Artificial Pasture Grazing System Attenuates Lipopolysaccharide-Induced Gut Barrier Dysfunction, Liver Inflammation, and Metabolic Syndrome by Activating ALP-Dependent Keap1-Nrf2 Pathway
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Animals
2.2. Sample Collection
2.3. Determination of ALP, LPS, and ROS Levels
2.4. Measurement of Gut Permeability
2.5. Determination of Antioxidant Activity
2.6. Determination of Metabolic Profiles
2.7. Staining with Hematoxylin and Eosin (H&E)
2.8. RNA Extraction and cDNA Synthesis for RT-qPCR Analysis
2.9. Statistical Analysis
3. Results
3.1. A Commercial Diet-Dependent Decline in ALP Activity Caused Gut Permeability and Systemic Inflammation
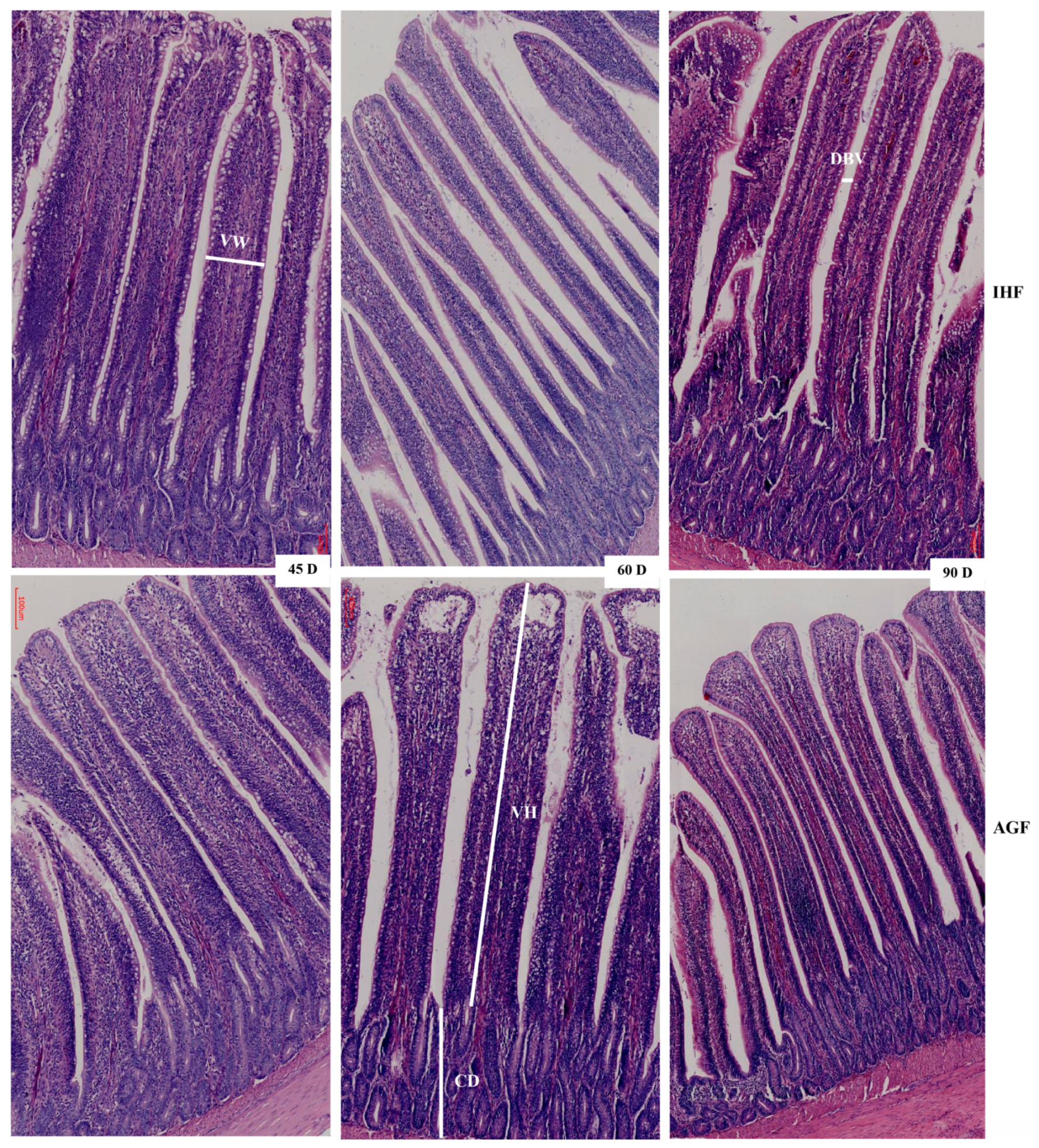
3.2. Commercial Diet Caused Deterioration of Nutrient Absorption
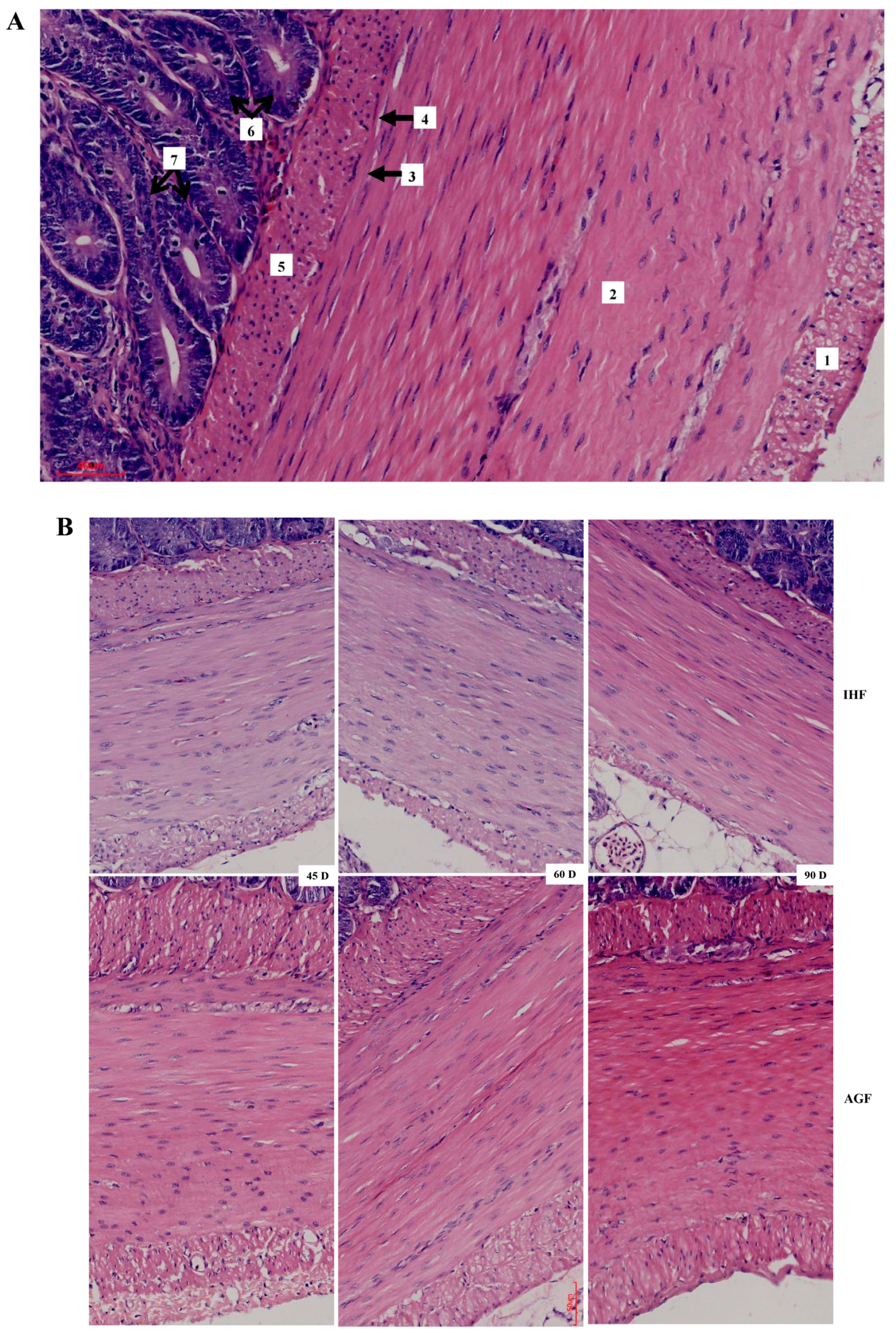
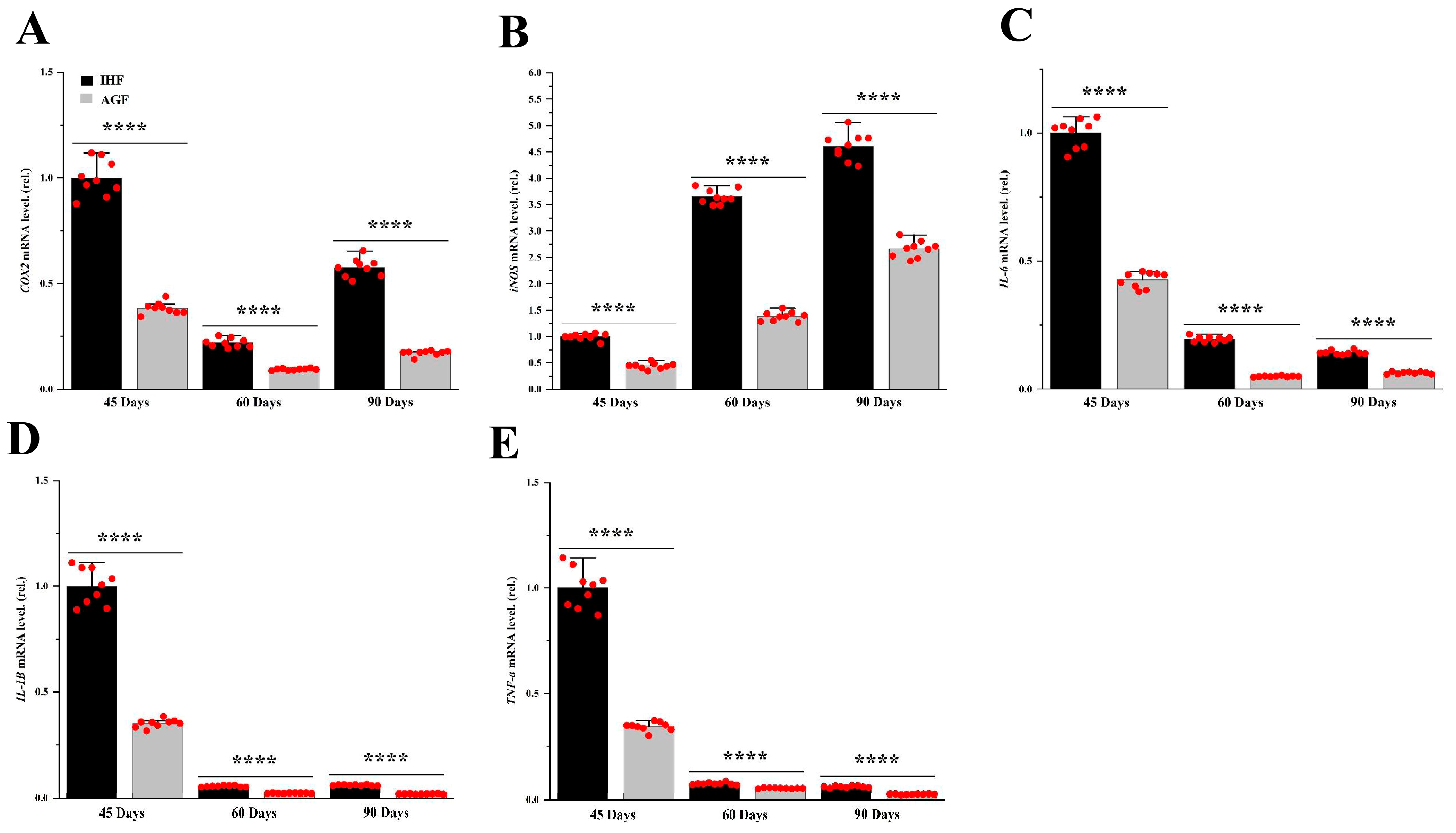
3.3. Commercial Diet-Dependent Gut Permeability Is Paralleled by Liver Inflammation
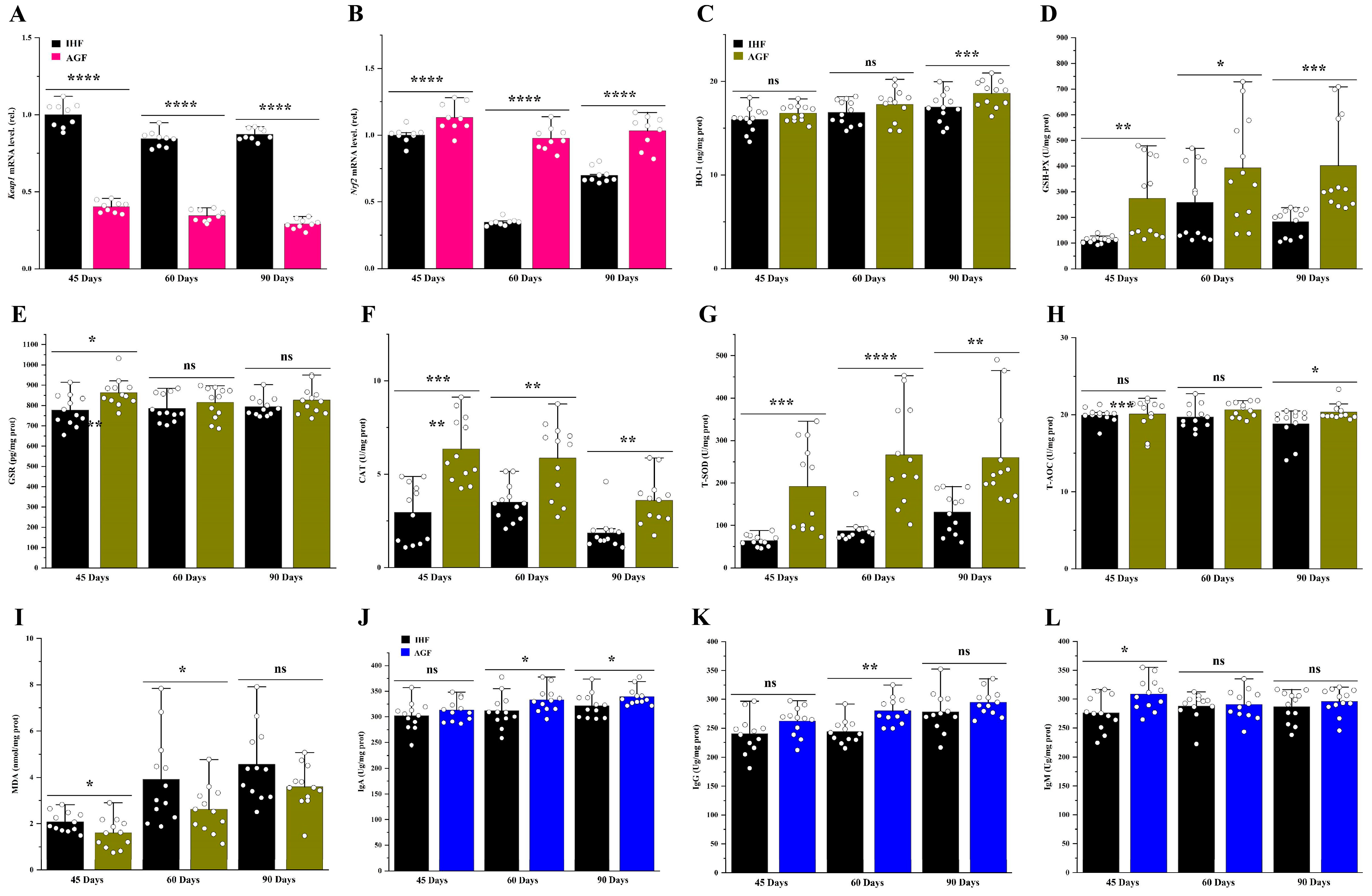
3.4. Long-Term Pasture Intake Causes Redox Signaling Pathway Activation
3.5. Long-Term Pasture Intake Caused Improved Growth Performance, Intestinal Organ Development, and the Metabolic Profile of Geese
3.6. Integrated Analysis of Host Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obianwuna, U.E.; Agbai Kalu, N.; Wang, J.; Zhang, H.; Qi, G.; Qiu, K.; Wu, S. Recent Trends on Mitigative Effect of Probiotics on Oxidative-Stress-Induced Gut Dysfunction in Broilers under Necrotic Enteritis Challenge: A Review. Antioxidants 2023, 12, 911. [Google Scholar] [CrossRef]
- Romano, K.P.; Hung, D.T. Targeting LPS biosynthesis and transport in gram-negative bacteria in the era of multi-drug resistance. Biochim. Biophys. Acta Mol. Cell Res. 2023, 1870, 119407. [Google Scholar] [CrossRef]
- Ali, Q.; Ma, S.; Farooq, U.; Niu, J.; Li, F.; Li, D.; Wang, Z.; Sun, H.; Cui, Y.; Shi, Y. Pasture intake protects against commercial diet-induced lipopolysaccharide production facilitated by gut microbiota through activating intestinal alkaline phosphatase enzyme in meat geese. Front. Immunol. 2022, 13, 1041070. [Google Scholar] [CrossRef] [PubMed]
