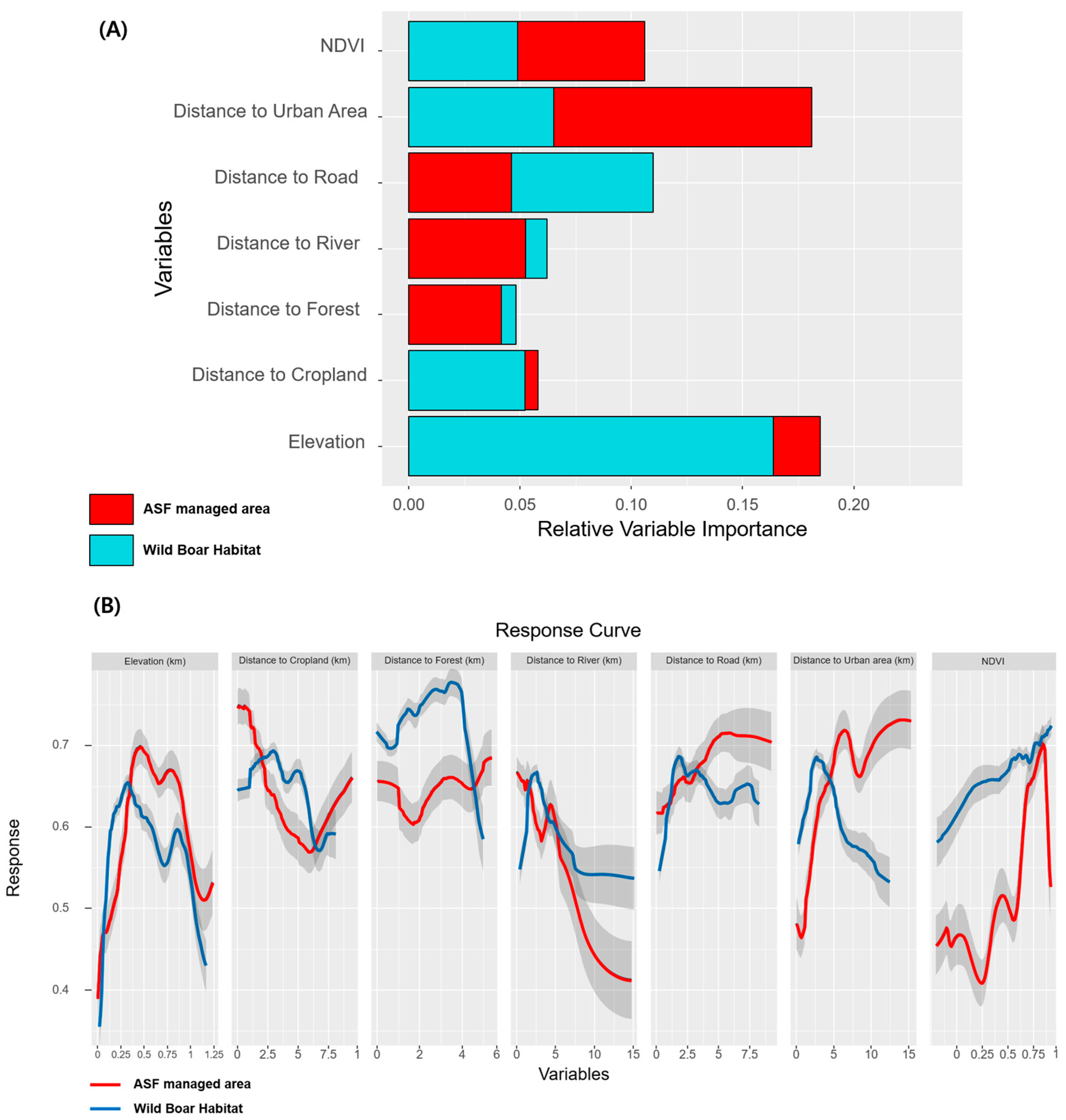
3.1. The Suitability of Wild Boar Habitats and ASF-Managed Areas
3.2. Estimation of Wild Boar Habitats and ASF-Managed Areas
3.3. Evaluating Disparity Areas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Granoff, A.; Webster, R.G. Encyclopedia of Virology; Elsevier: Memphis, TN, USA, 1999. [Google Scholar]
- Costard, S.; Wieland, B.; de Glanville, W.; Jori, F.; Rowlands, R.; Vosloo, W.; Roger, F.; Pfeiffer, D.U.; Dixon, L.K. African swine fever: How can global spread be prevented? Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2683–2696. [Google Scholar] [CrossRef]
- Manuja, B.K.; Manuja, A.; Singh, R.K. Globalization and livestock biosecurity. Agric. Res. 2014, 3, 22–31. [Google Scholar] [CrossRef]
- Fischer, M.; Hühr, J.; Blome, S.; Conraths, F.J.; Probst, C. Stability of African Swine Fever Virus in Carcasses of Domestic Pigs and Wild Boar Experimentally Infected with the ASFV “Estonia 2014” Isolate. Viruses 2020, 12, 1118. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, Y.J.; Lee, S.H.; Lee, E.H.; Park, J.J.; Park, K.H.; Sunwoo, S.Y.; Lee, J.G. A Study on Improvement Plan for Establishing a Farm Level ASF Control Strategies; Gyeonggi Research Institute: Suwon, Republic of Korea, 2020; pp. 1–133. (In Korean) [Google Scholar]
- Mebus, C.; Arias, M.; Pineda, J.M.; Tapiador, J.; House, C.; Sanchez Vizcaino, J.M. Survival of several porcine viruses in different Spanish dry-cured meat products. Food Chem. 1997, 59, 555–559. [Google Scholar] [CrossRef]
- Bosch, J.; Iglesias, I.; Muñoz, M.J.; De la Torre, A. A cartographic tool for managing African swine fever in Eurasia: Mapping wild boar distribution based on the quality of available habitats. Transbound. Emerg. Dis. 2017, 64, 1720–1733. [Google Scholar] [CrossRef]
