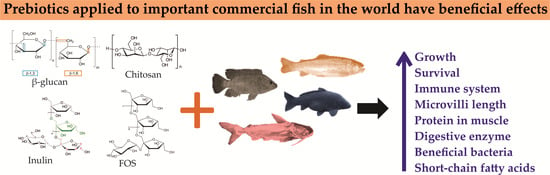
Prebiotics in Global and Mexican Fish Aquaculture: A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Importance and Types of Prebiotics
3. Feasibility and Future Demand for Prebiotics in the Global Industry
4. Characteristics of Prebiotics
4.1. Obligatory Characteristics of Prebiotics in Aquaculture
4.2. Mechanisms of Prebiotics
5. Use of Prebiotics in Global Fish Aquaculture
- I.
- In studies where β-glucan is added to different fish species, the results are mainly in improving growth and immune-related mechanisms such as phagocytosis, lysozyme, haemolytic complement, and bactericidal activity (Table 1).
- II.
- Regarding the application of inulin, in different studies, the effects are presented in an increase in the production of digestive enzymes, and short-chain fatty acids, as well as improvement of the immune system and growth. In contrast, in other studies, there are negative and null results in growth, survival, and the intestinal cells (Table 1).
- III.
- MOS has been found to have multiple beneficial effects such as improved growth, survival, immune system, length and density of microvilli of intestinal cells, as well as a reduction in potential intestinal pathogenic bacteria such as Aeromonas spp. and Vibrio spp. Similar results are found with the cell wall application of Saccharomyces cerevisiae, which is composed of β-glucan and MOS (Table 1).
- IV.
- Works where AXOS, GOS, oligofructose, and XOS are applied are less abundant compared to works studying β-glucan, inulin, and MOS. Despite this, the results have been beneficial for growth, survival, and the immune system, as well as an increased activity of the digestive enzymes (lipase and amylase) and microvilli length of the intestinal cells (Table 1).
6. Limitations of Use of Prebiotics in Fish Aquaculture
7. Potential New Prebiotics in the Fish Aquaculture Industry
8. Current Status and Potential Use of Prebiotics in Mexican Fish Aquaculture
8.1. Fish Production in Mexico
8.2. Use of Prebiotics in Mexican Fish Aquaculture Experiments
| Prebiotics | Fish Species | Dosage/ Application Time | Prebiotics Sources | Effects | References |
|---|---|---|---|---|---|
| Agavin | Totoaba macdonaldi | 2%/44 days | Agave tequilana | → Growth | [101] |
| Agavin | O. niloticus | 2, 4%/80 and 110 days | A. tequilana | 80 days → growth 110 days ↑ growth, ↓ cortisol and triglycerides level | [91] |
| Agavin | T. macdonaldi | 1%/56 days | A. tequilana | → Growth, trypsin and protease activity | [102] |
| β-glucan | Lutjanus peru | 0.1, 0.2%/42 days | S. cerevisiae | ↑ Growth, trypsin, and chymotrypsin activity | [93] |
| β-glucan | I. punctatus | 0.05, 0.1, 0.5%/28 days | S. cerevisiae | 0.05% ↑ immune system | [92] |
| β-glucan | Atractosteus tropicus | 0.5, 1.0, 1.5, 2.0%/62 days | S. cerevisiae | → Growthand protease, trypsin, and amylase activity | [94] |
| β-glucan | A. tropicus | 0.2, 0.4, 0.6, 0.8%%/21 days | S. cerevisiae | → Growth 0.6 and 0.8% ↑ trypsin and lipase activity | [95] |
| β-glucan, chitosan and inulin | T. macdonaldi | 0.1, 0.5, 1.0%/60 days | β-glucan from S. cerevisiae Chitosan from shrimp shells Inulin from Agave sp. | β-glucan ↑ gene immune system Chitosan ↑ respiratory burst ↓ Lipase activity Inulin ↑ trypsin and lipase activity | [103] |
| FOS | O. mykiss | 0.5%/70 days | Saccharum officinarum | → Growth ↑ Immune system and lipid and protein in muscle | [89] |
| FOS | A. tropicus | 0.5, 1.0, 1.5, 2.0%/45 days | A. tequilana | ↑ Growth ↓ Protease, trypsin, and lipase activity | [96] |
| FOS | A. tropicus | 0.5, 0.75%/15 days | A. tequilana | 0.5% ↑ protease and amylase activity 0.75% ↑ growth, survival | [97] |
| FOS y MOS | O. mykiss | 3.0%/60 days | FOS from S. officinarum MOS from S. cerevisiae | → Growth, protein in muscle MOS ↑ lipid in muscle | [90] |
| Inulin | Mycteroperca rosacea | 1%/56 days | A. tequilana | → Growth and lisozyme activity | [100] |
| Inulin | A. tropicus | 0.5, 1.0, 1.5, 2.0, 2.5%/45 days | A. tequilana | 1.0 and 1.5% ↓ growth 2.5% ↑ survival | [98] |
| MOS | A. tropicus | 0.2, 0.4 and 0.6%/20 days | S. cerevisiae | ↑ Growth and trypsin, lipase, and amylase activity | [99] |
9. Challenges and Opportunities
- I.
- The basis of known and applied prebiotics with beneficial effects in fish aquaculture with global commercial importance results in an opportunity to advise small, medium, and large producers in developing countries on their application and improvement of their activity.
- II.
- To make known the alternatives of new potential prebiotics to experiment and test the effects on commercially known and endemic fish that can be used for production.
- III.
- To consider the opportunity of taking advantage of undervalued natural sources of prebiotics, such as the large amount of Sargassum reaching the coasts of Mexico and the waste of organisms, such as crustaceans, potential sources of chitosan, to isolate these supplements and design experiments to determine their effects on the global and endemic commercial fish used in aquaculture from developing countries such as Mexico.
- IV.
- To experiment with the addition of prebiotics and consortia of prebiotics by varying their concentrations, time, and dose of application to determine whether there is synergy in the benefits.
- V.
- Make more timely legislation in developing countries on the use of prebiotics in the aquaculture sector, considering the laws applied in developed countries in this productive activity.
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation. 2022. Available online: https://www.fao.org/3/cc0461en/cc0461en.pdf (accessed on 30 March 2023).
- Mohammadian, T.; Alishahi, M.; Tabandeh, M.R.; Ghorbanpoor, M.; Gharibi, D. Effect of Lactobacillus plantarum and Lactobacillus delbrueckii subsp. bulgaricus on growth performance, gut microbial flora and digestive enzymes activities in Tor grypus (Karaman, 1971). Iran. J. Fish. Sci. 2017, 16, 296–317. [Google Scholar]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Ashley, P.J. Fish welfare: Current issues in aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Mo, W.Y.; Chen, Z.; Leung, H.M.; Leung, A.O.W. Application of veterinary antibiotics in China’s aquaculture industry and their potential human health risks. Environ. Sci. Pollut. Res. 2015, 24, 8978–8989. [Google Scholar] [CrossRef] [PubMed]
- FDA (Food and Drug Administration). Approved Aquaculture Drugs. 2023. Available online: https://www.fda.gov/animal-veterinary/aquaculture/approved-aquaculture-drugs (accessed on 12 April 2023).
