Comparison of Two Methods for the Measurement of Blood Plasma and Capillary Blood Glucose in Tropical Highland Grassing Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
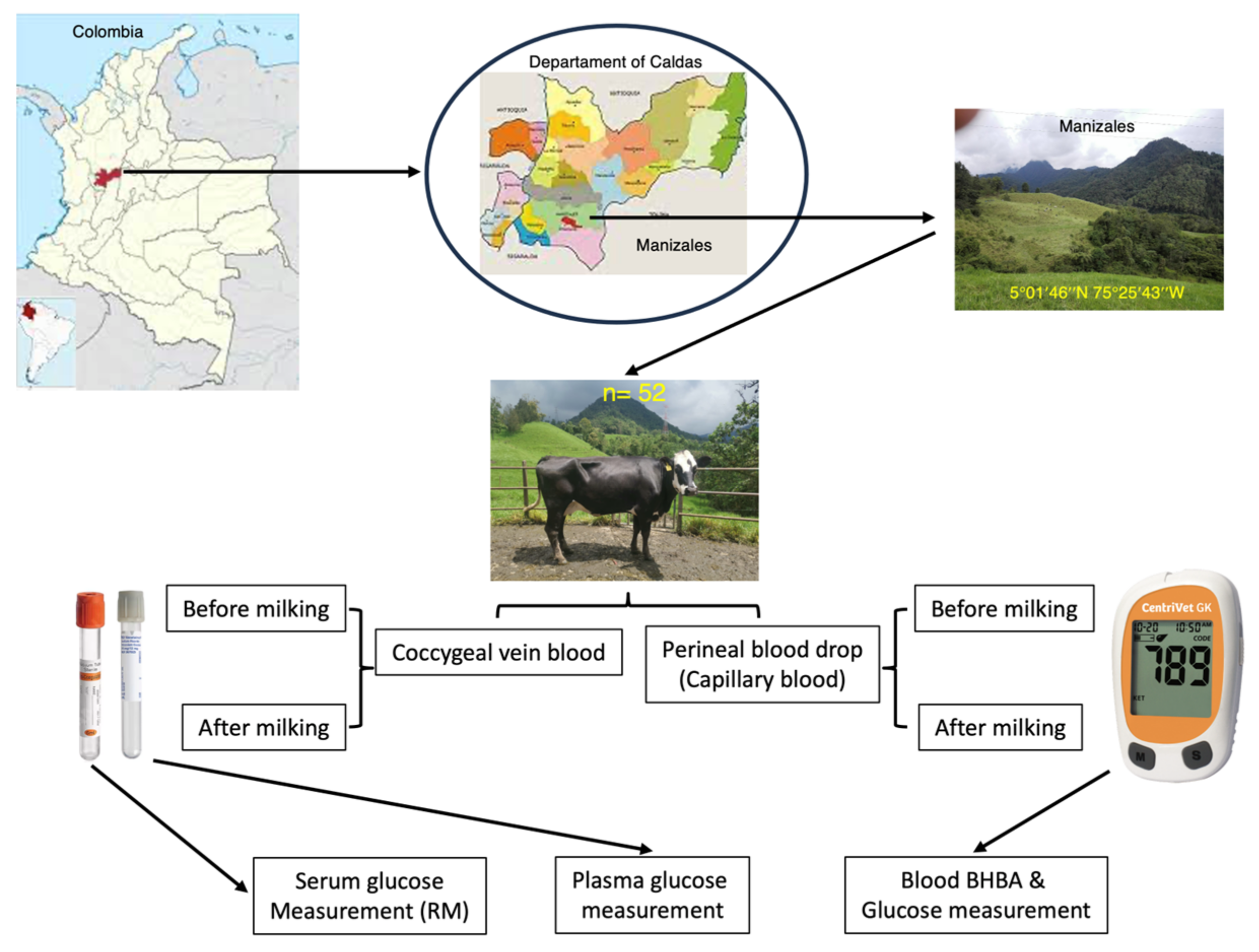
2.1. Farm Location and Animals
2.2. Design of the Study
2.3. Blood Sampling and Processing
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucy, M.C.; Butler, S.T.; Garverick, H.A. Endocrine and metabolic mechanisms linking postpartum glucose with early embryonic and foetal development in dairy cows. Animal 2014, 8 (Suppl. S1), 82–90. [Google Scholar] [CrossRef] [PubMed]
- Mair, B.; Drillich, M.; Klein-Jöbstl, D.; Kanz, P.; Borchardt, S.; Meyer, L.; Schwendenwein, I.; Iwersen, M. Glucose concentration in capillary blood of dairy cows obtained by a minimally invasive lancet technique and determined with three different hand-held devices. BMC Vet. Res. 2016, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.; Hasan, M.M.; Tanni, N.S.; Parvin, M.S.; Asaduzzaman, M.; Ehsan, M.A.; Islam, M.T. Metabolic profiling in periparturient dairy cows and its relation with metabolic diseases. BMC Res. Notes 2022, 15, 231. [Google Scholar] [CrossRef]
- Castro, N.; Kawashima, C.; van Dorland, H.A.; Morel, I.; Miyamoto, A.; Bruckmaier, R.M. Metabolic and energy status during the dry period is crucial for the resumption of ovarian activity postpartum in dairy cows. J. Dairy Sci. 2012, 95, 5804–5812. [Google Scholar] [CrossRef] [PubMed]
- Iwersen, M.; Klein-Jöbstl, D.; Pichler, M.; Roland, L.; Fidlschuster, B.; Schwendenwein, I.; Drillich, M. Comparison of 2 electronic cowside tests to detect subclinical ketosis in dairy cows and the influence of the temperature and type of blood sample on the test results. J. Dairy Sci. 2013, 96, 7719–7730. [Google Scholar] [CrossRef] [PubMed]
- Kanz, P.; Drillich, M.; Klein-Jöbstl, D.; Mair, B.; Borchardt, S.; Meyer, L.; Schwendenwein, I.; Iwersen, M. Suitability of capillary blood obtained by a minimally invasive lancet technique to detect subclinical ketosis in dairy cows by using 3 different electronic hand-held devices. J. Dairy Sci. 2015, 98, 6108–6118. [Google Scholar] [CrossRef]
- LeBlanc, S. Monitoring metabolic health of dairy cattle in the transition period. J. Reprod. Dev. 2010, 56, S29–S35. [Google Scholar] [CrossRef]
- Smith, G.L.; Friggens, N.C.; Ashworth, C.J.; Chagunda, M.G.G. Association between body energy content in the dry period and post-calving production disease status in dairy cattle. Animal 2017, 11, 1590–1598. [Google Scholar] [CrossRef]
- Tatone, E.H.; Gordon, J.L.; Hubbs, J.; LeBlanc, S.J.; DeVries, T.J.; Duffield, T.F. A systematic review and meta-analysis of the diagnostic accuracy of point-of-care tests for the detection of hyperketonemia in dairy cows. Prev. Vet. Med. 2016, 130, 18–32. [Google Scholar] [CrossRef]