- Nurmoja, I.; Petrov, A.; Breidenstein, C.; Zani, L.; Forth, J.H.; Beer, M.; Kristian, M.; Viltrop, A.; Blome, S. Biological characterization of African swine fever virus genotype II strains from north-eastern Estonia in European wild boar. Transbound. Emerg. Dis. 2017, 64, 2034–2041. [Google Scholar] [CrossRef]
- Blome, S.; Gabriel, C.; Beer, M. Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Res. 2013, 173, 122–130. [Google Scholar] [CrossRef]
- Laddomada, A.; Rolesu, S.; Loi, F.; Cappai, S.; Oggiano, A.; Madrau, M.P.; Sanna, M.L.; Pilo, G.; Bandino, E.; Brundu, D.; et al. Surveillance and control of African Swine Fever in free-ranging pigs in Sardinia. Transbound. Emerg. Dis. 2019, 66, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Gomez-Villamandos, J.C.; Carrasco, L. An Update on the Epidemiology and Pathology of African Swine Fever. J. Comp. Path. 2015, 152, 9–21. [Google Scholar] [CrossRef]
- Garcia, S.N.; Osburn, B.I.; Jay-Russell, M.T. One health for food safety, food security, and sustainable food production. Front. Sustain. Food Syst. 2020, 4, 1. [Google Scholar] [CrossRef]
- Kedkovid, R.; Sirisereewan, C.; Thanawongnuwech, R. Major swine viral diseases: An Asian perspective after the African swine fever introduction. Porc. Health Manag. 2020, 6, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Saenz, J.; Diaz, A.; Bonilla-Aldana, D.K.; Rodríguez-Morales, A.J.; Martinez-Gutierrez, M.; Aguilar, P.V. African swine fever virus: A re-emerging threat to the swine industry and food security in the Americas. Front. Microbiol. 2022, 13, 1011891. [Google Scholar] [CrossRef]
- OIE. Global Situation of ASF. Available online: https://www.oie.int/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/ASF/Report_47_Global_situation_ASF.pdf (accessed on 23 September 2023).