- Pepi, M.; Focardi, S. Antibiotic-resistant bacteria in aquaculture and climate change: A challenge for health in the Mediterranean area. Int. J. Environ. Res. Public Health 2021, 18, 5723. [Google Scholar] [CrossRef]
- Amenyogbe, E.; Chen, G.; Wang, Z.; Huang, J.S.; Huang, B.; Li, H. The exploitation of probiotics, prebiotics and synbiotics in aquaculture: Present study, limitations and future directions: A review. Aquac. Int. 2020, 28, 1017–1041. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Food and Agriculture Organization/World Health Organization. Probiotics in Food. Health and Nutritional Properties and Guidelines for Evaluation. FAO Food and Nutrition Paper, Rome. 2006. Available online: https://www.fao.org/3/a0512e/a0512e.pdf (accessed on 10 April 2023).
- Wong, J.M.W.; Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Yousefian, M.; Amiri, M.S. A review of the use of prebiotic in aquaculture for fish and shrimp. Afr. J. Biotechnol. 2009, 8, 7313–7318. [Google Scholar]
- Lauzon, H.L.; Dimitroglou, A.; Merrifield, D.L.; Ringø, E.; Davies, S.J. Probiotics and prebiotics: Concepts, definitions and history. In Aquaculture Nutrition: Gut Health, Probiotics and Prebiotics; Merrifield, D.L., Hossein, S., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 169–184. [Google Scholar]
- Ötles, S. Probiotics and Prebiotics in Food, Nutrition and Health; CRC Press: Bornova, Turkey, 2014. [Google Scholar]
- McLoughlin, R.F.; Berthon, B.S.; Jensen, M.E.; Baines, K.J.; Wood, L.G. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 930–945. [Google Scholar] [CrossRef]
- Kim, K.H.; Chun, B.H.; Baek, J.H.; Roh, S.W.; Lee, S.H. Genomic and metabolic features of Lactobacillus sakei as revealed by its pangenome and the metatranscriptome of kimchi fermentation. Food Microbiol. 2020, 86, 103341. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, I.; Oliva-Teles, A.; Enes, P. Prebiotics as functional ingredients: Focus on Mediterranean fish aquaculture. Rev. Aquac. 2017, 10, 800–832. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.A.; Oliveira, H.M.; Jesus, G.F.A.; Addam, K.G.S.; Silva, B.C.; Yamashita, M.M.; Lehmann, N.B.; Martins, M.L.; Mouriño, J.L.P. Can the minerals calcium and sodium, chelated to propionic acid, influence the health and zootechnical parameters of native silver catfish Rhamdia quelen? Aquaculture 2018, 496, 88–95. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Hendry, G. The ecological significance of fructan in a contemporary flora. New Phytol. 1987, 106, 201–216. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Ringø, E.; Masouleh, A.S.; Esteban, M.A. Probiotic, prebiotic and synbiotic supplements in sturgeon aquaculture: A review. Rev. Aquac. 2016, 8, 89–102. [Google Scholar] [CrossRef]
- Das, S.; Mondal, K.; Haque, S. A review on application of probiotic, prebiotic and synbiotic for sustainable development of aquaculture. J. Entomol. Zool. Stud. 2017, 5, 422–429. [Google Scholar]
- Galgano, F.; Condelli, N.; Caruso, M.C.; Colangelo, M.A.; Favati, F. Probiotics and Prebiotics in Fruits and Vegetables: Technological and Sensory Aspects. In Beneficial Microbes in Fermented and Functional Foods; Rai, V.R., Bai, J.A., Eds.; CRC Press Ltd.: London, UK, 2015; pp. 189–206. [Google Scholar]
- Consumer Goods. GVR Report Cover Prebiotics Market Size, Share & Trends Analysis Report By Ingredients (FOS, Inulin, GOS, MOS), by Application (Food & Beverages, Dietary Supplements, Animal Feed), by Region, and Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/prebiotics-market# (accessed on 3 March 2023).
- Rajan, K.; D’Souza, D.H.; Kim, K.; Moon-Choi, J.; Elder, T.; Carrier, D.J.; Labbé, N. Production and Characterization of High Value Prebiotics From Biorefinery-Relevant Feedstocks. Front. Microbiol. 2021, 12, 675314. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Geraylou, Z.; Souffreau, C.; Rurangwa, E.; D’Hondt, S.; Callewaert, L.; Courtin, C.M.; Delcour, J.A.; Buyse, J.; Ollevier, F. Effects of arabinoxylan-oligosaccharides (AXOS) on juvenile Siberian sturgeon (Acipenser baerii) performance, immune responses and gastrointestinal microbial community. Fish Shellfish Immunol. 2012, 33, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Misra, C.K.; Das, B.K.; Mukherjee, S.B.; Pattnaik, P. Effect of long term administration of dietary β-glucan on immunity, growth and survival of Labeo rohita fingerlings. Aquaculture 2006, 255, 82–94. [Google Scholar] [CrossRef]
- Ai, Q.; Mai, K.; Zhang, L.; Tan, B.; Zhang, W.; Xu, W.; Li, H. Effects of dietary β-1, 3 glucan on innate immune response of large yellow croaker, Pseudosciaena crocea. Fish Shellfish Immunol. 2007, 22, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, G.; Keyvanshokooh, S.; Azarm, H.M.; Akhlaghi, M. Effects of dietary β-glucan on maternal immunity and fry quality of rainbow trout (Oncorhynchus mykiss). Aquaculture 2015, 441, 78–83. [Google Scholar] [CrossRef]
- Amphan, S.; Unajak, S.; Printrakoon, C.; Areechon, N. Feeding-regimen of β-glucan to enhance innate immunity and disease resistance of Nile tilapia, Oreochromis niloticus Linn., against Aeromonas hydrophila and Flavobacterium columnare. Fish Shellfish Immunol. 2019, 87, 120–128. [Google Scholar] [CrossRef]
- Cao, H.; Yu, R.; Zhang, Y.; Hu, B.; Jian, S.; Wen, C.; Kajbaf, K.; Kumar, V.; Yang, G. Effects of dietary supplementation with β-glucan and Bacillus subtilis on growth, fillet quality, immune capacity, and antioxidant status of Pengze crucian carp (Carassius auratus var. Pengze). Aquaculture 2019, 508, 106–112. [Google Scholar] [CrossRef]