- Geishauser, T.; Leslie, K.; Tenhag, J.; Bashiri, A. Evaluation of eight cow-side ketone tests in milk for detection of subclinical ketosis in dairy cows. J. Dairy Sci. 2000, 83, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Bilić-Zulle, L. Comparison of methods: Passing and Bablok regression. Biochem. Med. 2011, 21, 49–52. [Google Scholar] [CrossRef]
- Nickles, K.R.; Relling, A.E.; Garcia-Guerra, A.; Fluharty, F.L.; Parker, A.J. Short communication: A comparison between two glucose measurement methods in beef steers during a glucose tolerance test. PLoS ONE 2022, 17, e0271673. [Google Scholar] [CrossRef] [PubMed]
- Karapinar, T.; Tumer, K.C.; Buczinski, S. Evaluation of the Freestyle Optium Neo H point-of-care device for measuring blood glucose concentrations in sick calves. J. Vet. Intern. Med. 2020, 34, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Katsoulos, P.D.; Minas, A.; Karatzia, M.A.; Pourliotis, K.; Christodoulopoulos, G. Evaluation of a portable glucose meter for use in cattle and sheep. Vet. Clin. Pathol. 2011, 40, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Wittrock, J.A.; Duffield, T.F.; LeBlanc, S.J. Short communication: Validation of a point-of-care glucometer for use in dairy cows. J. Dairy Sci. 2013, 96, 4514–4518. [Google Scholar] [CrossRef]
- Lopes, R.B.; Valldecabres, A.; Silva-Del-Río, N. Technical note: Glucose concentration in dairy cows measured using 6 handheld meters designed for human use. J. Dairy Sci. 2019, 102, 9401–9408. [Google Scholar] [CrossRef]
- McCabe, C.J.; Suarez-Trujillo, A.; Teeple, K.A.; Casey, T.M.; Boerman, J.P. Chronic prepartum light-dark phase shifts in cattle disrupt circadian clocks, decrease insulin sensitivity and mammary development, and are associated with lower milk yield through 60 days postpartum. J. Dairy Sci. 2021, 104, 2422–2437. [Google Scholar] [CrossRef] [PubMed]
- Urbutis, M.; Malašauskienė, D.; Televičius, M.; Juozaitienė, V.; Baumgartner, W.; Antanaitis, R. Evaluation of the Metabolic Relationship between Cows and Calves by Monitoring Calf Health and Cow Automatic Milking System and Metabolic Parameters. Animals 2023, 13, 2576. [Google Scholar] [CrossRef] [PubMed]
- Peakman, T.C.; Elliott, P. The UK Biobank sample handling and storage validation studies. Int. J. Epidemiol. 2008, 37 (Suppl. S1), i2–i6. [Google Scholar] [CrossRef]
- Doğan, N. Bland-Altman analysis: A paradigm to understand correlation and agreement. Turk. J. Emerg. Med. 2018, 18, 139–141. [Google Scholar] [CrossRef]
- Holtenius, P.; Holtenius, K. New aspects of ketone bodies in energy metabolism of dairy cows: A review. Zentralbl. Vet. Med. A 1996, 43, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, G.; Ravarotto, L.; Gottardo, F.; Stefani, A.L.; Contiero, B.; Moro, L.; Brscic, M.; Dalvit, P. Short communication: Reference values for blood parameters in Holstein dairy cows: Effects of parity, stage of lactation, and season of production. J. Dairy Sci. 2011, 94, 3895–3901. [Google Scholar] [PubMed]
