Genomic Analysis Reveals Candidate Genes Underlying Sex-Linked Eyelid Coloboma, Feather Color Traits, and Climatic Adaptation in Huoyan Geese
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sequencing and Variant Calling
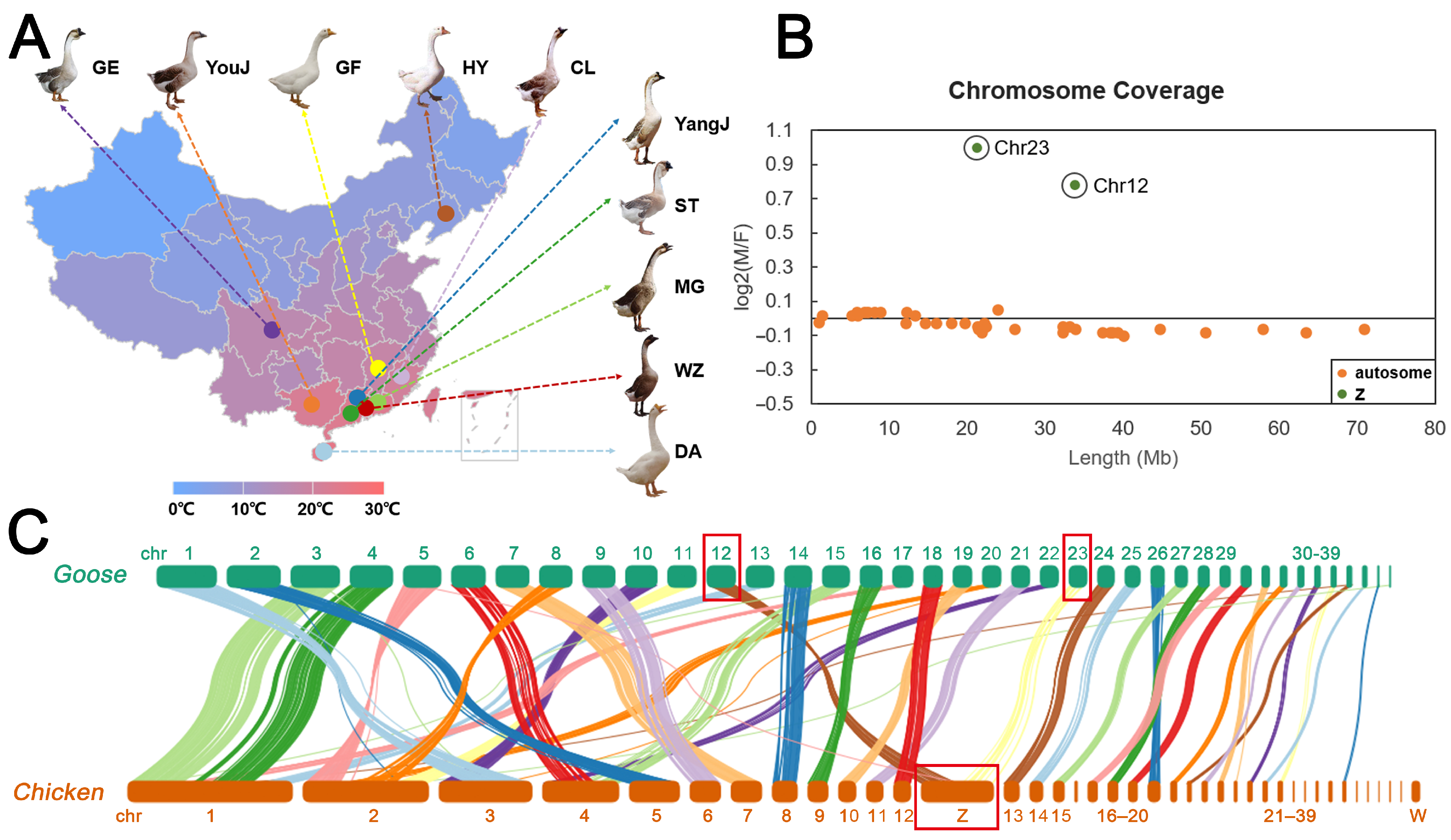
2.3. Identification of Sex Chromosomes in Goose
2.4. Population Structure Analysis
2.5. Selective Sweep Analyses
2.6. Functional Annotation of Candidate Genes
3. Results
3.1. Identification of Sex Chromosomes in Goose
3.2. Population Genetic Structure
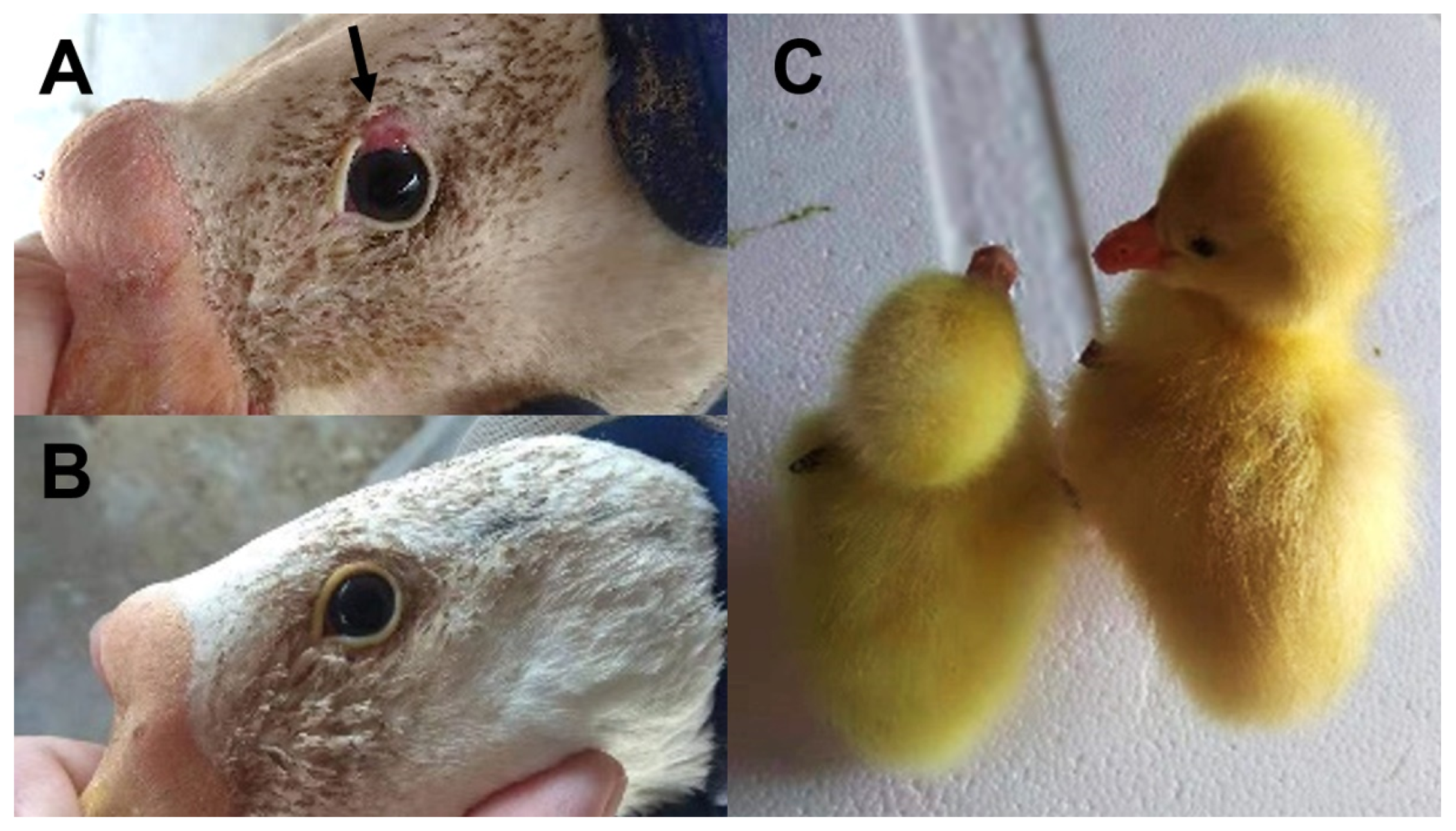
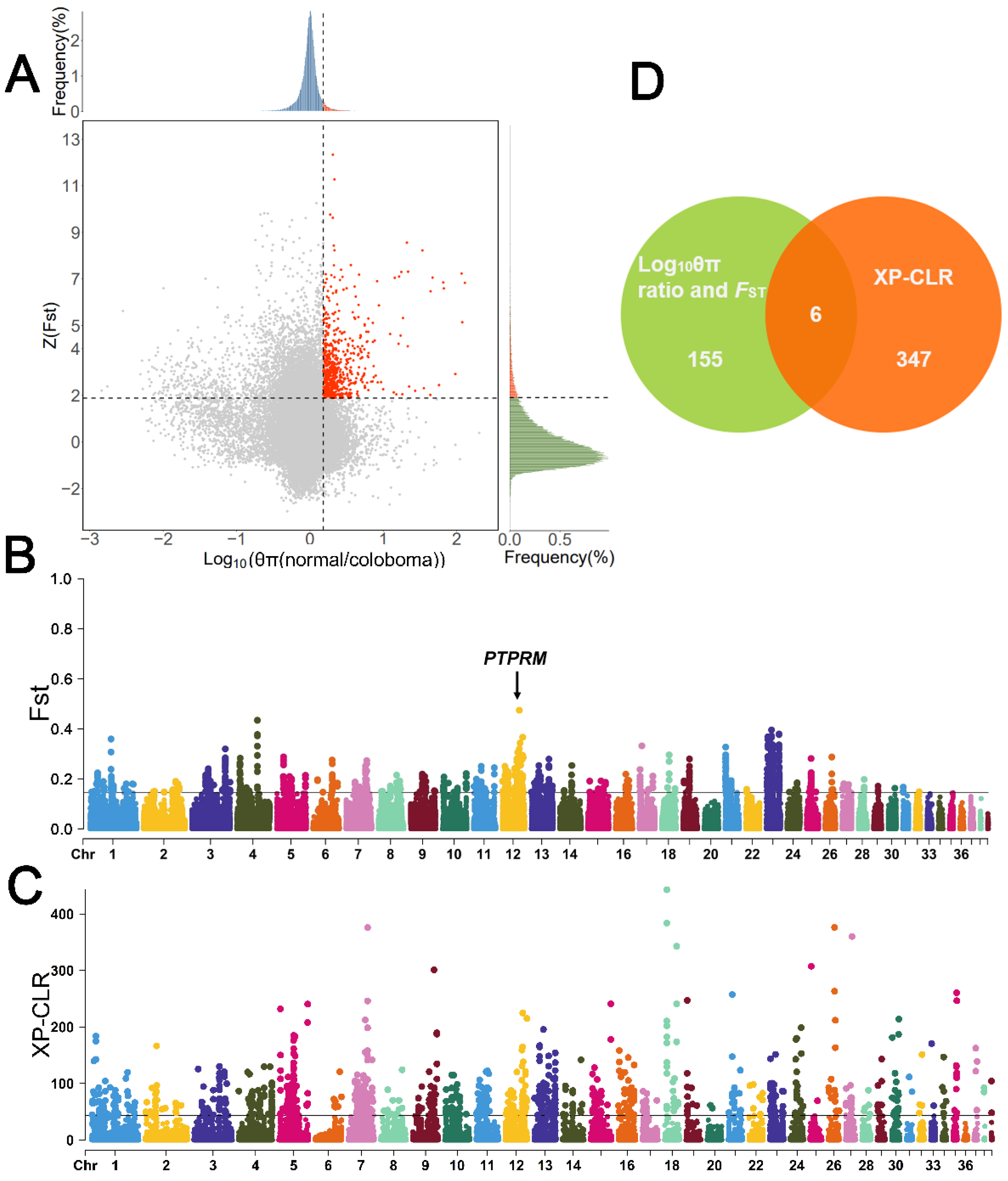
3.3. Selective Signatures of Eyelid Coloboma in Huoyan Geese
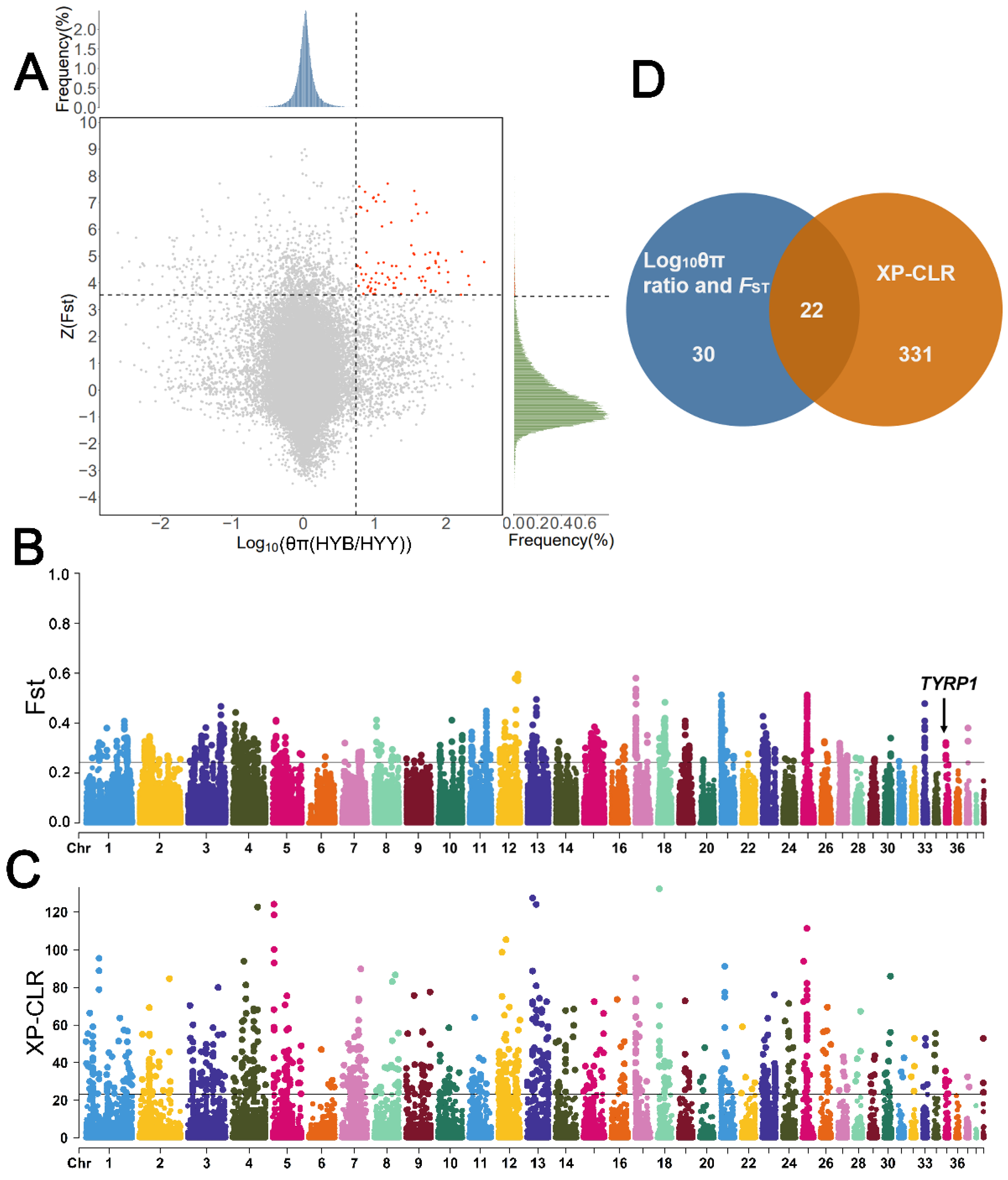
3.4. Selective Signatures of Feather Color in Huoyan Geese
3.5. Climate Adaptation of Chinese Local Geese
4. Discussion
4.1. Upper Eyelid Coloboma Trait
4.2. Feather Color Trait
4.3. Climate Adaptation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- China National Commission of Animal. Animal Genetic Resources of China—Poultry; China Agricultural Press: Beijing, China, 2011. [Google Scholar]