- Do-Huu, H. Influence of dietary β-glucan on length-weight relationship, condition factor and relative weight of pompano fish (Trachinotus ovatus, family Carangidae). Int. J. Fish. Aquat. Stud. 2020, 8, 85–91. [Google Scholar]
- Kazuń, B.; Małaczewska, J.; Kazuń, K.; Kamiński, R.; Adamek-Urbańska, D.; Żylińska-Urban, J. Dietary administration of β-1,3/1,6-glucan and Lactobacillus plantarum improves innate immune response and increases the number of intestine immune cells in roach (Rutilus rutilus). BMC Vet. Res. 2020, 16, 216. [Google Scholar] [CrossRef]
- Furlan-Murari, P.J.; Suzuki de Lima, E.C.; Pinheiro de Souza, F.; Urrea-Rojas, A.M.; Eugenio Pupim, A.C.; de Almeida Araújo, E.J.; Meletti, P.C.; Seino Leal, C.N.; Lima Fernandes, L.; Lopera-Barrero, N.M. Inclusion of β-1,3/1,6-glucan in the ornamental fish, Jewel tetra (Hyphessobrycon eques), and its effects on growth, blood glucose, and intestinal histology. Aquacult. Int. 2022, 30, 501–515. [Google Scholar] [CrossRef]
- Ortuño, J.; Cuesta, A.; Rodríguez, A.; Angeles-Esteban, M.; Meseguer, J. Oral administration of yeast, Saccharomyces cerevisiae, enhaces the cellular innate immnune response of gilthead seabream (Sparus aurata L.). Vet. Immunol. Immunopathol. 2002, 85, 41–50. [Google Scholar] [CrossRef]
- Pal, D.; Joardar, S.N.; Roy, B. Immunostimulatory Effects of a Yeast (Saccharomyces cerevisiae) Cell Wall Feed Supplement on Rohu (Labeo rohita), an Indian Major Carp. Isr. J. Aquac. Bamidgeh 2007, 59, 175–181. [Google Scholar] [CrossRef]
- Abu-Elala, N.M.; Younis, N.A.; AbuBakr, H.O.; Ragaa, N.M.; Borges, L.L.; Bonato, M.A. Efficacy of dietary yeast cell wall supplementation on the nutrition and immune response of Nile tilapia. Egypt. J. Aquat. Res. 2018, 44, 333–341. [Google Scholar] [CrossRef]
- Dhanasiri, A.K.S.; Jaramillo-Torres, A.; Chikwati, E.M.; Forberg, T.; Krogdahl, A.; Kortner, T.M. Effects of dietary supplementation with prebiotics and Pediococcus acidilactici on gut health, transcriptome, microbiota, and metabolome in Atlantic salmon (Salmo salar L.) after seawater transfer. Anim. Microbiome 2023, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, S.; Cai, Y.; Shi, H.; Zhou, Y.; Zhang, D.; Guo, W.; Wang, S. Effects of Five Prebiotics on Growth, Antioxidant Capacity, Non-Specific Immunity, Stress Resistance, and Disease Resistance of Juvenile Hybrid Grouper (Epinephelus fuscoguttatus × Epinephelus lanceolatus). Animals 2023, 13, 754. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, E.; Mazurkiewicz, J.; Slawinska, A. Innate Immune Responses of Skin Mucosa in Common Carp (Cyprinus Carpio) Fed a Diet Supplemented with Galactooligosaccharides. Animals 2020, 10, 438. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, G.H.; Ouraji, H.; Khalesi, M.K.; Sudagar, M.; Barari, A.; Zarei Dangesaraki, M.; Jani Khalili, K.H. Effects of a prebiotic, Immunogen®, on feed utilization, body composition, immunity and resistance to Aeromonas hydrophila infection in the common carp Cyprinus carpio (Linnaeus) fingerlings. J. Anim. Physiol. Anim. Nutr. 2012, 96, 591–599. [Google Scholar] [CrossRef]
- Romano, N.; Kanmani, N.; Ebrahimi, M.; Chong, C.M.; Teh, J.C.; Hoseinifar, S.H.; Amin, S.M.N.; Kamarudin, M.S.; Kumar, V. Combination of dietary pre-gelatinized starch and isomaltooligosaccharides improved pellet characteristics, subsequent feeding efficiencies and physiological status in African catfish, Clarias gariepinus, juveniles. Aquaculture 2018, 484, 293–302. [Google Scholar] [CrossRef]
- Olsen, R.E.; Myklebust, R.; Kryvi, H.; Mayhew, T.M.; Ringø, E. Damaging effect of dietary inulin on intestinal enterocytes in Arctic charr (Salvelinus alpinus L.). Aquac. Res. 2001, 32, 931–934. [Google Scholar] [CrossRef]
- Mahious, A.S.; Gatesoupe, F.J.; Hervi, M.; Metailler, R.; Ollevier, F. Effect of dietary inulin and oligosaccharides as prebiotics for weaning turbot, Psetta maxima (Linnaeus, C. 1758). Aquac. Int. 2006, 14, 219–229. [Google Scholar] [CrossRef]
- Reza, A.; Abdolmajid, H.; Abbas, M.; Abdolmohammad, K.A. Effect of dietary prebiotic inulin on growth performance, intestinal microflora, body composition and hematological parameters of juvenile beluga, Huso huso (Linnaeus, 1758). J. World. Aquac. Soc. 2009, 40, 771–779. [Google Scholar] [CrossRef]
- Ibrahem, M.D.; Fathi, M.; Mesalhy, S.; Abd El-Aty, A.M. Effect of dietary supplementation of inulin and vitamin C on the growth, hematology, innate immunity, and resistance of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2010, 29, 241–246. [Google Scholar] [CrossRef]
- Ortiz, L.T.; Rebolé, A.; Velasco, S.; Rodríguez, M.L.; Treviño, J.; Tejedor, J.L.; Alzueta, C. Effects of inulin and fructooligosaccharides on growth performance, body chemical composition and intestinal microbiota of farmed rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2012, 19, 475–482. [Google Scholar] [CrossRef]
- Eshaghzadeh, H.; Hoseinifar, S.H.; Vahabzadeh, H.; Ringø, E. The effects of dietary inulin on growth performances, survival and digestive enzyme activities of common carp (Cyprinus carpio) fry. Aquac. Nutr. 2015, 21, 242–247. [Google Scholar] [CrossRef]
- Mo, W.Y.; Cheng, Z.; Choi, W.M.; Lun, C.H.I.; Man, Y.B.; Wong, J.T.F.; Chen, X.W.; Lau, S.C.K.; Wong, M.H. Use of food waste as fish feeds: Effects of prebiotic fibers (inulin and mannanoligosaccharide) on growth and non-specific immunity of grass carp (Ctenopharyngodon idella). Environ. Sci. Pollut. Res. 2015, 22, 17663–17671. [Google Scholar] [CrossRef] [PubMed]