- Habel, J.; Sundrum, A. Mismatch of Glucose Allocation between Different Life Functions in the Transition Period of Dairy Cows. Animals 2020, 10, 1028. [Google Scholar] [PubMed]
- Habel, J.; Sundrum, A. Dairy Cows Are Limited in Their Ability to Increase Glucose Availability for Immune Function during Disease. Animals 2023, 13, 1034. [Google Scholar] [PubMed]
- Cao, Y.; Zhang, J.; Yang, W.; Xia, C.; Zhang, H.Y.; Wang, Y.H.; Xu, C. Predictive Value of Plasma Parameters in the Risk of Postpartum Ketosis in Dairy Cows. J. Vet. Res. 2017, 61, 91–95. [Google Scholar]
- Mohammed, S.E.; Ahmad, F.O.; Frah, E.A.M.; Elfaki, I. Determination of Blood Glucose, Total Protein, Certain Minerals, and Triiodothyronine during Late Pregnancy and Postpartum Periods in Crossbred Dairy Cows. Vet. Med. Int. 2021, 2021, 6610362. [Google Scholar] [CrossRef]
- Civiero, M.; Cabezas-Garcia, E.H.; Ribeiro-Filho, H.M.N.; Gordon, A.W.; Ferris, C.P. Relationships between energy balance during early lactation and cow performance, blood metabolites, and fertility: A meta-analysis of individual cow data. J. Dairy Sci. 2021, 104, 7233–7251. [Google Scholar] [PubMed]
- Ingvartsen, K.L.; Moyes, K.M. Factors contributing to immunosuppression in the dairy cow during the periparturient period. Jpn. J. Vet Res. 2015, 63 (Suppl. S1), S15–S24. [Google Scholar]
- Garverick, H.A.; Harris, M.N.; Vogel-Bluel, R.; Sampson, J.D.; Bader, J.; Lamberson, W.R.; Spain, J.N.; Lucy, M.C.; Youngquist, R.S. Concentrations of nonesterified fatty acids and glucose in blood of periparturient dairy cows are indicative of pregnancy success at first insemination. J. Dairy Sci. 2013, 96, 181–188. [Google Scholar] [CrossRef]
- Brscic, M.; Cozzi, G.; Lora, I.; Stefani, A.L.; Contiero, B.; Ravarotto, L.; Gottardo, F. Short communication: Reference limits for blood analytes in Holstein late-pregnant heifers and dry cows: Effects of parity, days relative to calving, and season. J. Dairy Sci. 2015, 98, 7886–7892. [Google Scholar] [CrossRef]
- Salawu, M.B.; Adesogan, A.T.; Dewhurst, R.J. Forage intake, meal patterns, and milk production of lactating dairy cows fed grass silage or pea-wheat bi-crop silages. J. Dairy Sci. 2002, 85, 3035–3044. [Google Scholar] [CrossRef] [PubMed]
- Doepel, L.; Lobley, G.E.; Bernier, J.F.; Dubreuil, P.; Lapierre, H. Differences in splanchnic metabolism between late gestation and early lactation dairy cows. J. Dairy Sci. 2009, 92, 3233–3243. [Google Scholar] [PubMed]
- Wieghart, M.; Slepetis, R.; Elliot, J.M.; Smith, D.F. Glucose absorption and hepatic gluconeogenesis in dairy cows fed diets varying in forage content. J. Nutr. 1986, 116, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Seal, C.J.; Parker, D.S.; Avery, P.J. The effect of forage and forage-concentrate diets on rumen fermentation and metabolism of nutrients by the mesenteric- and portal-drained viscera in growing steers. Br. J. Nutr. 1992, 67, 355–370. [Google Scholar] [CrossRef] [PubMed]