- Yu, J.; Li, Z.; Yu, N.; Zhao, H. Genetic Analysis of Huoyan Trait Based on F2 Resource Population in Huoyan Goose. Sci. Agric. Sin. 2016, 49, 3845–3851. [Google Scholar]
- Yu, J.; Li, Z.; Yu, N.; Liu, K.; Zhao, H. Genetic Analysis of Sex-Linked Plumage Color Traits of Goose. Sci. Agric. Sin. 2019, 52, 949–954. [Google Scholar]
- Wang, M.S.; Li, Y.; Peng, M.S.; Zhong, L.; Wang, Z.J.; Li, Q.Y.; Tu, X.L.; Dong, Y.; Zhu, C.L.; Wang, L.; et al. Genomic Analyses Reveal Potential Independent Adaptation to High Altitude in Tibetan Chickens. Mol. Biol. Evol. 2015, 32, 1880–1889. [Google Scholar] [CrossRef]
- Tian, S.; Zhou, X.; Phuntsok, T.; Zhao, N.; Zhang, D.; Ning, C.; Li, D.; Zhao, H. Genomic Analyses Reveal Genetic Adaptations to Tropical Climates in Chickens. iScience 2020, 23, 101644. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Wang, H.; Li, H.; Qu, C.; Wen, J.; Zhang, X.; Zhu, T.; Nie, C.; Li, X.; et al. Genomic and transcriptomic analyses reveal genetic adaptation to cold conditions in the chickens. Genomics 2022, 114, 110485. [Google Scholar] [CrossRef]