- Tiengtam, N.; Khempaka, S.; Paengkoum, P.; Boonanuntanasarn, S. Effects of inulin and Jerusalem artichoke (Helianthus tuberosus) as prebiotic ingredients in the diet of juvenile Nile tilapia (Oreochromis niloticus). Anim. Feed Sci. Technol. 2015, 207, 120–129. [Google Scholar] [CrossRef]
- Özlüer-Hunt, A.; Çetinkaya, M.; Özkan-Yılmaz, F.; Yıldırım, M.; Berköz, M.; Yalın, S. Effect of Dietary Supplementation of Inulin on Growth Performance, Digestion Enzyme Activities and Antioxidant Status of Rainbow Trout (Oncorhynchus mykiss). Turk. JAF Sci. Technol. 2019, 7, 1344–1353. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.S.M.Y.; Abdel-Monam, I.M.E.; Mahi-Abd, E.F.A.G.; Marzok, S.S. Effects of Dietary Inulin as Prebiotic on Growth Performance, Immuno-haematological Indices and Ectoparasitic Infection of Fingerlings Nile Tilapia, Oreochromis niloticus. Egypt. J. Histol. 2020, 43, 88–103. [Google Scholar]
- Oliveira, F.; Dichoff-Kasaia, R.Y.; Fernandes, C.E.; Souza, W.; Meldau de Campos, C. Probiotic, prebiotic and synbiotics supplementation on growth performance and intestinal histomorphometry Pseudoplatystoma reticulatum larvae. J. Appl. Aquac. 2020, 1, 1–15. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Rashidian, G.; Bagheri, T.; Hoseinifar, S.H.; Van Doan, H. Study on growth enhancement and the protective effects of dietary prebiotic inulin on immunity responses of rainbow trout (Oncorhynchus mykiss) fry infected with Aeromonas hydrophila. Ann. Anim. Sci. 2021, 21, 543–559. [Google Scholar] [CrossRef]
- Li, M.; Zhang, M.; Jiang, H.; Qin, C. Comparison of dietary arginine or/and inulin supplementation on growth, digestive ability and ammonia tolerance of juvenile yellow catfish Pelteobagrus fulvidraco. Aquac. Rep. 2023, 30, 101543. [Google Scholar] [CrossRef]
- Torrecillas, S.; Makol, A.; Caballero, M.J.; Montero, D.; Robaina, L.; Real, F.; Sweetman, J.; Tort, L.; Izquierdo, M.S. Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunol. 2007, 23, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Genc, M.A.; Genc, E. Effects of Dietary Mannan Oligosaccaharides (MOS) on growth, body composition, intestine and liver histology of rainbow trout, Onchoryncus mykiss (Walbaum). Isr. J. Aquac. Bamidgeh 2007, 59, 183–189. [Google Scholar]
- Grisdale-Helland, B.; Helland, S.J.; Gatlin, D.M., III. The effects of dietary supplementation with mannanoligosaccharide, fructooligosaccharide or galactooligosaccharide on the growth and feed utilization of Atlantic salmon (Salmo salar). Aquaculture 2008, 283, 163–167. [Google Scholar] [CrossRef]
- Dimitroglou, A.; Merrifield, D.L.; Moate, R.; Davies, S.J.; Spring, P.; Sweetman, J.; Bradley, G. Dietary mannan oligosaccharide supplementation modulates intestinal microbial ecology and improves gut morphology of rainbow trout, Oncorhynchus mykiss (Walbaum). J. Anim. Sci. 2009, 87, 3226–3234. [Google Scholar] [CrossRef]
- Salem, M.; Gaber, M.M.; Zaki, M.; Nour, A.A. Effects of dietary mannan oligosaccharides on growth, body composition and intestine of the sea bass (Dicentrarchus labrax L.). Aquac. Res. 2016, 47, 3516–3525. [Google Scholar] [CrossRef]
- Akter, M.N.; Hashim, R.; Sutriana, A.; Azizah, M.N.S.; Asaduzzaman, M. 2019. Effect of Lactobacillus acidophilus supplementation on growth performances, digestive enzyme activities and gut histomorphology of striped catfish (Pangasianodon hypophthalmus Sauvage, 1878) juveniles. Aquac. Res. 2019, 50, 786–797. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, S.; Cai, Y.; Wu, Y.; Tian, L.; Wang, S.; Jiang, L.; Guo, W.; Sun, Y.; Zhou, Y. Effects of dietary mannan oligosaccharide supplementation on growth performance, antioxidant capacity, nonspecific immunity and immune-related gene expression of juvenile hybrid grouper (Epinephelus lanceolatus♂ × Epinephelus fuscoguttatus♀). Aquaculture 2020, 523, 735195. [Google Scholar] [CrossRef]
- Trullàs, C.; Sewaka, M.; Rodkhum, C.; Chansue, N.; Boonanuntanasarn, S.; Kamble, M.T.; Pirarat, N. Effects of Jerusalem Artichoke (Helianthus tuberosus) as a Prebiotic Supplement in the Diet of Red Tilapia (Oreochromis spp.). Animals 2022, 12, 2882. [Google Scholar] [CrossRef]
- Abdelmalek, B.E.; Driss, D.; Kallel, F.; Guargouri, M.; Missaoui, H.; Chaabouni, S.E.; Ayadi, M.A.; Bougatef, A. Effect of xylan oligosaccharides generated from corncobs on food acceptability, growth performance, haematology and immunological parameters of Dicentrarchus labrax fingerlings. Fish Physiol. Biochem. 2015, 41, 1587–1596. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Z.; Lu, S.; Jiang, H.; Li, J.; Wang, L.; Fan, Z.; Wu, D.; Zhang, Y.; Liu, Y.; et al. Effects of dietary xylooligosaccharide on growth, digestive enzymes activity, intestinal morphology, and the expression of inflammatory cytokines and tight junctions genes in triploid Oncorhynchus mykiss fed a low fishmeal diet. Aquac. Rep. 2022, 22, 100941. [Google Scholar] [CrossRef]
- Burr, G.; Hume, M.; Ricke, S.; Nisbet, D.; Gatlin, D., III. In Vitro and In Vivo Evaluation of the prebiotics GroBiotic®-A, Inulin, Mannanoligosaccharide, and Galactooligosaccharide on the digestive microbiota and performance of hybrid striped bass (Morone chrysops × Morone saxatilis). Microb. Ecol. 2010, 59, 187–198. [Google Scholar] [CrossRef]
- Hahor, W.; Thongprajukaew, K.; Suanyuk, N. Effects of dietary supplementation of oligosaccharides on growth performance, gut health and immune response of hybrid catfish (Pangasianodon gigas × Pangasianodon hypophthalmus). Aquaculture 2019, 507, 97–107. [Google Scholar] [CrossRef]