| Statistical Parameter | Method Used for Glucose Measurement (mg/mL) | ||
|---|---|---|---|
| Serum EPA (Reference Method) | Plasma EPA | Hand-Held Device (Capillary Blood) | |
| Before milking | |||
| Mean | 81.07 a | 78.92 b | 71.25 c |
| 95% IC mean upper | 84.35 | 82.40 | 73.64 |
| 95% IC mean lower | 77.98 | 75.44 | 68.85 |
| Standard deviation | 11.73 | 11.78 | 15.10 |
| Median | 81.00 | 78.00 | 70.00 |
| Interquartile range | 55.00–113.00 | 51.00–105.00 | 50.00–91.00 |
| After milking | |||
| Mean | 82.00 a | 73.72 b | 69.83 c |
| 95% IC mean upper | 86.19 | 86.19 | 72.73 |
| 95% IC mean lower | 77.81 | 77.80 | 66.91 |
| Standard deviation | 15.11 | 10.69 | 10.71 |
| Median | 81.50 | 72.50 | 67.00 |
| Interquartile range | 52.00–117.00 | 53.00 | 47.00–107.00 |
| Method for Glucose Determination | Statistical Method | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Passing–Bablok Regression Values | Bland–Altman Calculations Values | ||||||||
| Slope (β) | CI95% (β) | Intercept (α) | CI95% (α) | p Value | Mean Diff | SD Diff | Lower | Upper | |
| Plasma (EPA) | 0.72 | 0.61–0.92 | 14.81 | 0.27–26.6 | 0.09 | 5.27 | 11.73 | −17.73 | 28.28 |
| Hand-held device (capillary blood) | 2.00 | 1.33–3.20 | −57.00 | 3.20–138.8 | 0.01 | 11.01 | 15.74 | −19.87 | 41.87 |
| GLMM Number | Effect | p-Value |
|---|---|---|
| 1 | Intercept | <0.001 |
| Method | <0.001 | |
| Age group | 0.035 | |
| Method × Age group | 0.075 | |
| 2 | Intercept | <0.001 |
| Method | <0.001 | |
| Breed | 0.752 | |
| Method × Breed | 0.913 | |
| 3 | Intercept | <0.001 |
| Method | <0.001 | |
| Reproductive status | 0.696 | |
| Method × Reproductive status | 0.610 | |
| 4 | Intercept | <0.001 |
| Method | <0.001 | |
| Pregnancy status | 0.679 | |
| Method × Pregnancy status | 0.697 | |
| 5 | Intercept | <0.001 |
| Method | <0.001 | |
| Days of lactation | 0.940 | |
| Method × Days of lactation | 0.533 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, C.; Hincapié, V.; Carmona, J.U. Comparison of Two Methods for the Measurement of Blood Plasma and Capillary Blood Glucose in Tropical Highland Grassing Dairy Cows. Animals 2023, 13, 3536. https://doi.org/10.3390/ani13223536
López C, Hincapié V, Carmona JU. Comparison of Two Methods for the Measurement of Blood Plasma and Capillary Blood Glucose in Tropical Highland Grassing Dairy Cows. Animals. 2023; 13(22):3536. https://doi.org/10.3390/ani13223536
Chicago/Turabian StyleLópez, Catalina, Valentina Hincapié, and Jorge U. Carmona. 2023. "Comparison of Two Methods for the Measurement of Blood Plasma and Capillary Blood Glucose in Tropical Highland Grassing Dairy Cows" Animals 13, no. 22: 3536. https://doi.org/10.3390/ani13223536
APA StyleLópez, C., Hincapié, V., & Carmona, J. U. (2023). Comparison of Two Methods for the Measurement of Blood Plasma and Capillary Blood Glucose in Tropical Highland Grassing Dairy Cows. Animals, 13(22), 3536. https://doi.org/10.3390/ani13223536