- Choi, J.H.; Namgung, H.; Lim, S.J.; Kim, E.K.; Oh, Y.; Park, Y.C. Predicting Suitable Areas for African Swine Fever Outbreaks in Wild Boars in South Korea and Their Implications for Managing High-Risk Pig Farms. Animals 2023, 13, 2148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hayes, D.J.; Ji, Y.; Li, M.; Zlong, T. African swine fever in China: An update. Agric. Policy Rev. 2019, 1, 1–3. [Google Scholar]
- Vergne, T.; Chen-Fu, C.; Li, S.; Cappelle, J.; Edwards, J.; Martin, V.; Pfeiffer, D.U.; Fusheng, G.; Roger, F.L. Pig empire under infectious threat: Risk of African swine fever introduction into the People’s Republic of China. Vet. Rec. 2017, 181, 117. [Google Scholar] [CrossRef]
- Ministry of Environment. Wild Boar Population Reduction Outcomes against African Swine Fever. Department of Biodiversity, Ministry of Environment, 2020. Available online: http://www.me.go.kr/home/web/board/read.do?pagerOffset=0&maxPageItems=10&maxIndexPages=10&searchKey=&searchValue=&menuId=286&orgCd=&boardMasterId=1&boardCategoryId=&boardId=1407580&decorator=/ (accessed on 14 September 2023).
- Morelle, K.; Bubnicki, J.; Churski, M.; Gryz, J.; Podgorski, T.; Kuijper, D.P.J. Disease-induced mortality outweighs hunting in causing wild boar population crash after African swine fever outbreak. Front. Vet. Sci. 2020, 7, 378. [Google Scholar] [CrossRef]
- Salazar, L.G.; Rose, N.; Hayes, B.; Hammami, P.; Baubet, E.; Desvaux, S.; Andraud, M. Effects of habitat fragmentation and hunting activities on African swine fever dynamics among wild boar populations. Prev. Vet. Med. 2022, 208, 105750. [Google Scholar] [CrossRef]
- Lim, S.J.; Han, S.H.; Park, J.Y.; Nam, G.H.; Choi, K.T.; Kwon, J.H.; Choi, J.H.; Park, Y.C. Wild boar habitat in the border areas of Gangwon-do (Yanggu, Hwacheon) and south of the border (Hongcheon, Hwaseong). In Proceedings of the 2021 Annual Conference of the Korean Society for Intergrative Biology, Pyeongchang, Republic of Korea, 20–22 December 2021. [Google Scholar]
- Woźniakowski, G.; Pejsak, Z.; Jabłoński, A. Emergence of African Swine Fever in Poland (2014–2021). Successes and Failures in Disease Eradication. Agriculture 2021, 11, 738. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Bonardi, A.; Mairota, P.; Leronni, V.; Padoa-Schioppa, E. Predicting wild boar damages to croplands in a mosaic of agricultural and natural areas. Curr. Zool. 2014, 60, 170–179. [Google Scholar] [CrossRef]
- Bosch, J.; Mardones, F.; Pérez, A.; De la Torre, A.; Muñoz, M.J. A maximum entropy model for predicting wild boar distribution in Spain. Span. J. Agric. Res. 2014, 12, 984–999. [Google Scholar] [CrossRef]
- Morelle, K.; Lejeune, P. Seasonal variations of wild boar Sus scrofa distribution in agricultural landscapes: A species distribution modelling approach. Eur. J. Wildl. Res. 2015, 61, 45–56. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Thuiller, W.; Zimmermann, N.E. Habitat Suitability and Distribution Models: With Applications in R; Cambridge University Press: Cambridge, UK, 2017; Volume 12, pp. 217–219. [Google Scholar]
- Ma, J.; Chen, H.; Gao, X.; Xiao, J.; Wang, H. African Swine Fever Emerging in China: Distribution Characteristics and High-Risk Areas. Prev. Vet. Med. 2020, 175, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Andraud, M.; Bougeard, S.; Chesnoiu, T.; Rose, N. Spatiotemporal clustering and Random Forest models to identify risk factors of African swine fever outbreak in Romania in 2018–2019. Sci. Rep. 2021, 11, 2098. [Google Scholar] [CrossRef]