- Yousefi, S.; Hoseinifar, S.H.; Paknejad, H.; Hajimoradloo, A. The effects of dietary supplement of galactooligosaccharide on innate immunity, immune related genes expression and growth performance in zebrafish (Danio rerio). Fish Shellfish Immunol. 2018, 73, 192–196. [Google Scholar] [CrossRef]
- Cerezuela, R.; Fumanal, M.; Tapia-Paniagua, S.T.; Meseguer, J.; Moriñigo, M.A.; Esteban, M.A. Changes in intestinal morphology and microbiota caused by dietary administration of inulin and Bacillus subtilis in gilthead sea bream (Sparus aurata L.) specimens. Fish Shellfish Immunol. 2013, 34, 1063–1070. [Google Scholar] [CrossRef]
- Ferrara, E.; Gustinelli, A.; Fioravanti, M.L.; Restucci, B.; Quaglio, F.; Marono, S.; Piccolo, G. Histological and micro-/macro-morphological evaluation of intestine in sharpsnout seabream (Diplodus puntazzo) fed soybean meal-based diets added with MOS and inulin as prebiotics. Aquac. Int. 2015, 23, 1525–1537. [Google Scholar] [CrossRef]
- Sanders, M.E.; Tompkins, T.; Heimbach, J.T.; Kolida, S. Weight of evidence needed to substantiate a health effect for probiotics and prebiotics. Eur. J. Nutr. 2005, 44, 303–310. [Google Scholar] [CrossRef]
- Pacurucu-Reyes, A.R.; Chiluiza-Ramos, P.; Argüello-Hernández, P. Probióticos y prebióticos: Una revisión de la normativa internacional. Rev. Perspect. 2017, 18, 158–167. [Google Scholar]
- Hoseinifar, S.H.; Mirvaghefi, A.; Amoozegar, M.A.; Merrifield, D.L.; Ringø, E. In vitro selection of a synbiotic and in vivo evaluation on intestinal microbiota, performance and physiological response of rainbow trout (Oncorhynchus mykiss) fingerlings. Aquac. Nutr. 2017, 23, 111–118. [Google Scholar] [CrossRef]
- Van der Marel, M.; Adamek, M.; Gonzalez, S.F.; Frost, P.; Rombout, J.H.W.M.; Wiegertjes, G.F.; Savelkoul, H.F.J.; Steinhagen, D. Molecular cloning and expression of two β-defensin and two mucin genes in common carp (Cyprinus carpio L.) and their up-regulation after β-glucan feeding. Fish Shellfish Immunol. 2012, 32, 494–501. [Google Scholar] [CrossRef]
- Hernández-Cocoletzi, H.; Águila-Almanza, E.; Flores-Agustin, O.; Viveros-Nava, E.L.; Ramos-Cassellis, E. Obtención y caracterización de quitosano a partir de exoesqueletos de camarón. Superf. Vacio 2009, 22, 57–60. [Google Scholar]
- Cruz-Jiménez, K.I. Obtención de Quitosano Por Medio de Escamas de Pescado Para Tratamiento de Agua Potable. Bachelor’s Thesis, Universidad de la Costa, Barranquilla, Colombia, 2021. [Google Scholar]
- Alishahi, A.; Aïder, M. Applications of Chitosan in the Seafood Industry and Aquaculture: A Review. Food. Bioproc. Technol. 2012, 5, 817–830. [Google Scholar] [CrossRef]
- Ortega-Cardona, C.E.; Aparicio-Fernández, X. Quitosano: Una alternativa sostenible para el empaque de alimentos. Rev. Dig. Univ. 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Corona-Rangel, M.L.; Correa-Pacheco, Z.N. Conservación de productos hortofrutícolas mediante el uso de nanopartículas de quitosano y agentes naturales. CIENCIA Ergo-Sum 2022, 29, 1–7. [Google Scholar] [CrossRef]
- Geng, X.; Dong, X.H.; Tan, B.P.; Yang, Q.H.; Chi, S.Y.; Liu, H.Y.; Liu, X.Q. Effects of dietary chitosan and Bacillus subtilis on the growth performance, non-specific immunity and disease resistance of cobia, Rachycentron canadum. Fish Shellfish Immunol. 2011, 31, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Soliman, F.M.; Adly, M.A.; Soliman, H.A.M.; El-Matbouli, M.; Saleh, M. Recent progress in biomedical applications of chitosan and its nanocomposites in aquaculture: A review. Res. Vet. Sci. 2019, 126, 68–82. [Google Scholar] [CrossRef]
- Yildirim-Aksoy, M.; Beck, B.H. Antimicrobial activity of chitosan and a chitosan oligomer against bacterial pathogens of warmwater fish. J. Appl. Microbiol. 2017, 122, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Usoltseva, R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Surits, V.V.; Silchenko, A.S.; Isakov, V.V.; Zvyagintseva, T.N.; Thinh, P.D.; Ermakova, S.P. Polysaccharides from brown algae Sargassum duplicatum: The structure and anticancer activity in vitro. Carbohydr. Polym. 2017, 175, 547–556. [Google Scholar] [CrossRef]
- El-Boshy, M.E.; El-Ashram, A.M.; Abdel-Hamid, F.M.; Gadalla, H.A. Immunomodulatory effect of dietary Saccharomyces cerevisiae, β-glucan and laminaran in mercuric chloride treated Nile tilapia (Oreochromis niloticus) and experimentally infected with Aeromonas hydrophila. Fish Shellfish Immunol. 2010, 28, 802–808. [Google Scholar] [CrossRef]
- Immanuel, G.; Sivagnanavelmurugan, M.; Marudhupandi, T.; Radhakrishnan, S.; Palavesam, A. The effect of fucoidan from brown seaweed Sargassum wightii on WSSV resistance and immune activity in shrimp Penaeus monodon (Fab). Fish Shellfish Immunol. 2012, 32, 551–564. [Google Scholar] [CrossRef]
- Raposo, M.F.J.; Bernardo de Morais, A.M.M.; Santos Costa de Morais, R.M. Emergent Sources of Prebiotics: Seaweeds and Microalgae. Mar. Drugs 2016, 14, 1–27. [Google Scholar]
- Allsopp, P.; Paul, C.; Strain, C.; Yadav, S.; Smyth, T.; Ross, P.; McSorley, E.; Stanton, C. An in-vitro investigation into the prebiotic potential of xylan derived from the edible red seaweed Palmaria palmata. Proc. Nutr. Soc. 2020, 79, E111. [Google Scholar] [CrossRef]
- Comisión Nacional de Acuacultura y Pesca (CONAPESCA). Edición. 2021. Available online: https://nube.conapesca.gob.mx/sites/cona/dgppe/2021/ANUARIO_ESTADISTICO_DE_ACUACULTURA_Y_PESCA_2021.pdf (accessed on 25 March 2023).
