Dietary Curcumin Modulating Effect on Performance, Antioxidant Status, and Immune-Related Response of Broiler Chickens Exposed to Imidacloprid Insecticide
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Compounds and Chemical Reagents
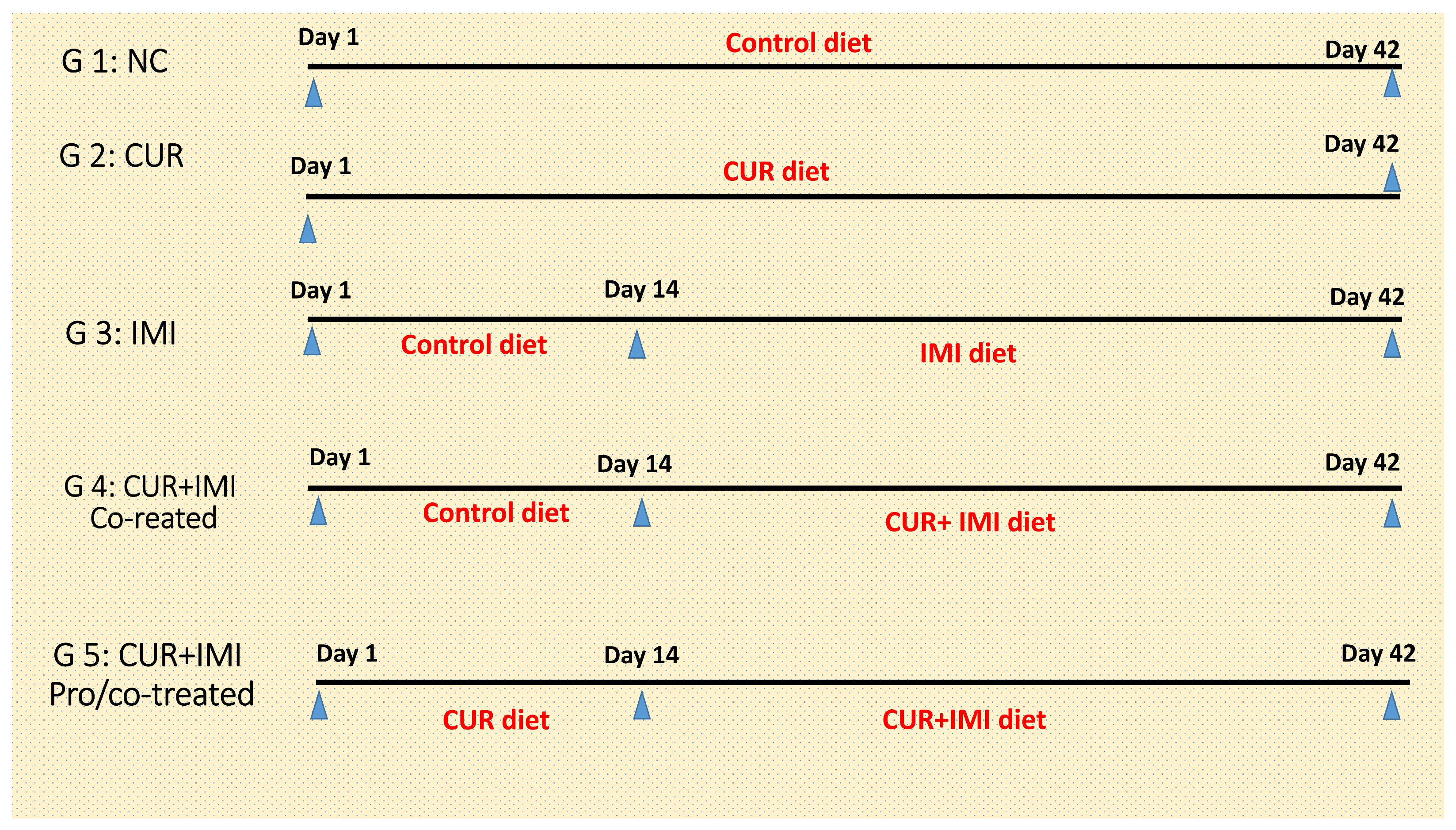
2.2. Experimental Birds, Design, and Diet Preparation
2.3. Total Growth Performance Related Indices
2.4. Collection of Samples
2.5. Hematological Analysis and Phagocytosis Assay
2.6. Biochemical Measurements of the Serum and Splenic Tissue
2.7. Transcriptional Analysis of Immune-Related Genes IL-1β, TLR-4, and IL-10 in Spleen Tissue Using Quantitative Real-Time PCR
2.8. Histopathological and Immunohistochemical Investigations
2.9. Statistical Analysis of Data
3. Results
3.1. Effect of Dietary Supplementation of CUR, IMI, and Their Combinations on Overall Performance-Related Indices of Ross 308 Broiler Chickens
3.2. Effect of Dietary Supplementation of CUR, IMI, and Their Combinations on Blood Hematological-Related Indices of Ross 308 Broiler Chickens
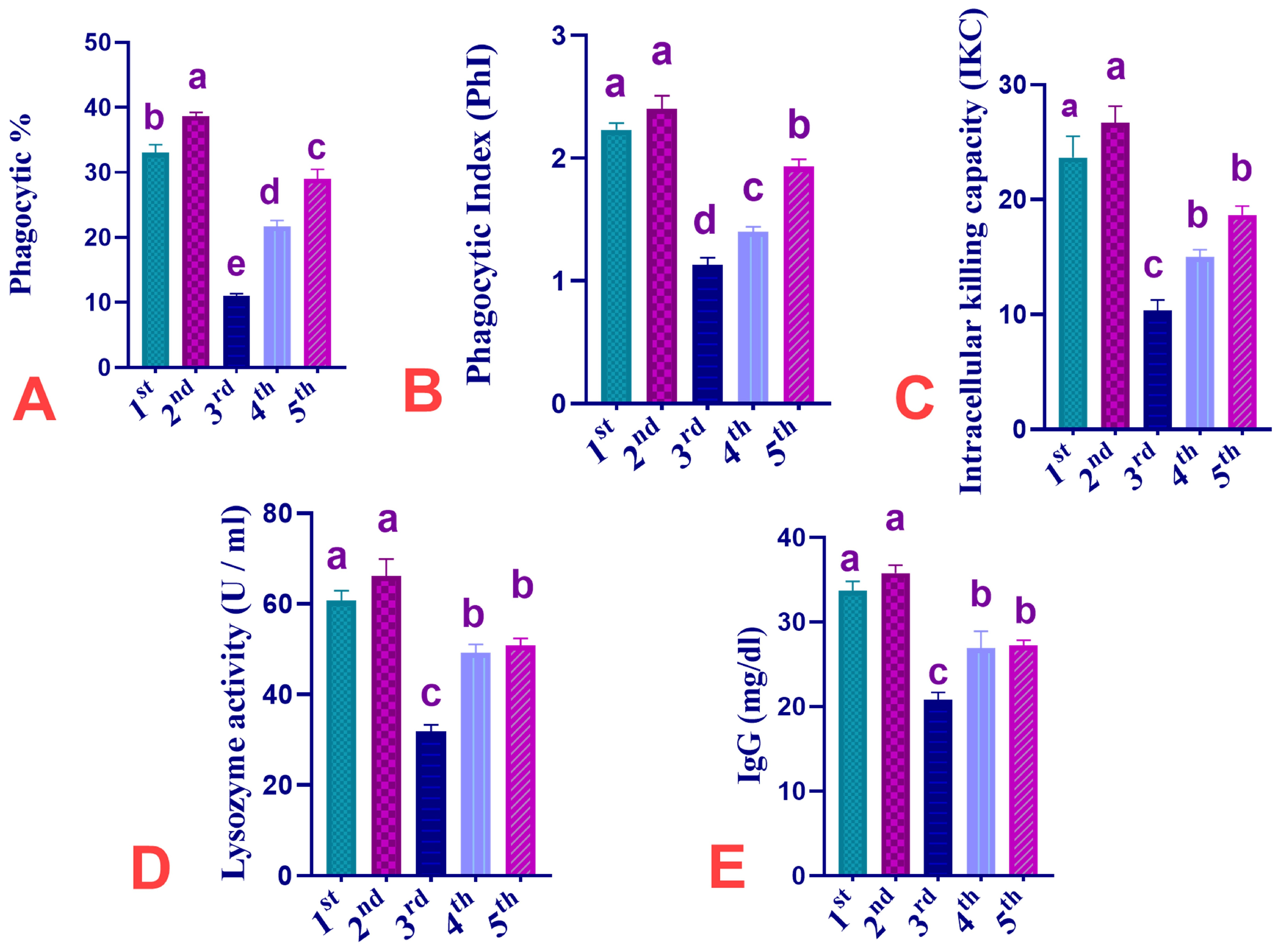
3.3. Effect of Dietary Supplementation of CUR, IMI, and Their Combinations on Phagocytosis %, Phagocytic Index, Intracellular Killing Capacity, Lysozyme Activity, and IgG of Ross 308 Broiler Chickens
3.4. Effect of Dietary Supplementation of CUR, IMI, and Their Combinations on Serum Biochemical Measurements of Ross 308 Broiler Chickens
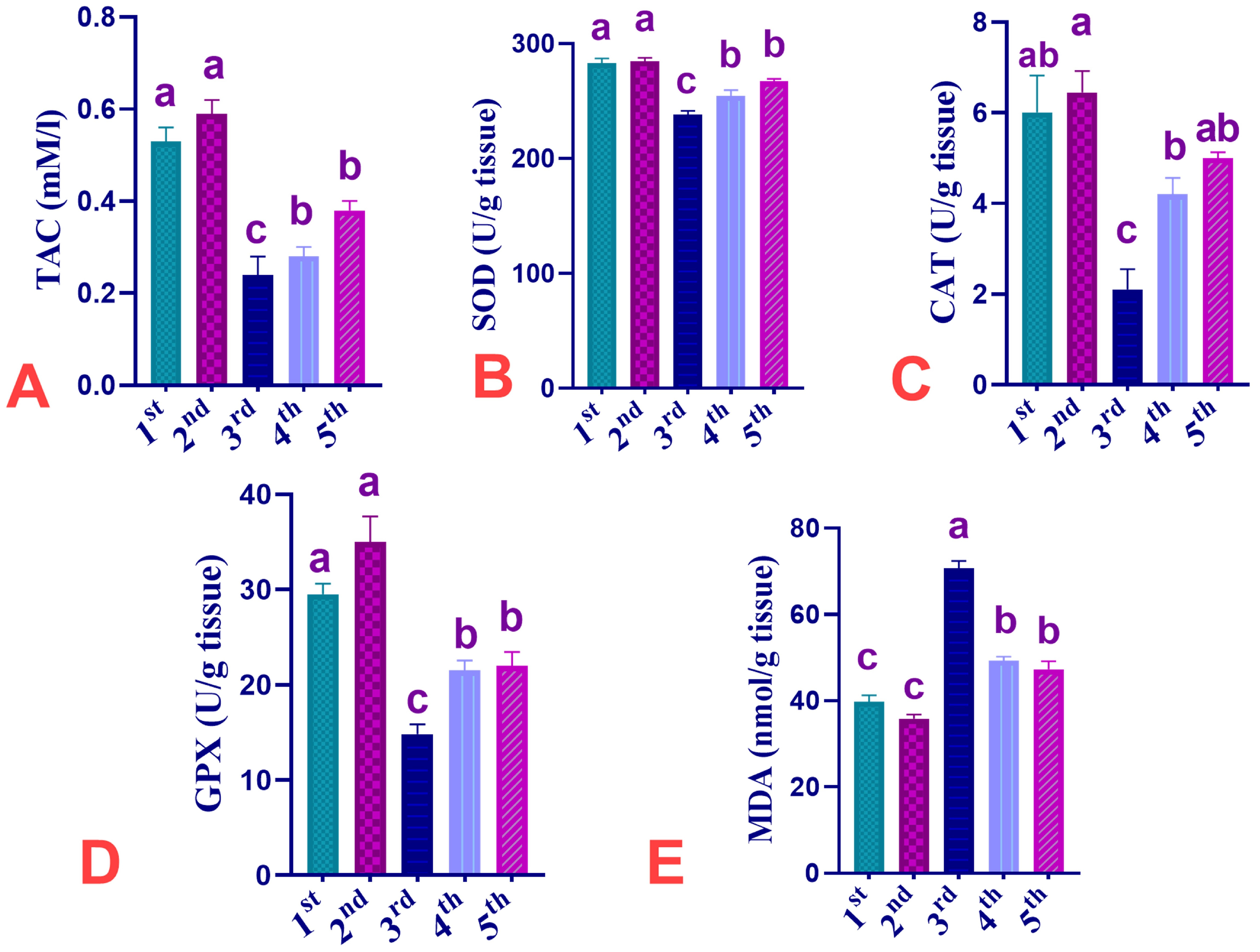