- Patel, R.K.; Jain, M. NGS QC Toolkit: A toolkit for quality control of next generation sequencing data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef]
- Li, Y.; Gao, G.; Lin, Y.; Hu, S.; Luo, Y.; Wang, G.; Jin, L.; Wang, Q.; Wang, J.; Tang, Q.; et al. Pacific Biosciences assembly with Hi-C mapping generates an improved, chromosome-level goose genome. Gigascience 2020, 9, giaa114. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Broad Institute. Picard Toolkit. Available online: https://broadinstitute.github.io/picard/ (accessed on 10 May 2022).
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernandez-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Wan, F.; Yin, C.; Tang, R.; Chen, M.; Wu, Q.; Huang, C.; Qian, W.; Rota-Stabelli, O.; Yang, N.; Wang, S.; et al. A chromosome-level genome assembly of Cydia pomonella provides insights into chemical ecology and insecticide resistance. Nat. Commun. 2019, 10, 4237. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef]
- Mongue, A.J.; Nguyen, P.; Volenikova, A.; Walters, J.R. Neo-sex Chromosomes in the Monarch Butterfly, Danaus plexippus. G3 2017, 7, 3281–3294. [Google Scholar] [CrossRef]
- He, W.; Yang, J.; Jing, Y.; Xu, L.; Yu, K.; Fang, X. NGenomeSyn: An easy-to-use and flexible tool for publication-ready visualization of syntenic relationships across multiple genomes. Bioinformatics 2023, 39, btad121. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef]
- Francis, R.M. Pophelper: An R package and web app to analyse and visualize population structure. Mol. Ecol. Resour. 2017, 17, 27–32. [Google Scholar] [CrossRef]
- Lee, T.H.; Guo, H.; Wang, X.; Kim, C.; Paterson, A.H. SNPhylo: A pipeline to construct a phylogenetic tree from huge SNP data. BMC Genomics 2014, 15, 162. [Google Scholar] [CrossRef]
- Interactive Tree of Life. Available online: https://itol.embl.de/ (accessed on 25 October 2022).
- Weir, B.S.; Cockerham, C.C. Estimating F-Statistics for the Analysis of Population Structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef]
- Chen, H.; Patterson, N.; Reich, D. Population differentiation as a test for selective sweeps. Genome Res. 2010, 20, 393–402. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 10 January 2023).