- Liang, R.; Lu, Y.; Qu, X.; Su, Q.; Li, C.; Xia, S.; Liu, Y.; Zhang, Q.; Cao, X.; Chen, Q.; et al. Prediction for global African swine fever outbreaks based on a combination of random forest algorithms and meteorological data. Transbound. Emerg. Dis. 2020, 67, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-P.; Gao, X.; An, Q.; Sun, Z.; Wang, H.-B. Ecological niche modeling based on ensemble algorithms to predicting current and future potential distribution of African swine fever virus in China. Sci. Rep. 2022, 12, 15614. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cao, J.M.; Grossmann, N.; Goodman, S.M.; Bosch, J.; Guis, H.; Rasamoelina, M.; Rakotoarivony, R.; Jori, F.; Martínez-López, B. A maximum entropy species distribution model to estimate the distribution of bushpigs on Madagascar and its implications for African Swine Fever. Transbound. Emerg. Dis. 2023, 2023, 7976252. [Google Scholar] [CrossRef]
- Fekede, R.J.; van Gils, H.; Huang, L.Y.; Wang, X.L. High probability areas for ASF infection in China along the Russian and Korean borders. Transbound. Emerg. Dis. 2019, 66, 852–864. [Google Scholar] [CrossRef]
- Lim, J.-S.; Kim, E.; Ryu, P.-D.; Pak, S.-I. Basic reproduction number of African swine fever in wild boars (Sus scrofa) and its spatiotemporal heterogeneity in South Korea. J. Vet. Sci. 2021, 22, e71. [Google Scholar] [CrossRef]
- Bergmann, H.; Schulz, K.; Conraths, F.J.; Sauter-Louis, C. A review of environmental risk factors for African Swine Fever in European wild boar. Animals 2021, 11, 2692. [Google Scholar] [CrossRef]
- Ko, C.; Cho, W.; Hwang, B.; Chang, B.; Kang, W.; Ko, D.W. Simulating Hunting Effects on the Wild Boar Population and African Swine Fever Expansion Using Agent-Based Modeling. Animals 2023, 13, 298. [Google Scholar] [CrossRef] [PubMed]
- ESRI, How Kernel Density Works. Available online: https://pro.arcgis.com/en/pro-app/latest/tool-reference/spatial-analyst/how-kernel-density-works.htm (accessed on 14 September 2023).
- Grenouillet, G.; Buisson, L.; Casajus, N.; Lek, S. Ensemble modelling of species distribution: The effects of geographical and environmental ranges. Ecography 2011, 34, 9–17. [Google Scholar] [CrossRef]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. Evaluation of consensus methods in predictive species distribution modelling. Divers. Distrib. 2009, 15, 59–69. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, Y.; Jang, R.; Jeon, S. Mapping the Species Richness of Woody Plants in Republic of Korea. Sustainability 2023, 15, 5718. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-vector network. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef]
- Norberg, A.; Abrego, N.; Blanchet, F.G.; Adler, F.R.; Anderson, B.J.; Anttila, J.; Araujo, M.B.; Dallas, T.; Dunson, D.; Elith, J.; et al. A comprehensive evaluation of predictive performance of 33 species distribution models at species and community levels. Ecol. Monogr. 2019, 89, e01370. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Ahmadi, M. A comparison of absolute performance of different correlative and mechanistic species distribution models in an independent area. Ecol. Evol. 2016, 6, 5973–5986. [Google Scholar] [CrossRef]
- Hastie, T.J.; Tibshirani, R.J. Generalized Additive Models; Routledge: London, UK, 2017. [Google Scholar]
- Ho, T.K. Random decision forests. In Proceedings of the 3rd International Conference on Document Analysis and Recognition, Montreal, QC, Canada, 14–15 August 1995; Volume 1, pp. 278–282. [Google Scholar]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Jaynes, E.T. Information theory and statistical mechanics I. Phys. Rev. 1957, 106, 620. [Google Scholar] [CrossRef]
- Jaynes, E.T. Information theory and statistical mechanics II. Phys. Rev. 1957, 108, 171. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Forestry, and Fisheries. Disclose the Status of an Outbreak of Animal Spotted Fever (ASF). Available online: https://www.mafra.go.kr/FMD-AI2/2145/subview.do (accessed on 4 September 2023).