3.5. Effect of Dietary Supplementation of CUR, IMI, and Their Combinations on Oxidative Stress-Related Indices in Spleen of Ross 308 Broiler Chickens
3.6. Effect of Dietary Supplementation of CUR, IMI, and Their Combinations on mRNA Expression of IL-1β, TLR-4, and IL-10 Genes in Spleen of Ross 308 Broiler Chickens
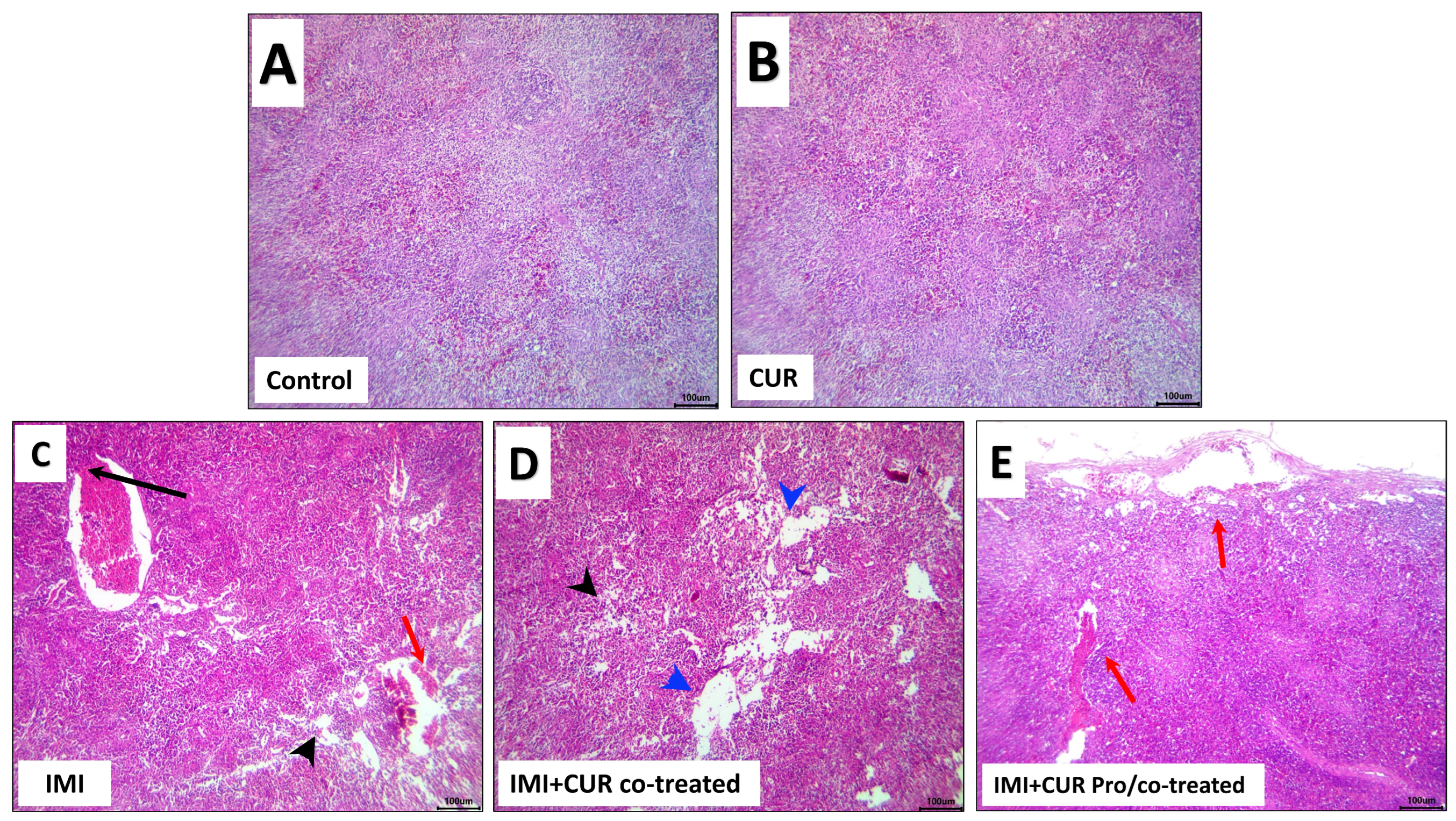
3.7. Histopathological and Immunohistochemical Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nabi, F.; Arain, M.A.; Rajput, N.; Alagawany, M.; Soomro, J.; Umer, M.; Soomro, F.; Wang, Z.; Ye, R.; Liu, J. Health benefits of carotenoids and potential application in poultry industry: A review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.; Taha, A.E.; Soliman, S.M.; Ahmed, A.E.; El-Kott, A.F. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef] [PubMed]
- Abou-Kassem, D.E.; El-Abasy, M.M.; Al-Harbi, M.S.; Abol-Ela, S.; Salem, H.M.; El-Tahan, A.M.; El-Saadony, M.T.; Abd El-Hack, M.E.; Ashour, E.A. Influences of total sulfur amino acids and photoperiod on growth, carcass traits, blood parameters, meat quality and cecal microbial load of broilers. Saudi J. Biol. Sci. 2022, 29, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; El-Naggar, K.; Taha, A.E.; Khafaga, A.F.; Madkour, M.; Salem, H.M.; El-Tahan, A.M.; El-Saadony, M.T. Betaine and related compounds: Chemistry, metabolism and role in mitigating heat stress in poultry. J. Therm. Biol. 2022, 104, 103168. [Google Scholar] [CrossRef] [PubMed]