- Volk, A.; Lee, J. Cyanobacterial blooms: A player in the freshwater environmental resistome with public health relevance? Environ. Res. 2023, 216, 114612. [Google Scholar] [CrossRef]
- Kumar, A.; Ali, A.; Kapardar, R.K.; Dar, G.M.; Nimisha; Apurva; Sharma, A.K.; Verma, R.; Sattar, R.S.A.; Ahmad, E.; et al. Implication of gut microbes and its metabolites in colorectal cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 441–465. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.; Abbas, W.; Huang, J.; Guo, F.; Zhang, K.; Kong, L.; Zhen, W.; Guo, Y.; Wang, Z. Dietary coated essential oil and organic acid mixture supplementation improves health of broilers infected with avian pathogenic Escherichia coli. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2023, 12, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Moradi Vastegani, S.; Hajipour, S.; Sarkaki, A.; Basir, Z.; Parisa Navabi, S.; Farbood, Y.; Khoshnam, S.E. Curcumin mitigates lipopolysaccharide-induced anxiety/depression-like behaviors, blood-brain barrier dysfunction and brain edema by decreasing cerebral oxidative stress in male rats. Neurosci. Let. 2022, 782, 136697. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, N.M.; Beukema, M.; Tian, L.; Troost, B.; Scholte, J.; Bruininx, E.; Bruggeman, G.; van den Berg, M.; Scheurink, A.; Schols, H.A.; et al. Dietary Fiber Pectin Directly Blocks Toll-Like Receptor 2-1 and Prevents Doxorubicin-Induced Ileitis. Front. Immunol. 2018, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Wellington, M.O.; Hamonic, K.; Krone, J.E.C.; Htoo, J.K.; Van Kessel, A.G.; Columbus, D.A. Effect of dietary fiber and threonine content on intestinal barrier function in pigs challenged with either systemic E. coli lipopolysaccharide or enteric Salmonella Typhimurium. J. Anim. Sci. Biotech. 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Islam, A.U.; Prakash, H.; Singh, S. Phytochemicals targeting NF-κB signaling: Potential anti-cancer interventions. J. Pharmaceut. Anal. 2022, 12, 394–405. [Google Scholar] [CrossRef]
- Okazaki, Y.; Katayama, T. Glucomannan consumption elevates colonic alkaline phosphatase activity by up-regulating the expression of IAP-I, which is associated with increased production of protective factors for gut epithelial homeostasis in high-fat diet-fed rats. Nutr. Res. 2017, 43, 43–50. [Google Scholar] [CrossRef]