- Ito, S.; Bosch, J.; Jeong, H.; Aguilar-Vega, C.; Park, J.; Martínez-Avilés, M.; Sánchez-Vizcaíno, J.M. Spatio-Temporal Epidemiology of the Spread of African Swine Fever in Wild Boar and the Role of Environmental Factors in South Korea. Viruses 2022, 14, 2779. [Google Scholar] [CrossRef] [PubMed]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Gurrutxaga, M.; Rubio, L.; Saura, S. Key connectors in protected forest area networks and the impact of highways: A transnational case study from the Cantabrian Range to the Western Alps (SW Europe). Landsc. Urban Plan. 2011, 101, 310–320. [Google Scholar] [CrossRef]
- Stillfried, M.; Gras, P.; Börner, K.; Göritz, F.; Painer, J.; Röllig, K.; Wenzler, M.; Hofer, H.; Ortmann, S.; Kramer-Schadt, S. Secrets of Success in a Landscape of Fear: Urban Wild Boar Adjust Risk Perception and Tolerate Disturbance. Front. Ecol. Evol. 2017, 5, 157. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Radomski, T.; Beamer, D.; Babineau, A.; Wilson, C.; Pechmann, J.; Kozak, K.H. Finding what you don’t know: Testing SDM methods for poorly known species. Divers. Distrib. 2022, 28, 1769–1780. [Google Scholar] [CrossRef]
- Scherrer, D.; D’Amen, M.; Fernandes, R.F.; Mateo, R.G.; Guisan, A. How to best threshold and validate stacked species assemblages? Community optimisation might hold the answer. Methods Ecol. Evol. 2018, 9, 2155–2166. [Google Scholar] [CrossRef]
- Liu, G.R.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2015, 6, 337–348. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- MAFRA (Ministry of Agriculture, Food, and Rural Affairs). Standard Operating Procedure (SOP) for African Swine Fever. Available online: https://www.mafra.go.kr/home/5248/subview.do (accessed on 10 September 2023).
- Lim, J.S.; Andraud, M.; Kim, E.; Vergne, T. Three years of African swine fever in South Korea (2019–2021): A scoping review of epidemiological understanding. Transbound. Emerg. Dis. 2023, 2023, 4686980. [Google Scholar] [CrossRef]
- Lim, S.J.; Namgung, H.; Kim, N.H.; Oh, Y.; Park, Y.C. Prediction of potential spread areas of African swine fever virus through wild boars using Maxent model. J. Ecol. Environ. 2022, 46, 6. [Google Scholar] [CrossRef]
- Laguna, E.; Barasona, J.A.; Carpio, A.J.; Vicente, J.; Acevedo, P. Permeability of artificial barriers (fences) for wild boar Sus scrofa in Mediterranean mixed landscapes. Pest Manag. Sci. 2022, 78, 2277–2286. [Google Scholar] [CrossRef]
- Merli, E.; Grignolio, S.; Marcon, A.; Apollonio, M. Wild boar under fire: The effect of spatial behaviour, habitat use and social class on hunting mortality. J. Zool. 2017, 303, 155–164. [Google Scholar] [CrossRef]
- Sodeikat, G.; Pohlmeyer, K. Impact of drive hunts on daytime resting site areas of wild boar family groups (Sus scrofa L.). Wildl. Biol. Pract. 2007, 3, 28–38. [Google Scholar] [CrossRef]

| GAM | BRT | SVM | MaxEnt | RF | Ensemble | |
|---|---|---|---|---|---|---|
| Wild boar habitat | 0.71 | 0.75 | 0.76 | 0.72 | 0.89 | 0.76 |
| ASF-managed area | 0.75 | 0.79 | 0.77 | 0.75 | 0.92 | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, C.; Ko, D.W.; Cho, W. Predicting Disparity between ASF-Managed Areas and Wild Boar Habitats: A Case of South Korea. Animals 2023, 13, 3482. https://doi.org/10.3390/ani13223482
Ko C, Ko DW, Cho W. Predicting Disparity between ASF-Managed Areas and Wild Boar Habitats: A Case of South Korea. Animals. 2023; 13(22):3482. https://doi.org/10.3390/ani13223482
Chicago/Turabian StyleKo, Chanwoo, Dongwook W. Ko, and Wonhee Cho. 2023. "Predicting Disparity between ASF-Managed Areas and Wild Boar Habitats: A Case of South Korea" Animals 13, no. 22: 3482. https://doi.org/10.3390/ani13223482
APA StyleKo, C., Ko, D. W., & Cho, W. (2023). Predicting Disparity between ASF-Managed Areas and Wild Boar Habitats: A Case of South Korea. Animals, 13(22), 3482. https://doi.org/10.3390/ani13223482