- Righi, F.; Pitino, R.; Manuelian, C.L.; Simoni, M.; Quarantelli, A.; De Marchi, M.; Tsiplakou, E. Plant feed additives as natural alternatives to the use of synthetic antioxidant vitamins on poultry performances, health, and oxidative status: A review of the literature in the last 20 years. Antioxidants 2021, 10, 659. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Ismail, T.A.; Khalifa, E.; El-Kader, A.; Shaimaa, A.; Mohamed, D.I.; Mohamed, D.T.; Shahin, S.E.; El-Hamid, A.; Marwa, I. Supplementing Garlic Nanohydrogel Optimized Growth, Gastrointestinal Integrity and Economics and Ameliorated Necrotic Enteritis in Broiler Chickens Using a Clostridium perfringens Challenge Model. Animals 2021, 11, 2027. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Eldemery, F.; Metwally, A.S.; Abd-Allah, E.M.; Mohamed, D.T.; Ismail, T.A.; Hamed, T.A.; Al Sadik, G.M.; Neamat-Allah, A.N.; El-Hamid, A. Dietary Eugenol Nanoemulsion Potentiated Performance of Broiler Chickens: Orchestration of Digestive Enzymes, Intestinal Barrier Functions and Cytokines Related Gene Expression with a Consequence of Attenuating the Severity of E. coli O78 Infection. Front. Vet. Sci. 2022, 9, 847580. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Shahin, S.; Alqahtani, L.; Hassan, Z.; Althobaiti, F.; Albogami, S.; Soliman, M.; El-Malt, R.; Al-Harthi, H.; Alqadri, N. Exploring the Interactive Effects of Thymol and Thymoquinone: Moving towards an Enhanced Performance, Gross Margin, Immunity and Aeromonas sobria Resistance of Nile Tilapia (Oreochromis niloticus). Animals 2022, 12, 3034. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; Khater, S.I.; Abdelfattah-Hassan, A.; Alqahtani, L.S.; Metwally, A.S.M.; Bazeed, S.M.; Elgamal, A.; Sheraiba, N.I.; Hussein, E.M.; Alasmary, F.A. Prospects of new targeted nanotherapy combining liponiosomes with berberine to combat colorectal cancer development: An in vivo experimental model. Int. J. Pharm. 2023, 647, 123511. [Google Scholar] [CrossRef] [PubMed]
- El-Ghareeb, W.R.; Kishawy, A.T.; Anter, R.G.; Aboelabbas Gouda, A.; Abdelaziz, W.S.; Alhawas, B.; Meligy, A.M.; Abdel-Raheem, S.M.; Ismail, H.; Ibrahim, D. Novel Antioxidant Insights of Myricetin on the Performance of Broiler Chickens and Alleviating Experimental Infection with Eimeria spp.: Crosstalk between Oxidative Stress and Inflammation. Antioxidants 2023, 12, 1026. [Google Scholar] [CrossRef]
- Ibrahim, D.; Arisha, A.H.; Khater, S.I.; Gad, W.M.; Hassan, Z.; Abou-Khadra, S.H.; Mohamed, D.I.; Ahmed Ismail, T.; Gad, S.A.; Eid, S.A. Impact of Omega-3 Fatty Acids Nano-Formulation on Growth, Antioxidant Potential, Fillet Quality, Immunity, Autophagy-Related Genes and Aeromonas hydrophila Resistance in Nile Tilapia (Oreochromis niloticus). Antioxidants 2022, 11, 1523. [Google Scholar] [CrossRef]
- Ibrahim, D.; Moustafa, A.; Metwally, A.S.; Nassan, M.A.; Abdallah, K.; Eldemery, F.; Tufarelli, V.; Laudadio, V.; Kishawy, A.T. Potential Application of Cornelian Cherry Extract on Broiler Chickens: Growth, Expression of Antioxidant Biomarker and Glucose Transport Genes, and Oxidative Stability of Frozen Meat. Animals 2021, 11, 1038. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Du, M.; Xu, Q.; Chen, Y.; Wen, C.; Zhou, Y. Dietary mannan oligosaccharide improves growth performance, muscle oxidative status, and meat quality in broilers under cyclic heat stress. J. Therm. Biol. 2018, 75, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.; Izzo, A.A. Principles of pharmacological research of nutraceuticals. Br. J. Pharmacol. 2017, 174, 1177. [Google Scholar] [CrossRef] [PubMed]
- Alhawas, B.; Abd El-Hamid, M.I.; Hassan, Z.; Ibrahim, G.A.; Neamat-Allah, A.N.; El-Ghareeb, W.R.; Alahmad, B.A.-H.Y.; Meligy, A.M.; Abdel-Raheem, S.M.; Ismail, H.A.-M.A. Curcumin loaded liposome formulation: Enhanced efficacy on performance, flesh quality, immune response with defense against Streptococcus agalactiae in Nile tilapia (Orechromis niloticus). Fish Shellfish Immunol. 2023, 138, 108776. [Google Scholar] [CrossRef] [PubMed]
- Dono, N.D. Turmeric (Curcuma longa Linn.) supplementation as an alternative to antibiotics in poultry diets. WARTAZOA. Indones. Bull. Anim. Vet. Sci. 2014, 23, 41–49. [Google Scholar] [CrossRef]
- Ashraf, K. A comprehensive review on Curcuma longa Linn.: Phytochemical, pharmacological, and molecular study. Int. J. Green Pharm. 2017, 11, S671–S685. [Google Scholar] [CrossRef]
- Choudhury, D. Study on the nutrient composition of local variety of turmeric (Curcuma longa). J. Pharm. Innov. 2019, 8, 205–207. [Google Scholar]
- Attia, Y.A.; Al-Harthi, M.A.; Hassan, S.S. Turmeric (Curcuma longa Linn.) as a phytogenic growth promoter alternative for antibiotic and comparable to mannan oligosaccharides for broiler chicks. Rev. Mex. Cienc. Pecu. 2017, 8, 11–21. [Google Scholar] [CrossRef]