- Cid-García, R.A.; Hernández-Hernández, L.H.; Carrillo-Longoria, J.A.; Fernández-Araiza, M.A. Inclusion of yeast and/or fructooligosaccharides in diets with plant-origin protein concentrates for rainbow trout (Oncorhynchus mykiss) fingerlings. J. World Aquac. Soc. 2019, 51, 970–981. [Google Scholar] [CrossRef]
- Segura-Campos, J.M.; Trujano-Rodríguez, A.A.; Hernández-Hernández, L.H.; Macedo-Garzón, B.; Cardenas-Reygadas, R. Inclusion of fructooligosaccharides and mannanoligosaccharides in plant-protein based diets for rainbow trout (Oncorhynchus mykiss) fingerlings and its effects on the growth and blood serum biochemistry. Hidrobiológica 2021, 31, 163–169. [Google Scholar] [CrossRef]
- Flores-Méndez, L.C.; Lizárraga-Velázquez, C.E.; Sánchez-Gutiérrez, E.Y.; Arrizon, J.; Leyva-López, N.; Hernández, C. Study of the Effect of Dietary Agavin Supplementation in Blood Parameters and Antioxidant Enzymes of Juvenile Nile Tilapia (Oreochromis niloticus) under Stress Conditions. Fishes 2022, 7, 340. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.G.; Rábago-Castro, J.L.; Vázquez-Sauceda, M.L.; Pérez-Castañeda, R.; Blanco-Martínez, Z.; Benavides-González, F. Effect of β-glucan dietary levels on immune response and hematology of cannel catfish Ictalurus punctatus juveniles. Lat. Am. J. Aquat. Res. 2017, 45, 690–698. [Google Scholar] [CrossRef]
- Guzmán-Villanueva, L.T.; Ascencio-Valle, F.; Macías-Rodríguez, M.E.; Tovar-Ramírez, D. Effects of dietary β-1,3/1,6-glucan on the antioxidant and digestive enzyme activities of Pacific red snapper (Lutjanus peru) after exposure to lipopolysaccharides. Fish Physiol. Biochem. 2013, 40, 827–837. [Google Scholar] [CrossRef]
- Nieves-Rodríguez, K.N.; Álvarez-González, C.A.; Peña-Marín, E.S.; Vega-Villasante, F.; Martínez-García, R.; Camarillo-Coop, S.; Tovar-Ramírez, D.; Guzmán-Villanueva, L.T.; Andree, K.B.; Gisbert, E. Effect of β-Glucans in Diets on Growth, Survival, Digestive Enzyme Activity, and Immune System and Intestinal Barrier Gene Expression for Tropical Gar (Atractosteus tropicus) Juveniles. Fishes 2018, 3, 27. [Google Scholar] [CrossRef]
- Cigarroa-Ruiz, L.A.; Toledo-Solís, F.J.; Frías-Gómez, S.A.; Guerrero-Zárate, R.; Camarillo-Coop, S.; Alvarez-Villagómez, C.S.; Peña-Marín, E.S.; Galaviz, M.A.; Martínez-García, R.; Álvarez-González, C.A. Addition of β-glucans in diets for tropical gar (Atractosteus tropicus) larvae: Effects on growth, digestive enzymes and gene expression of intestinal epithelial integrity and immune system. Fish Physiol. Biochem. 2023, 49, 613–626. [Google Scholar] [CrossRef]
- Sepúlveda-Quiroz, C.A.; Peña-Marín, E.S.; Pérez-Morales, A.; Martínez-García, R.; Alvarez-Villagomez, C.S.; Maytorena-Verdugo, C.I.; Camarillo-Coop, S.; Vissio, P.G.; Pérez-Sirkin, D.; Tovar-Ramírez, D.; et al. Fructooligosaccharide supplementation in diets for tropical gar (Atractosteus tropicus) juvenile: Effects on morphophysiology and intestinal barrier function. Aquac. Res. 2020, 52, 37–50. [Google Scholar] [CrossRef]
- Pérez-Jiménez, G.M.; Peña-Marín, E.S.; Maytorena-Verdugo, C.I.; Sepúlveda-Quiroz, C.A.; Jiménez-Martínez, L.D.; De la Rosa-García, S.; Asencio-Alcudia, G.G.; Martínez, R.; Tovar-Ramírez, D.; Galaviz, M.A.; et al. Incorporation of Fructooligosaccharides in Diets Influence Growth Performance, Digestive Enzyme Activity, and Expression of Intestinal Barrier Function Genes in Tropical Gar (Atractosteus tropicus) Larvae. Fishes 2022, 7, 137. [Google Scholar] [CrossRef]
- De La Cruz-Marín, E.; Martínez-García, R.; López-Hernández, J.F.; Méndez-Marín, O.; De la Rosa-García, S.C.; Peña-Marín, E.S.; Tovar-Ramírez, D.; Sepúlveda-Quiroz, C.A.; Pérez-Jiménez, G.M.; Jiménez-Martínez, L.D.; et al. Inulin Supplementation in Diets for Tropical Gar (Atractosteus tropicus) Larvae: Effects on Growth, Survival, and Digestive and Antioxidant Enzyme Activities. Aquac. J. 2023, 3, 43–55. [Google Scholar] [CrossRef]
- Maytorena-Verdugo, C.I.; Peña-Marín, E.S.; Alvarez-Villagómez, C.S.; Pérez-Jiménez, G.M.; Sepúlveda-Quiroz, C.A.; Alvarez-González, C.A. Inclusion ofMannan-Oligosaccharides in Diets for Tropical Gar Atractosteus tropicus Larvae: Effects on Growth, Digestive Enzymes, and Expression of Intestinal Barrier Genes. Fishes 2022, 7, 127. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Ascencio, F.; Gracia-Lopez, V.; Macias, M.E.; Cadena-Roa, M.; Ángeles-Esteban, M. Single or combined effects of Lactobacillus sakei and inulin on growth, non-specific immunity and IgM expression in leopard grouper (Mycteroperca rosacea). Fish Physiol. Biochem. 2014, 40, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Quesada, J.P.; Cornejo-Granados, F.; Mata-Sotres, J.A.; Ochoa-Romo, J.P.; Rombenso, A.N.; Guerrero-Rentería, Y.; Lazo, J.P.; Pohlenz, C.; Ochoa-Leyva, A.; Viana, M.T. Prebiotic agavin in juvenile totoaba, Totoaba macdonaldi diets, to relieve soybean meal-induced enteritis: Growth performance, gut histology and microbiota. Aquac. Nutr. 2020, 26, 2115–2134. [Google Scholar] [CrossRef]
- Fuentes-Quesada, J.P.; Viana, M.T.; Mata-Sotres, J.A.; Campos, A.; Pohlenz, C.; Lazo, J.P. Dietary glutamine enhances growth performance and gut integrity of Totoaba macdonaldi juveniles fed low fishmeal diets but has limited synergetic effects in combination with a prebiotic. Aquaculture 2023, 576, 739834. [Google Scholar] [CrossRef]