- Fawley, J.; Gourlay, D.M. Intestinal alkaline phosphatase: A summary of its role in clinical disease. J. Surg. Res. 2016, 202, 225–234. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Gehr, T.W.; Ghosh, S. Curcumin and chronic kidney disease (CKD): Major mode of action through stimulating endogenous intestinal alkaline phosphatase. Molecules 2014, 19, 20139–20156. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Correas, S.; Broadbent, J.A.; Andraszek, A.; Stockwell, S.; Howitt, C.A.; Juhász, A.; Colgrave, M.L. Perennial Ryegrass Contains Gluten-Like Proteins That Could Contaminate Cereal Crops. Front. Nutr. 2021, 8, 708122. [Google Scholar] [CrossRef]
- Cartoni Mancinelli, A.; Mattioli, S.; Dal Bosco, A.; Piottoli, L.; Ranucci, D.; Branciari, R.; Cotozzolo, E.; Castellini, C. Rearing Romagnola geese in vineyard: Pasture and antioxidant intake, performance, carcass and meat quality. Ital. J. Anim. Sci. 2019, 18, 372–380. [Google Scholar] [CrossRef]
- Barati, M.T.; Caster, D.J. The potential of Nrf2 activation as a therapeutic target in systemic lupus erythematosus. Metabolites 2022, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.; Battigaglia, M.S.; Murone, E.; Dozio, E.; Pensabene, L.; Agosti, M. Dietary Fibers in Healthy Children and in Pediatric Gastrointestinal Disorders: A Practical Guide. Nutrients 2023, 15, 2208. [Google Scholar] [CrossRef]
- Huyghebaert, G.; Ducatelle, R.; Van Immerseel, F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Chen, Y.; Wang, H.Y.; Wang, R.F. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum. Vac. Immunotherap. 2014, 10, 3270–3285. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Amer. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef]
- Kühn, F.; Adiliaghdam, F.; Cavallaro, P.M.; Hamarneh, S.R.; Tsurumi, A.; Hoda, R.S.; Munoz, A.R.; Dhole, Y.; Ramirez, J.M.; Liu, E.; et al. Intestinal alkaline phosphatase targets the gut barrier to prevent aging. JCI Insight 2020, 5, e134049. [Google Scholar] [CrossRef]