- Zhong, W.; Qian, K.; Xiong, J.; Ma, K.; Wang, A.; Zou, Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via PI3K/AKT and NF-κB related signaling. Biomed. Pharmacother. 2016, 83, 302–313. [Google Scholar] [CrossRef]
- Abou-Elkhair, R.; Ahmed, H.; Selim, S. Effects of black pepper (Piper nigrum), turmeric powder (Curcuma longa) and coriander seeds (Coriandrum sativum) and their combinations as feed additives on growth performance, carcass traits, some blood parameters and humoral immune response of broiler chickens. Asian-Australas. J. Anim. Sci. 2014, 27, 847. [Google Scholar] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives–A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Nawab, A.; Li, G.; Liu, W.; Lan, R.; Wu, J.; Zhao, Y.; Kang, K.; Kieser, B.; Sun, C.; Tang, S. Effect of dietary curcumin on the antioxidant status of laying hens under high-temperature condition. J. Therm. Biol. 2019, 86, 102449. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yuan, Y.; Sun, C.; Balasubramanian, B.; Zhao, Z.; An, L. Effects of dietary betaine on growth performance, digestive function, carcass traits, and meat quality in indigenous yellow-feathered broilers under long-term heat stress. Animals 2019, 9, 506. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Min, T. Curcumin, curcumin nanoparticles and curcumin nanospheres: A review on their pharmacodynamics based on monogastric farm animal, poultry and fish nutrition. Pharmaceutics 2020, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Paw, M.; Gogoi, R.; Sarma, N.; Pandey, S.K.; Borah, A.; Begum, T.; Lal, M. Study of anti-oxidant, anti-inflammatory, genotoxicity, and antimicrobial activities and analysis of different constituents found in rhizome essential oil of Curcuma caesia Roxb., collected from north east India. Curr. Pharm. Biotechnol. 2020, 21, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.J.; Goulson, D. The environmental risks of neonicotinoid pesticides: A review of the evidence post 2013. Environ. Sci. Pollut. Res. 2017, 24, 17285–17325. [Google Scholar] [CrossRef]
- Lu, C.; Lu, Z.; Lin, S.; Dai, W.; Zhang, Q. Neonicotinoid insecticides in the drinking water system–Fate, transportation, and their contributions to the overall dietary risks. Environ. Pollut. 2020, 258, 113722. [Google Scholar] [CrossRef]
- Anadón, A.; Ares, I.; Martínez, M.; Martínez-Larrañaga, M.-R.; Martínez, M.-A. Neurotoxicity of neonicotinoids. Adv. Neurotoxicology 2020, 4, 167–207. [Google Scholar]
- Gibbons, D.; Morrissey, C.; Mineau, P. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ. Sci. Pollut. Res. 2015, 22, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Mineau, P.; Palmer, C. The Impact of the Nation’s Most Widely Used Insecticides on Birds. Neonicotinoid Insecticides and Birds. Report by American Bird Conservancy. 2013. Available online: www.abcbirds.org/abcprograms/policy/toxins/Neonic_FINAL.pdf (accessed on 23 November 2023).