- da Huang, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Lu, L.; Chen, Y.; Wang, Z.; Li, X.; Chen, W.; Tao, Z.; Shen, J.; Tian, Y.; Wang, D.; Li, G.; et al. The goose genome sequence leads to insights into the evolution of waterfowl and susceptibility to fatty liver. Genome Biol. 2015, 16, 89. [Google Scholar] [CrossRef]
- Li, J.; Bed’Hom, B.; Marthey, S.; Valade, M.; Dureux, A.; Moroldo, M.; Pechoux, C.; Coville, J.L.; Gourichon, D.; Vieaud, A.; et al. A missense mutation in TYRP1 causes the chocolate plumage color in chicken and alters melanosome structure. Pigment. Cell Melanoma Res. 2019, 32, 381–390. [Google Scholar] [CrossRef]
- Nadeau, N.J.; Mundy, N.I.; Gourichon, D.; Minvielle, F. Association of a single-nucleotide substitution in TYRP1 with roux in Japanese quail (Coturnix japonica). Anim. Genet. 2007, 38, 609–613. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Feng, Z.; Song, Y.; Zhou, Y.; Mabrouk, I.; Cao, H.; Hu, X.; Li, H.; Sun, Y. Sex Identification of Feather Color in Geese and the Expression of Melanin in Embryonic Dorsal Skin Feather Follicles. Animals 2022, 12, 1427. [Google Scholar] [CrossRef]
- Friedman, E.A.; Harris, P.A.; Wood, A.J.; Stein, C.M.; Kurnik, D. The alpha2C-adrenoceptor deletion322-325 variant and cold-induced vasoconstriction. Clin. Auton. Res. 2009, 19, 247–254. [Google Scholar] [CrossRef]
- Pauter, A.M.; Olsson, P.; Asadi, A.; Herslof, B.; Csikasz, R.I.; Zadravec, D.; Jacobsson, A. Elovl2 ablation demonstrates that systemic DHA is endogenously produced and is essential for lipid homeostasis in mice. J. Lipid. Res. 2014, 55, 718–728. [Google Scholar] [CrossRef]
- Schroeder, B.; Vander, S.T.; Espinoza, I.; Venkatapoorna, C.M.K.; Hu, Z.; Silva, F.M.; Regan, K.; Cuyas, E.; Meng, X.W.; Verdura, S.; et al. Fatty acid synthase (FASN) regulates the mitochondrial priming of cancer cells. Cell Death Dis. 2021, 12, 977. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Vaz, F.M.; Waterham, H.R.; Ferdinandusse, S. Fatty Acid Oxidation in Peroxisomes: Enzymology, Metabolic Crosstalk with Other Organelles and Peroxisomal Disorders. In Peroxisome Biology: Experimental Models, Peroxisomal Disorders and Neurological Diseases; Lizard, G., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 55–70. [Google Scholar]
- Breuer, M.E.; Willems, P.H.; Smeitink, J.A.; Koopman, W.J.; Nooteboom, M. Cellular and animal models for mitochondrial complex I deficiency: A focus on the NDUFS4 subunit. IUBMB Life 2013, 65, 202–208. [Google Scholar] [CrossRef]
- Lutkewitte, A.J.; Finck, B.N. Regulation of Signaling and Metabolism by Lipin-mediated Phosphatidic Acid Phosphohydrolase Activity. Biomolecules 2020, 10, 1386. [Google Scholar] [CrossRef]
- Christensen, A.H.; Chatelain, F.C.; Huttner, I.G.; Olesen, M.S.; Soka, M.; Feliciangeli, S.; Horvat, C.; Santiago, C.F.; Vandenberg, J.I.; Schmitt, N.; et al. The two-pore domain potassium channel, TWIK-1, has a role in the regulation of heart rate and atrial size. J. Mol. Cell Cardiol. 2016, 97, 24–35. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, J.; Chen, P.; Lu, T.; Jiao, K.; Tester, D.J.; Giudicessi, J.R.; Jiang, K.; Ackerman, M.J.; Li, Y.; et al. Knockout of SORBS2 Protein Disrupts the Structural Integrity of Intercalated Disc and Manifests Features of Arrhythmogenic Cardiomyopathy. J. Am. Heart. Assoc. 2020, 9, e017055. [Google Scholar] [CrossRef]
- Subramanian, H.; Froese, A.; Jonsson, P.; Schmidt, H.; Gorelik, J.; Nikolaev, V.O. Distinct submembrane localisation compartmentalises cardiac NPR1 and NPR2 signalling to cGMP. Nat. Commun. 2018, 9, 2446. [Google Scholar] [CrossRef]
- Wang, D.; Ma, H.; Zhao, Y.; Zhao, J. Ubiquitin-specific protease 14 is a new therapeutic target for the treatment of diseases. J. Cell Physiol. 2021, 236, 3396–3405. [Google Scholar] [CrossRef]
- Sinha, R.K.; Bojjireddy, N.; Kulkarni, D.; Ratheesh, A.; Chiplunkar, S.V.; Gude, R.; Subrahmanyam, G. Type II phosphatidylinositol 4-kinase beta is an integral signaling component of early T cell activation mechanisms. Biochimie 2013, 95, 1560–1566. [Google Scholar] [CrossRef]