- Koyama, I.; Matsunaga, T.; Harada, T.; Hokari, S.; Komoda, T. Alkaline phosphatases reduce toxicity of lipopolysaccharides in vivo and in vitro through dephosphorylation. Clin. Bioch. 2002, 35, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lim, B.S.; Lee, Y.K.; Yang, H.C. Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Bio. Toxicol. 2006, 22, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Huang, Y.; Li, Y.; Huang, Y.; Yan, J.; Shi, Z. The effects of dietary protein and fiber levels on growth performance, gout occurrence, intestinal microbial communities, and immunoregulation in the gut-kidney axis of goslings. Poult. Sci. 2022, 101, 101780. [Google Scholar] [CrossRef]
- Liu, W.; Hu, D.; Huo, H.; Zhang, W.; Adiliaghdam, F.; Morrison, S.; Ramirez, J.M.; Gul, S.S.; Hamarneh, S.R.; Hodin, R.A. Intestinal Alkaline Phosphatase Regulates Tight Junction Protein Levels. J. Amer. Coll. Surg. 2016, 222, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ma, S.; La, S.; Guo, Z.; Liu, B.; Gao, Z.; Farooq, U.; Wang, Z.; Zhu, X.; Cui, Y.; et al. Microbial short-chain fatty acids: A bridge between dietary fibers and poultry gut health—A review. Anim. Biosci. 2022, 35, 1461–1478. [Google Scholar] [CrossRef]
- Alemka, A.; Whelan, S.; Gough, R.; Clyne, M.; Gallagher, M.E.; Carrington, S.D.; Bourke, B. Purified chicken intestinal mucin attenuates Campylobacter jejuni pathogenicity in vitro. J. Med. Microb. 2010, 59, 898–903. [Google Scholar] [CrossRef]
- Pourabedin, M.; Chen, Q.; Yang, M.; Zhao, X. Mannan-and xylooligosaccharides modulate caecal microbiota and expression of inflammatory-related cytokines and reduce caecal Salmonella Enteritidis colonisation in young chickens. FEMS Microb. Ecol. 2017, 93, fiw226. [Google Scholar] [CrossRef] [PubMed]
- Mappley, L.J.; La Ragione, R.M.; Woodward, M.J. Brachyspira and its role in avian intestinal spirochaetosis. Vet. Microb. 2014, 168, 245–260. [Google Scholar] [CrossRef] [PubMed]
- McNabney, S.M.; Henagan, T.M. Short chain fatty acids in the colon and peripheral tissues: A focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef]
- Guerville, M.; Boudry, G. Gastrointestinal and hepatic mechanisms limiting entry and dissemination of lipopolysaccharide into the systemic circulation. Am. J. Physiol. Gastroint. Liver Physiol. 2016, 311, G1–G15. [Google Scholar] [CrossRef]