- Juárez, O.E.; Galindo-Sánchez, C.E.; Lafarga-De la Cruz, F.; Enciso, S.; López-Landavery, E.A.; Muñoz, C.; Aguilera, F.; Lazo, J.P. Physiological and transcriptomic effects of formulated diets including the prebiotics inulin, β-glucan, and chitosan on juveniles of Totoaba macdonaldi. Aquac. Int. 2023. [Google Scholar] [CrossRef]


| Prebiotics | Fish Species | Country (Experiment Conducted) | Dosage/ Application Time | Prebiotics Sources | Effects | References |
|---|---|---|---|---|---|---|
| Arabinoxylan-oligosaccharides-3-0.25, Arabinoxylan-oligosaccharides-32-0.30 | Acipenser baerii | Belgium | 2%/84 days | Wheat bran via extraction with endoxylanases | ↑ Survival and phagocytic activity → Lysozyme activity ↑ Lactic acid bacteria and Clostridium sp. Arabinoxylan-oligosaccharides-32-0.30 ↑ growth Arabinoxylan-oligosaccharide-32-0.30 ↑ short-chain fatty acids (acetate and butyrate) | [29] |
| β-glucan | Labeo rohita | India | 0.01, 0.025, 0.05%/56 days | S. cerevisiae | ↑ Growth, phagocytosis, lysozyme, haemolytic complement, bactericidal activity | [30] |
| β-glucan | Pseudosciaena crocea | China | 0.5 y 1%/56 days | S. cerevisiae | ↑ Growth, lysozyme, phagocytosis, protection against Vibrio harveyi | [31] |
| β-glucan | Oncorhynchus mykiss | Iran | 0.1 and 0.2%/60 days | S. cerevisiae | ↑ Growth and lysozyme | [32] |
| β-glucan | Oreochromis niloticus | Thailand | 0.1%/56 days | S. cerevisiae | ↓ Bacteria Aeromonas hydrophila and Flavobacterium columnare | [33] |
| β-glucan | Carassius auratus var. Pengze | China | 0.1%/70 days | S. cerevisiae | → Growth, ↑ immune system, and microvilli size | [34] |
| β-glucan | Trachinotus ovatus | Vietnam | 0.1, 0.2% and 0.4%/56 days | S. cerevisiae | ↑ Growth in 0.1% | [35] |
| β-glucan | Rutilus rutilus | Poland | 1%/14 days | S. cerevisiae | → Growth ↑ Immune system | [36] |
| β-glucan | Hyphessobrycon eques | Brazil | 0.05, 0.1 and 0.2%/42 days | S. cerevisiae | ↑ Immune system in 0.2% | [37] |
| Cell wall (β-glucan and MOS) | Sparus aurata | Spain | 0.1, 0.5 and 1%/28 days | S. cerevisiae | 0.5 and 1% ↑ phagocytic activity 0.1% ↑ cytotoxic activity | [38] |
| Cell wall (β-glucan and MOS) | Labeo rohita | India | 0.5%/15 days | S. cerevisiae | ↑Phagocytic activity | [39] |
| Cell wall (β-glucan and MOS) | O. niloticus | Egypt | 0.1 and 0.2%/60 days | S. cerevisiae | ↑ Growth, white blood cell count ↑ Phagocytic activity and gene expression related to immune system in 0.2% | [40] |
| FOS | Salmo salar L. | Norway | 0.1%/70 days | Biomar AS, Brande, Denmark | → Growth | [41] |
| FOS, chitosan, MOS, β-glucan, XOS | Hybrid Epinephelus lanceolatus x Epinephelus fuscoguttatus | China | 0.2, 0.5, 0.2, 0.1, 0.05%/28 days | Shandong Shengyuan Biotechnology Co., Ltd., Qingdao, China | MOS and XOS ↑ growth, survival of XOS ↑ protein in muscle | [42] |
| GOS | Cyprinus carpio | Poland | 2%/50 days | Bi2tos®, Clasado Biosciences Ltd., Jersey, UK | ↑ Immune system | [43] |
| Immunogen® provided by Soroush Radian Co., Ltd., Tehran, Iran | C. carpio | Iran | 0.05, 0.1, 0.15, and 0.25%/56 days | Immunogen® provided by Soroush Radian Co., Ltd., Tehran, Iran | → Growth Immunogen® 0.15 y 0.25%, ↑ leucocytes | [44] |
| IMO | Clarias gariepinus | Malaysia | 0.5%/56 days | Composed by combination of isomaltose, isomaltotriose, maltose, panose, maltotriose, glucose and others (24.5, 12.0, 6.1, 1.6, 1.5, 0.3, and 43.7%) | → Growth | [45] |
| Inulin | Salvelinus alpinus | Norway | 15%/28 days | Not available | ↓ Enterocytes of hindgut | [46] |
| Inulin, Oligofructose (type of FOS), Lactosucrose | Psetta maxima | France | 2%/26 days | Inulin obtained from chicory Cichorium intybus. Oligofructose produced via partial enzymatic hydrolysis of chicory Inulin. Lactosucrose obtained from the Ensuiko Sugar Refining Co. (Yokohama, Japan) | → Survival of oligofructose, ↑ growth of inulin and lactosucrose, ↑ gut microbiota | [47] |
| Inulin | Huso huso | Iran | 1, 2 and 3%/56 days | Chicory Cichorium intybus | ↓ Growth and survival, ↓ Total bacteria, ↑ lactic acid bacteria | [48] |
| Inulin | O. niloticus | Egypt | 0.5%/60 days | Chicory Cichorium intybus | ↑ Growth and survival | [49] |
| Inulin, FOS | O. mykiss | Spain | 0.5 and 1%/49 days | Inulin obtained from chicory Cichorium intybus roots (PREBIOFEED 88; Qualivet, Las Rozas, Spain). Fructooligosaccharides obtained viq partial enzymatic hydrolysis of inulin (Oligofructose from Beneo P95; Beneo-Orafti España SL, Barcelona, Spain) | ↑ Growth, → gut bacteria (Aeromonas spp., Pseudomonas spp. and Gram-positive bacteria) | [50] |
| Inulin | C. carpio | Iran | 0.5 and 1%/49 days | Provided by Orafti (Raffinerie Tirlemontoise, Tienen, Belgium) | → Growth and enzymatic activity (lipase, protease, and amylase) ↑ Survival | [51] |
| Inulin, MOS | Ctenopharyngodon idella | China | 0.2 and 2%/56 days | Inulin from chicory Cichorium intybus (Sigma, Saint Louis, MO, USA). MOS from yeast Saccharomyces cerevisiae (Fubon, Yichang, China) | Inulin and MOS 2% ↑ growth and bactericidal activity | [52] |
| Inulin | O. niloticus | Thailand | 0.25 and 0.5%, 0.5 and 1%/56 days | Inulin from chicory Cichorium intybus (PREBIOFEED 88; Warcoing, Belgium) | ↑ Growth, blood cell number, and lysozyme activity → Survival | [53] |
| Inulin | O. mykiss | Turkey | 1 and 2%/56 days | Chicory roots Cichorium intybus | ↑ Growth and survival in 1%, ↑ Digestive enzyme activities in 1% | [54] |