- Munoz-Braceras, S.; Tornero-Ecija, A.R.; Vincent, O.; Escalante, R. VPS13A is closely associated with mitochondria and is required for efficient lysosomal degradation. Dis. Model. Mech. 2019, 12, dmm036681. [Google Scholar] [CrossRef]
- Asadollahi, H.; Vaez, T.R.; Ehsani, A.; Masoudi, A.A. An association of CEP78, MEF2C, VPS13A and ARRDC3 genes with survivability to heat stress in an F(2) chicken population. J. Anim. Breed Genet. 2022, 139, 574–582. [Google Scholar] [CrossRef]
- Lu, J.; He, L.; Behrends, C.; Araki, M.; Araki, K.; Jun, W.Q.; Catanzaro, J.M.; Friedman, S.L.; Zong, W.X.; Fiel, M.I.; et al. NRBF2 regulates autophagy and prevents liver injury by modulating Atg14L-linked phosphatidylinositol-3 kinase III activity. Nat. Commun. 2014, 5, 3920. [Google Scholar] [CrossRef]
- Blessing, N.A.; Kasturirangan, S.; Zink, E.M.; Schroyer, A.L.; Chadee, D.N. Osmotic and heat stress-dependent regulation of MLK4beta and MLK3 by the CHIP E3 ligase in ovarian cancer cells. Cell Signal 2017, 39, 66–73. [Google Scholar] [CrossRef]
- Wei, Y.; An, Z.; Zou, Z.; Sumpter, R.; Su, M.; Zang, X.; Sinha, S.; Gaestel, M.; Levine, B. The stress-responsive kinases MAPKAPK2/MAPKAPK3 activate starvation-induced autophagy through Beclin 1 phosphorylation. Elife 2015, 4, e05289. [Google Scholar] [CrossRef]
- Puppala, A.K.; French, R.L.; Matthies, D.; Baxa, U.; Subramaniam, S.; Simonovic, M. Structural basis for early-onset neurological disorders caused by mutations in human selenocysteine synthase. Sci. Rep. 2016, 6, 32563. [Google Scholar] [CrossRef]
- Lalonde, R.; Strazielle, C. The AGTPBP1 gene in neurobiology. Gene 2022, 809, 146001. [Google Scholar] [CrossRef]
- Starheim, K.K.; Gromyko, D.; Evjenth, R.; Ryningen, A.; Varhaug, J.E.; Lillehaug, J.R.; Arnesen, T. Knockdown of human N alpha-terminal acetyltransferase complex C leads to p53-dependent apoptosis and aberrant human Arl8b localization. Mol. Cell Biol. 2009, 29, 3569–3581. [Google Scholar] [CrossRef]
- Guan, K.L.; Dixon, J.E. Evidence for protein-tyrosine-phosphatase catalysis proceeding via a cysteine-phosphate intermediate. J. Biol. Chem. 1991, 266, 17026–17030. [Google Scholar] [CrossRef]
- Macho, A.P.; Lozano-Duran, R.; Zipfel, C. Importance of tyrosine phosphorylation in receptor kinase complexes. Trends Plant Sci. 2015, 20, 269–272. [Google Scholar] [CrossRef]
- Stanoev, A.; Mhamane, A.; Schuermann, K.C.; Grecco, H.E.; Stallaert, W.; Baumdick, M.; Bruggemann, Y.; Joshi, M.S.; Roda-Navarro, P.; Fengler, S.; et al. Interdependence between EGFR and Phosphatases Spatially Established by Vesicular Dynamics Generates a Growth Factor Sensing and Responding Network. Cell Syst. 2018, 7, 295–309.e211. [Google Scholar] [CrossRef]
- Lim, W.A.; Pawson, T. Phosphotyrosine signaling: Evolving a new cellular communication system. Cell 2010, 142, 661–667. [Google Scholar] [CrossRef]
- Sun, P.H.; Ye, L.; Mason, M.D.; Jiang, W.G. Protein tyrosine phosphatase micro (PTP micro or PTPRM), a negative regulator of proliferation and invasion of breast cancer cells, is associated with disease prognosis. PLoS ONE 2012, 7, e50183. [Google Scholar] [CrossRef]
- Ciccolini, F.; Mandl, C.; Holzl-Wenig, G.; Kehlenbach, A.; Hellwig, A. Prospective isolation of late development multipotent precursors whose migration is promoted by EGFR. Dev. Biol. 2005, 284, 112–125. [Google Scholar] [CrossRef]
- Feng, Y. The Effects of Epidermal Growth Factor Receptor and Its Signal Transduction Pathway in Proliferative Vitreoretinopathy (PVR). Master’s Thesis, Fourth Military Medical University, Xi’an, China, 2004. [Google Scholar]
- Bing, L. Associations of Genetic Variants in Endocytic Trafficking of EGFR Super Pathway with Risk of Nonsyndromic Cleft Lip with or without Cleft Palate. Ph.D. Thesis, Nanjing Medical University, Nanjing, China, 2018. [Google Scholar]
- Gu, S.; Khoong, Y.; Huang, X.; Zan, T. Facial cleft? The first case of manitoba-oculo-tricho-anal syndrome with novel mutations in China: A case report. BMC Pediatr. 2021, 21, 46. [Google Scholar] [CrossRef]
- Chacon-Camacho, O.F.; Zenker, M.; Schanze, D.; Ledesma-Gil, J.; Zenteno, J.C. Novel FREM1 mutations in a patient with MOTA syndrome: Clinical findings, mutation update and review of FREM1-related disorders literature. Eur. J. Med. Genet. 2017, 60, 190–194. [Google Scholar] [CrossRef]