- Estaki, M.; DeCoffe, D.; Gibson, D.L. Interplay between intestinal alkaline phosphatase, diet, gut microbes and immunity. World J. Gastroenterol. 2014, 20, 15650–15656. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed]
- Varadhan, R.; Yao, W.; Matteini, A.; Beamer, B.A.; Xue, Q.L.; Yang, H.; Manwani, B.; Reiner, A.; Jenny, N.; Parekh, N.; et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all-cause mortality in older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014, 69, 165–173. [Google Scholar] [CrossRef]
- Horie, Y.; Suzuki, T.; Inoue, J.; Iso, T.; Wells, G.; Moore, T.W.; Mizushima, T.; Dinkova-Kostova, A.T.; Kasai, T.; Kamei, T.; et al. Molecular basis for the disruption of Keap1–Nrf2 interaction via Hinge & Latch mechanism. Commun. Biol. 2021, 4, 576. [Google Scholar] [CrossRef]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xing, T.; Li, J.; Zhang, L.; Jiang, Y.; Gao, F. Oxidative stress impairs the meat quality of broiler by damaging mitochondrial function, affecting calcium metabolism and leading to ferroptosis. Anim. Biosci. 2022, 35, 1616–1627. [Google Scholar] [CrossRef]
- Ling, J.; Gao, Y.-Y.; Hui, Y.; Wang, W.-C.; Lin, Z.-P.; Yang, H.-Y.; Huang, S.-B.; Lin, Y. Effects of dietary fiber and grit on performance, gastrointestinal tract development, lipometabolism, and grit retention of goslings. J. Integr. Agric. 2014, 13, 2731–2740. [Google Scholar] [CrossRef]
- Chen, J.; Weng, K.; Liu, J.; Gu, W.; Luo, S.; Zheng, M.; Cao, Z.; Zhang, Y.; Zhang, Y.; Chen, G. Effect of different free-range systems on the growth performance, carcass traits, and meat quality of Yangzhou geese. Anim. Biotech. 2022, 1–7. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wu, H.; Lv, Y.; You, H.; Zha, L.; Li, Q.; Huang, Y.; Tian, J.; Chen, Q.; Shen, Y. Gastrointestinal development and microbiota responses of geese to honeycomb flavonoids supplementation. Front. Vet. Sci. 2021, 8, 739237. [Google Scholar] [CrossRef]