| Inulin | O. niloticus | Egypt | 0.25, 0.5 and 1%/90 days | Chicory roots Cichorium intybus | ↑ Growth in 0.25% | [55] |
| Inulin | Pseudoplatystoma reticulatum | Brazil | 0.7%/12 days | Chicory roots Cichorium intybus | → Growthand ↓ microvilli size | [56] |
| Inulin | O. mykiss | Iran | 1, 2 and 3%/60 days | Inulin Orafti® GR (Beneo Company, Tienen, Belgium) | ↑ Growth and lysozyme activity | [57] |
| Inulin | Pelteobagrus fulvidraco | China | 0.4%/70 days | Inulin Orafti® GR (Beneo Company, Tienen, Belgium) | ↑ Growth and butyric acid | [58] |
| MOS | Dicentrarchus labrax | Spain | 0.2 and 0.4%/63 days | S. cerevisiae | ↑ Growth | [59] |
| MOS | O. mykiss | Turkey | 0.15, 0.3 and 0.45%/90 days | MOS were derived from the outer cell wall of the yeast S. cerevisiae | ↑ Growth in MOS 0.15% ↑ Microvilli length in MOS 0.15 and 0.3% | [60] |
| MOS, FOS, GOS | S. salar | Norway | 1%/120 days | MOS (Bio-Mos, Alltech Inc., Nicholasville, KY, USA), FOS from inulin (Encore Technologies, Plymouth, MN, USA), GOS (Friesland Foods Domo, Zwolle, The Netherland) | → Growth and survival MOS 1% ↓ Lysozyme activity | [61] |
| MOS | O. mykiss | United Kingdom | 0.2%/58 days | MOS (Bio-Mos, Alltech Inc., Lexington, KY, USA)were derived from the outer cell wall of the yeast S. cerevisiae strain 1026 | ↑ Microvilli length and density ↓ Gut bacteria Aeromonas spp. and Vibrio spp. | [62] |
| MOS | D. labrax | Egypt | 0.1, 0.2, 0.3 and 0.4%/75 days | S. cerevisiae | ↑ Growth y microvilli size ↑ Survival (0.1%) | [63] |
| MOS | Pangasianodon hypophthalmus | Malaysia | 0.2, 0.4, 0.6 and 0.8%/84 days | S. cerevisiae | ↑ Survival and lysozyme activity against pathogen A. hydrophila | [64] |
| MOS | Hybrid E. lanceolatus ♂ and E. fuscoguttatus ♀ | China | 0.3, 0.6, 1.0 and 2.0%/63 days | S. cerevisiae | ↑ Lysozyme activity and microvilli length | [65] |
| Oligofructose | Oreochromis spp. | Thailand | 0.5, 1.0%/28 days | Jerusalem Artichoke Helianthus tuberosus | ↑ Growth, immune system | [66] |
| XOS | D. labrax | Tunisia | 0.5 and 1%/84 days | Corncob Zea mays | ↑ Growth (0.5%) and survival against pathogen A. hydrophila (1%) | [67] |
| XOS | O. mykiss | China | 0.25, 0.5, 0.75 and 1.0%/56 days | Henan Hebi Taixin Technology Co., Ltd., Zibo, China | ↑ Growth (1%) ↑ Microvilli height ↑ Lipase and amylase activity | [68] |
| Family | Common Name, Species | Production (Million Tons) | Percentage |
|---|---|---|---|
| Inland aquaculture | |||
| Centrarchidae | Largemouth black bass, Micropterus salmoides | 0.62 | 1.3 |
| Cichlidae | Nile tilapia, O. niloticus Tilapias nei, Oreochromis spp. | 4.41 1.07 | 9 2.2 |
| Clariidae | Clarias catfishes, Clarias spp. | 1.25 | 2.5 |
| Cyprinidae | Grass carp, Ctenopharyngodon idellus | 5.79 | 11.8 |
| Silver carp, Hypophthalmichthys molitrix | 4.9 | 10 | |
| Common carp, C. carpio | 4.24 | 8.6 | |
| Catla, Catla catla | 3.54 | 7.2 | |
| Bighead carp, Hypophthalmichthys nobilis | 3.19 | 6.5 | |
| Carassius spp. | 2.74 | 5.6 | |
| Roho labeo, L. rohita | 2.48 | 5.1 | |
| Wuchang bream, Megalobrama amblycephala | 0.78 | 1.6 | |
| Black carp, Mylopharyngodon piceus | 0.7 | 1.4 | |
| Pangasiidae | Striped catfish, P. hypophthalmus | 2.52 | 5.1 |
| Salmonidae | Rainbow trout, O. mykiss | 0.74 | 1.5 |
| Subtotal of 15 major species | 38.97 | 79.3 | |
| Subtotal other species | 10.15 | 20.7 | |
| Total | 49.12 | 100 | |
| Coastal and marine aquaculture | |||
| Carangidae | Pompano, T. ovatus | 0.16 | 1.9 |
| Japanese amberjack, Seriola quinqueradiata | 0.14 | 1.6 | |
| Chanidae | Milkfish, Chanos chanos | 1.17 | 14 |
| Cichlidae | Nile tilapia, O. niloticus | 0.11 | 1.3 |
| Lateolabracidae | Japanese seabass, Lateolabrax japonicus | 0.2 | 2.4 |
| Latidae | Barramundi(=Giant seaperch), Lates calcarifer | 0.11 | 1.3 |
| Moronidae | European seabass, D. labrax | 0.24 | 2.9 |
| Mugilidae | Mullets nei, Mugilidae | 0.29 | 3.5 |
| Salmonidae | Atlantic salmon, S. salar | 2.72 | 32.6 |
| Coho(=Silver) salmon, Oncorhynchus kisutch | 0.22 | 2.7 | |
| Rainbow trout, O. mykiss | 0.22 | 2.6 | |
| Sciaenidae | Red drum, Sciaenops ocellatus | 0.08 | 1 |
| Large yellow croaker, Larimichthys croceus | 0.25 | 3 | |
| Serranidae | Groupers nei, Epinephelus spp. | 0.23 | 2.7 |
| Sparidae | Gilthead seabream, S. aurata | 0.28 | 3.4 |
| Subtotal of 15 major species | 6.42 | 77 | |
| Subtotal other species | 1.92 | 23 | |
| Total | 8.34 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amillano-Cisneros, J.M.; Fuentes-Valencia, M.A.; Leyva-Morales, J.B.; Davizón, Y.A.; Marquéz-Pacheco, H.; Valencia-Castañeda, G.; Maldonado-Coyac, J.A.; Ontiveros-García, L.A.; Badilla-Medina, C.N. Prebiotics in Global and Mexican Fish Aquaculture: A Review. Animals 2023, 13, 3607. https://doi.org/10.3390/ani13233607
Amillano-Cisneros JM, Fuentes-Valencia MA, Leyva-Morales JB, Davizón YA, Marquéz-Pacheco H, Valencia-Castañeda G, Maldonado-Coyac JA, Ontiveros-García LA, Badilla-Medina CN. Prebiotics in Global and Mexican Fish Aquaculture: A Review. Animals. 2023; 13(23):3607. https://doi.org/10.3390/ani13233607
Chicago/Turabian StyleAmillano-Cisneros, Jesús Mateo, María Anel Fuentes-Valencia, José Belisario Leyva-Morales, Yasser A. Davizón, Henri Marquéz-Pacheco, Gladys Valencia-Castañeda, Juan Antonio Maldonado-Coyac, Luz Adriana Ontiveros-García, and Cesar Noé Badilla-Medina. 2023. "Prebiotics in Global and Mexican Fish Aquaculture: A Review" Animals 13, no. 23: 3607. https://doi.org/10.3390/ani13233607