- Kobayashi, T.; Imokawa, G.; Bennett, D.C.; Hearing, V.J. Tyrosinase stabilization by Tyrp1 (the brown locus protein). J. Biol. Chem. 1998, 273, 31801–31805. [Google Scholar] [CrossRef]
- Carefoot, W.C. Chocolate: A sex-linked recessive plumage colour mutant of the domestic fowl. Br. Poult. Sci. 1996, 37, 867–868. [Google Scholar] [CrossRef]
- Jiang, B.; Huang, M.; Bai, T.; Zeng, T.; Chen, L.; Shen, J.; Tao, Z.; Zhou, W.; Liu, G.; Lu, L.; et al. Transcriptome Analysis of Differential Gene Expression Associated with White and Black (Grey) Feather Follicles in Ducks (Anas platyrhynchos). J. Agric. Biotechnol. 2020, 28, 1623–1634. [Google Scholar]
- Pritchard, J.K.; Pickrell, J.K.; Coop, G. The genetics of human adaptation: Hard sweeps, soft sweeps, and polygenic adaptation. Curr. Biol. 2010, 20, R208–R215. [Google Scholar] [CrossRef]
- Zhang, Z.; Boelen, A.; Kalsbeek, A.; Fliers, E. TRH Neurons and Thyroid Hormone Coordinate the Hypothalamic Response to Cold. Eur. Thyroid. J. 2018, 7, 279–288. [Google Scholar] [CrossRef]
- Kim, E.S.; Elbeltagy, A.R.; Aboul-Naga, A.M.; Rischkowsky, B.; Sayre, B.; Mwacharo, J.M.; Rothschild, M.F. Multiple genomic signatures of selection in goats and sheep indigenous to a hot arid environment. Heredity 2016, 116, 255–264. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.R.; Lv, F.H.; He, S.G.; Tian, S.L.; Peng, W.F.; Sun, Y.W.; Zhao, Y.X.; Tu, X.L.; Zhang, M.; et al. Whole-Genome Sequencing of Native Sheep Provides Insights into Rapid Adaptations to Extreme Environments. Mol. Biol. Evol. 2016, 33, 2576–2592. [Google Scholar] [CrossRef]
- Wu, H.; Guang, X.; Al-Fageeh, M.B.; Cao, J.; Pan, S.; Zhou, H.; Zhang, L.; Abutarboush, M.H.; Xing, Y.; Xie, Z.; et al. Camelid genomes reveal evolution and adaptation to desert environments. Nat. Commun. 2014, 5, 5188. [Google Scholar] [CrossRef]
- Gu, J.; Liang, Q.; Liu, C.; Li, S. Genomic Analyses Reveal Adaptation to Hot Arid and Harsh Environments in Native Chickens of China. Front. Genet. 2020, 11, 582355. [Google Scholar] [CrossRef]
- van den Bout, I.; Divecha, N. PIP5K-driven PtdIns(4,5)P2 synthesis: Regulation and cellular functions. J. Cell Sci. 2009, 122, 3837–3850. [Google Scholar] [CrossRef]
- Bayot, A.; Reichman, S.; Lebon, S.; Csaba, Z.; Aubry, L.; Sterkers, G.; Husson, I.; Rak, M.; Rustin, P. Cis-silencing of PIP5K1B evidenced in Friedreich’s ataxia patient cells results in cytoskeleton anomalies. Hum. Mol. Genet. 2013, 22, 2894–2904. [Google Scholar] [CrossRef]
- Halstead, J.R.; van Rheenen, J.; Snel, M.H.; Meeuws, S.; Mohammed, S.; D’Santos, C.S.; Heck, A.J.; Jalink, K.; Divecha, N. A role for PtdIns(4,5)P2 and PIP5Kalpha in regulating stress-induced apoptosis. Curr. Biol. 2006, 16, 1850–1856. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Niu, M.; Hu, L.; Chen, L. Cold Acclimation for Enhancing the Cold Tolerance of Zebrafish Cells. Front. Physiol. 2021, 12, 813451. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Xu, P.; Tang, Y.; Su, S.; Liu, G.; Wu, N.; Xue, M.; Yu, F.; Feng, W.; et al. Hypothermia-Mediated Apoptosis and Inflammation Contribute to Antioxidant and Immune Adaption in Freshwater Drum, Aplodinotus grunniens. Antioxidants 2022, 11, 1657. [Google Scholar] [CrossRef]
- Lian, S.; Wang, D.; Xu, B.; Guo, W.; Wang, L.; Li, W.; Ji, H.; Wang, J.; Kong, F.; Zhen, L.; et al. Prenatal cold stress: Effect on maternal hippocampus and offspring behavior in rats. Behav. Brain Res. 2018, 346, 1–10. [Google Scholar] [CrossRef]
- Zonia, L.; Munnik, T. Cracking the green paradigm: Functional coding of phosphoinositide signals in plant stress responses. Subcell. Biochem. 2006, 39, 207–237. [Google Scholar]
- Mishkind, M.; Vermeer, J.E.; Darwish, E.; Munnik, T. Heat stress activates phospholipase D and triggers PIP accumulation at the plasma membrane and nucleus. Plant J. 2009, 60, 10–21. [Google Scholar] [CrossRef]
- Prieto, J.A.; Estruch, F.; Corcoles-Saez, I.; Del, P.M.; Rieger, R.; Stenzel, I.; Randez-Gil, F. Pho85 and PI(4,5)P(2) regulate different lipid metabolic pathways in response to cold. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158557. [Google Scholar] [CrossRef]