- Zheng, M.; Mao, P.; Tian, X.; Meng, L. Effects of grazing mixed-grass pastures on growth performance, immune responses, and intestinal microbiota in free-range Beijing-you chickens. Poult. Sci. 2021, 100, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Kawanabe, Y.; Shinozaki, F.; Sato, S.; Motoyoshi, Y.; Sugiyama, T.; Yamamoto, S.; Sueishi, M. Triglyceride is strongly associated with nonalcoholic fatty liver disease among markers of hyperlipidemia and diabetes. Biomed. Rep. 2014, 2, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Feng, P.; Xu, Y.; Hou, Y.Y.; Ojo, O.; Wang, X.H. The Role of Dietary Fiber Supplementation in Regulating Uremic Toxins in Patients with Chronic Kidney Disease: A Meta-Analysis of Randomized Controlled Trials. J. Renal Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2021, 31, 438–447. [Google Scholar] [CrossRef]
- Yu, J.; Yang, Z.; Yang, H.; Wang, Z. Effects of cottonseed meal on growth performance, liver redox status, and serum biochemical parameters in goslings at 1 to 28 days of age. BMC Vet. Res. 2022, 18, 347. [Google Scholar] [CrossRef]



| Parameters | 45 Days | 60 Days | 90 Days | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IHF | AGF | p > 0.05 | IHF | AGF | p > 0.05 | IHF | AGF | p > 0.05 | |
| Inner layer (µm) | 51.82 ± 5.76 | 59.5 ± 6.13 | 0.020 | 54.51 ± 5.77 | 80 ± 8.62 | 0.000 | 48.6 ± 7.3 | 70.29 ± 5.69 | 0.000 |
| Outer layer (µm) | 10.32 ± 2.22 | 14.36 ± 3.51 | 0.020 | 10.97 ± 2.25 | 19.25 ± 3.63 | 0.000 | 8.53 ± 1.18 | 15.48 ± 2.13 | 0.000 |
| Total (µm) | 62.14 ± 5 | 73.86 ± 9.04 | 0.010 | 65.48 ± 3.93 | 99.26 ± 9.26 | 0.000 | 57.13 ± 7.57 | 85.77 ± 6.8 | 0.000 |
| Relative thickness of muscular tonic (µm) | 26.98 ± 3.13 | 45.6 ± 6.55 | 0.000 | 15.34 ± 1.33 | 29.81 ± 2.44 | 0.000 | 10.69 ± 1.91 | 19.76 ± 2.71 | 0.000 |
| Inner layer (µm) | 8.89 ± 1.82 | 14.25 ± 3.66 | 0.005 | 8.5 ± 1.11 | 21.37 ± 3.68 | 0.000 | 6.53 ± 1.27 | 11.68 ± 3.4 | 0.003 |
| Outer layer (µm) | 1.07 ± 0.17 | 1.58 ± 0.21 | 0.000 | 1.16 ± 0.25 | 1.61 ± 0.31 | 0.010 | 0.99 ± 0.15 | 1.68 ± 0.33 | 0.000 |
| Total (µm) | 9.95 ± 1.94 | 15.83 ± 3.49 | 0.002 | 9.65 ± 1.01 | 22.97 ± 3.64 | 0.000 | 7.52 ± 1.32 | 13.35 ± 3.48 | 0.002 |
| Relative thickness of muscularis mucosa (µm) | 4.33 ± 0.99 | 9.76 ± 2.21 | 0.000 | 2.25 ± 0.19 | 6.89 ± 0.99 | 0.000 | 1.41 ± 0.32 | 3.13 ± 1.05 | 0.002 |