- Williams, M.E.; Torabinejad, J.; Cohick, E.; Parker, K.; Drake, E.J.; Thompson, J.E.; Hortter, M.; Dewald, D.B. Mutations in the Arabidopsis phosphoinositide phosphatase gene SAC9 lead to overaccumulation of PtdIns(4,5)P2 and constitutive expression of the stress-response pathway. Plant Physiol. 2005, 138, 686–700. [Google Scholar] [CrossRef]
- Lau, C.; Niere, M.; Ziegler, M. The NMN/NaMN adenylyltransferase (NMNAT) protein family. Front. Biosci. 2009, 14, 410–431. [Google Scholar] [CrossRef]
- Hikosaka, K.; Ikutani, M.; Shito, M.; Kazuma, K.; Gulshan, M.; Nagai, Y.; Takatsu, K.; Konno, K.; Tobe, K.; Kanno, H.; et al. Deficiency of nicotinamide mononucleotide adenylyltransferase 3 (nmnat3) causes hemolytic anemia by altering the glycolytic flow in mature erythrocytes. J. Biol. Chem. 2014, 289, 14796–14811. [Google Scholar] [CrossRef]
- Navas, L.E.; Carnero, A. NAD(+) metabolism, stemness, the immune response, and cancer. Signal Transduct. Target. Ther. 2021, 6, 2. [Google Scholar] [CrossRef]
- Nikiforov, A.; Dolle, C.; Niere, M.; Ziegler, M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: From entry of extracellular precursors to mitochondrial NAD generation. J. Biol. Chem. 2011, 286, 21767–21778. [Google Scholar] [CrossRef]
- Gulshan, M.; Yaku, K.; Okabe, K.; Mahmood, A.; Sasaki, T.; Yamamoto, M.; Hikosaka, K.; Usui, I.; Kitamura, T.; Tobe, K.; et al. Overexpression of Nmnat3 efficiently increases NAD and NGD levels and ameliorates age-associated insulin resistance. Aging Cell 2018, 17, e12798. [Google Scholar] [CrossRef]
- Fernandez, J.R.; Byrne, B.; Firestein, B.L. Phylogenetic analysis and molecular evolution of guanine deaminases: From guanine to dendrites. J. Mol. Evol. 2009, 68, 227–235. [Google Scholar] [CrossRef]
- Saint-Marc, C.; Daignan-Fornier, B. GUD1 (YDL238c) encodes Saccharomyces cerevisiae guanine deaminase, an enzyme expressed during post-diauxic growth. Yeast 2004, 21, 1359–1363. [Google Scholar] [CrossRef]
- Chen, M.; Lucas, K.G.; Akum, B.F.; Balasingam, G.; Stawicki, T.M.; Provost, J.M.; Riefler, G.M.; Jornsten, R.J.; Firestein, B.L. A novel role for snapin in dendrite patterning: Interaction with cypin. Mol. Biol. Cell 2005, 16, 5103–5114. [Google Scholar] [CrossRef]
- Chen, H.; Firestein, B.L. RhoA regulates dendrite branching in hippocampal neurons by decreasing cypin protein levels. J. Neurosci. 2007, 27, 8378–8386. [Google Scholar] [CrossRef]
- Cheong, K.A.; Lee, A.Y. Guanine Deaminase Stimulates Ultraviolet-induced Keratinocyte Senescence in Seborrhoeic Keratosis via Guanine Metabolites. Acta Derm. Venereol. 2020, 100, adv00109. [Google Scholar] [CrossRef]
- Noh, T.K.; Choi, S.J.; Chung, B.Y.; Kang, J.S.; Lee, J.H.; Lee, M.W.; Chang, S.E. Inflammatory features of melasma lesions in Asian skin. J. Dermatol. 2014, 41, 788–794. [Google Scholar] [CrossRef]
- Chung, B.Y.; Noh, T.K.; Yang, S.H.; Kim, I.H.; Lee, M.W.; Yoon, T.J.; Chang, S.E. Gene expression profiling in melasma in Korean women. Dermatology 2014, 229, 333–342. [Google Scholar] [CrossRef]
- Jung, J.M.; Noh, T.K.; Jo, S.Y.; Kim, S.Y.; Song, Y.; Kim, Y.H.; Chang, S.E. Guanine Deaminase in Human Epidermal Keratinocytes Contributes to Skin Pigmentation. Molecules 2020, 25, 2637. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, J.; Yu, J.; Zhang, L.; Li, H.; Wang, H.; Gu, H.; Zhao, X.; Zhang, X.; Ren, X.; Wang, G.; et al. Genomic Analysis Reveals Candidate Genes Underlying Sex-Linked Eyelid Coloboma, Feather Color Traits, and Climatic Adaptation in Huoyan Geese. Animals 2023, 13, 3608. https://doi.org/10.3390/ani13233608
Wen J, Yu J, Zhang L, Li H, Wang H, Gu H, Zhao X, Zhang X, Ren X, Wang G, et al. Genomic Analysis Reveals Candidate Genes Underlying Sex-Linked Eyelid Coloboma, Feather Color Traits, and Climatic Adaptation in Huoyan Geese. Animals. 2023; 13(23):3608. https://doi.org/10.3390/ani13233608
Chicago/Turabian StyleWen, Junhui, Jincheng Yu, Li Zhang, Haiying Li, Huie Wang, Hongchang Gu, Xiurong Zhao, Xinye Zhang, Xufang Ren, Gang Wang, and et al. 2023. "Genomic Analysis Reveals Candidate Genes Underlying Sex-Linked Eyelid Coloboma, Feather Color Traits, and Climatic Adaptation in Huoyan Geese" Animals 13, no. 23: 3608. https://doi.org/10.3390/ani13233608