| Parameters | 45 Days | 60 Days | 90 Days | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IHF | AGF | p > 0.05 | IHF | AGF | p > 0.05 | IHF | AGF | p > 0.05 | |
| Inner layer (µm) | 51.82 ± 5.76 | 59.5 ± 6.13 | 0.020 | 54.51 ± 5.77 | 80 ± 8.62 | 0.000 | 48.6 ± 7.3 | 70.29 ± 5.69 | 0.000 |
| Outer layer (µm) | 10.32 ± 2.22 | 14.36 ± 3.51 | 0.020 | 10.97 ± 2.25 | 19.25 ± 3.63 | 0.000 | 8.53 ± 1.18 | 15.48 ± 2.13 | 0.000 |
| Total (µm) | 62.14 ± 5 | 73.86 ± 9.04 | 0.010 | 65.48 ± 3.93 | 99.26 ± 9.26 | 0.000 | 57.13 ± 7.57 | 85.77 ± 6.8 | 0.000 |
| Relative thickness of muscular tonic (µm) | 26.98 ± 3.13 | 45.6 ± 6.55 | 0.000 | 15.34 ± 1.33 | 29.81 ± 2.44 | 0.000 | 10.69 ± 1.91 | 19.76 ± 2.71 | 0.000 |
| Inner layer (µm) | 8.89 ± 1.82 | 14.25 ± 3.66 | 0.005 | 8.5 ± 1.11 | 21.37 ± 3.68 | 0.000 | 6.53 ± 1.27 | 11.68 ± 3.4 | 0.003 |
| Outer layer (µm) | 1.07 ± 0.17 | 1.58 ± 0.21 | 0.000 | 1.16 ± 0.25 | 1.61 ± 0.31 | 0.010 | 0.99 ± 0.15 | 1.68 ± 0.33 | 0.000 |
| Total (µm) | 9.95 ± 1.94 | 15.83 ± 3.49 | 0.002 | 9.65 ± 1.01 | 22.97 ± 3.64 | 0.000 | 7.52 ± 1.32 | 13.35 ± 3.48 | 0.002 |
| Relative thickness of muscularis mucosa (µm) | 4.33 ± 0.99 | 9.76 ± 2.21 | 0.000 | 2.25 ± 0.19 | 6.89 ± 0.99 | 0.000 | 1.41 ± 0.32 | 3.13 ± 1.05 | 0.002 |
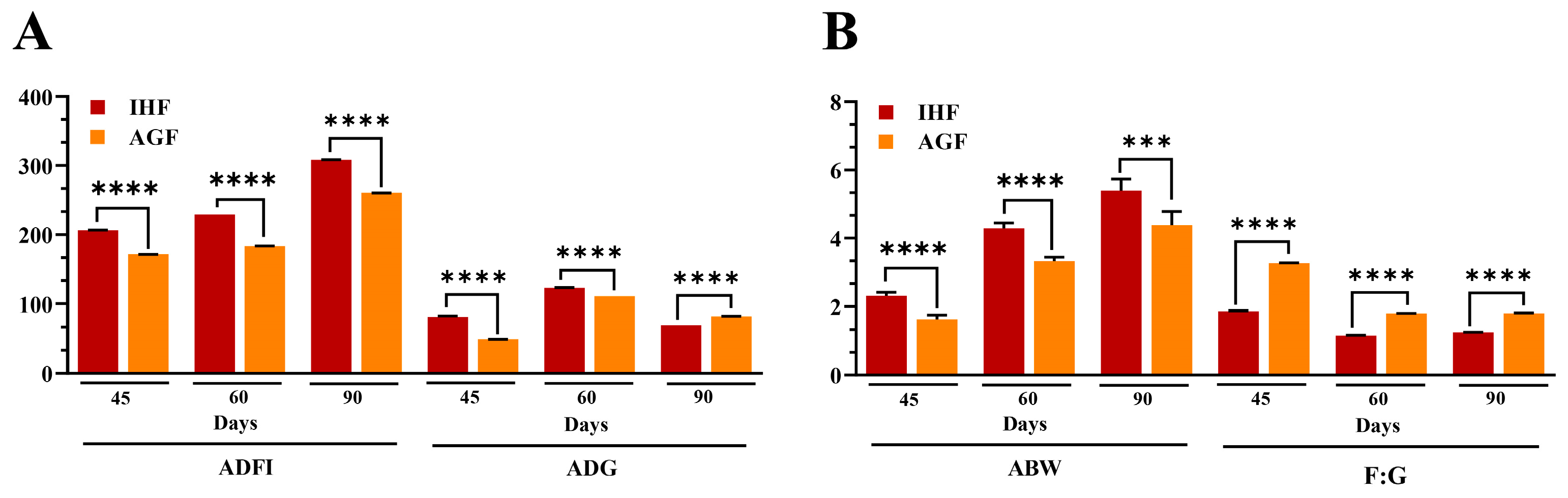
| Age, d | Parameters | IHF | * AGF | ** p-Value |
|---|---|---|---|---|
| 45 d | ADFI (g/d) | 206.3 ± 0.27 | 171.37 ± 0.23 | 0.000 |
| 60 d | 229.21 ± 0.01 | 183.3 ± 0.27 | 0.000 | |
| 90 d | 308.3 ± 0.09 | 260.4 ± 0.09 | 0.000 | |
| 45 d | ABW (kg) | 2.31 ± 0.13 | 1.63 ± 0.14 | 0.000 |
| 60 d | 4.28 ± 0.16 | 3.33 ± 0.11 | 0.000 | |
| 90 d | 5.39 ± 0.34 | 4.38 ± 0.4 | 0.000 | |
| 45 d | ADG (g/d) | 81.5 ± 1.26 | 49.06 ± 0.12 | 0.000 |
| 60 d | 123.15 ± 0.35 | 110.88 ± 0.02 | 0.000 | |
| 90 d | 69.38 ± 0.02 | 82.3 ± 0.09 | 0.000 | |
| 45 d | F:G | 1.87 ± 0.01 | 3.27 ± 0.01 | 0.000 |
| 60 d | 1.16 ± 0.01 | 1.8 ± 0.01 | 0.000 | |
| 90 d | 1.25 ± 0.01 | 1.81 ± 0.01 | 0.000 |
| Age, d | Parameters | IHF | AGF | p-Value |
|---|---|---|---|---|
| 45 d | Rectum | 7.04 ± 0.6 | 8.9 ± 0.84 | 0.001 |
| 60 d | 3.94 ± 0.53 | 5.22 ± 0.46 | 0.001 | |
| 90 d | 2.99 ± 0.3 | 3.99 ± 0.37 | 0.000 | |
| 45 d | Cecum | 10.02 ± 0.63 | 13.87 ± 1.1 | 0.001 |
| 60 d | 5.08 ± 0.36 | 7.93 ± 0.35 | 0.000 | |
| 90 d | 3.94 ± 0.17 | 6.19 ± 0.3 | 0.000 | |
| 45 d | Ileum | 39.9 ± 4.16 | 47.78 ± 4.24 | 0.004 |
| 60 d | 23.12 ± 1.43 | 29.57 ± 0.56 | 0.000 | |
| 90 d | 16.42 ± 1 | 24.31 ± 1.75 | 0.000 | |
| 45 d | Jejunum | 42.13 ± 3.18 | 49.34 ± 8.1 | 0.030 |
| 60 d | 23.2 ± 0.73 | 29.49 ± 1.7 | 0.000 | |
| 90 d | 17.23 ± 1.41 | 22.95 ± 1.13 | 0.000 | |
| 45 d | Duodenum | 20.04 ± 1.1 | 27.26 ± 2.11 | 0.000 |
| 60 d | 13.98 ± 2.42 | 16.29 ± 1.21 | 0.030 | |
| 90 d | 9.36 ± 1.71 | 12.18 ± 1.77 | 0.010 | |
| 45 d | Large intestine | 17.06 ± 0.9 | 22.77 ± 1.69 | 0.000 |
| 60 d | 9.02 ± 0.67 | 13.15 ± 0.68 | 0.000 | |
| 90 d | 6.94 ± 0.44 | 10.18 ± 0.58 | 0.000 | |
| 45 d | Small intestine | 102.07 ± 5.98 | 124.38 ± 12.34 | 0.001 |
| 60 d | 60.31 ± 3.55 | 75.35 ± 1.99 | 0.000 | |
| 90 d | 43.01 ± 1.44 | 59.43 ± 3.06 | 0.000 | |
| 45 d | Total intestine | 119.13 ± 6.26 | 147.15 ± 13.93 | 0.001 |
| 60 d | 69.32 ± 4.12 | 88.5 ± 2.41 | 0.000 | |
| 90 d | 49.94 ± 1.74 | 69.62 ± 3.51 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, Q.; Ma, S.; Liu, B.; Mustafa, A.; Wang, Z.; Sun, H.; Cui, Y.; Li, D.; Shi, Y. Artificial Pasture Grazing System Attenuates Lipopolysaccharide-Induced Gut Barrier Dysfunction, Liver Inflammation, and Metabolic Syndrome by Activating ALP-Dependent Keap1-Nrf2 Pathway. Animals 2023, 13, 3574. https://doi.org/10.3390/ani13223574
Ali Q, Ma S, Liu B, Mustafa A, Wang Z, Sun H, Cui Y, Li D, Shi Y. Artificial Pasture Grazing System Attenuates Lipopolysaccharide-Induced Gut Barrier Dysfunction, Liver Inflammation, and Metabolic Syndrome by Activating ALP-Dependent Keap1-Nrf2 Pathway. Animals. 2023; 13(22):3574. https://doi.org/10.3390/ani13223574
Chicago/Turabian StyleAli, Qasim, Sen Ma, Boshuai Liu, Ahsan Mustafa, Zhichang Wang, Hao Sun, Yalei Cui, Defeng Li, and Yinghua Shi. 2023. "Artificial Pasture Grazing System Attenuates Lipopolysaccharide-Induced Gut Barrier Dysfunction, Liver Inflammation, and Metabolic Syndrome by Activating ALP-Dependent Keap1-Nrf2 Pathway" Animals 13, no. 22: 3574. https://doi.org/10.3390/ani13223574