- Cestonaro, L.V.; Macedo, S.M.D.; Piton, Y.V.; Garcia, S.C.; Arbo, M.D. Toxic effects of pesticides on cellular and humoral immunity: An overview. Immunopharmacol. Immunotoxicol. 2022, 44, 816–831. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, A.; Smits, J.; Grasman, K.A. Avian immunotoxicology. J. Toxicol. Environ. Health Part B 2004, 7, 105–137. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef]
- Kawashima, K.; Fujii, T. Extraneuronal cholinergic system in lymphocytes. Pharmacol. Ther. 2000, 86, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Badgujar, P.C.; Jain, S.; Singh, A.; Punia, J.; Gupta, R.; Chandratre, G.A. Immunotoxic effects of imidacloprid following 28 days of oral exposure in BALB/c mice. Environ. Toxicol. Pharmacol. 2013, 35, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Gorenflo, A.; Siede, R.; Meixner, M.; Büchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J. Insect Physiol. 2016, 86, 40–47. [Google Scholar] [CrossRef]
- Kammon, A.; Brar, R.; Banga, H.; Sodhi, S. Ameliorating effects of vitamin E and selenium on immunological alterations induced by imidacloprid chronic toxicity in chickens. J. Environ. Anal. Toxicol. S 2012, 4. [Google Scholar] [CrossRef]
- Macaulay, S.J.; Hageman, K.J.; Piggott, J.J.; Matthaei, C.D. Time-cumulative effects of neonicotinoid exposure, heat waves and food limitation on stream mayfly nymphs: A multiple-stressor experiment. Sci. Total Environ. 2021, 754, 141941. [Google Scholar] [CrossRef]
- Abu Zeid, E.H.; Alam, R.T.; Ali, S.A.; Hendawi, M.Y. Dose-related impacts of imidacloprid oral intoxication on brain and liver of rock pigeon (Columba livia domestica), residues analysis in different organs. Ecotoxicol. Environ. Saf. 2019, 167, 60–68. [Google Scholar] [CrossRef]
- Mo, Q.; Kulyar, M.F.-e.-A.; Ding, Y.; Zhang, Y.; Pan, H.; Li, J. Thiram induces myocardial oxidative damage and apoptosis in broilers via interfering their cardiac metabolism. Ecotoxicol. Environ. Saf. 2022, 247, 114225. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, Y.; Su, G. Comparative study of neonicotinoid insecticides (NNIs) and NNI-Related substances (r-NNIs) in foodstuffs and indoor dust. Environ. Int. 2022, 166, 107368. [Google Scholar] [CrossRef] [PubMed]
- Mahai, G.; Wan, Y.; Xia, W.; Wang, A.; Shi, L.; Qian, X.; He, Z.; Xu, S. A nationwide study of occurrence and exposure assessment of neonicotinoid insecticides and their metabolites in drinking water of China. Water Res. 2021, 189, 116630. [Google Scholar] [CrossRef] [PubMed]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.-M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef] [PubMed]
- Emam, H.; Ahmed, E.; Abdel-Daim, M. Antioxidant capacity of omega-3-fatty acids and vitamin E against imidacloprid-induced hepatotoxicity in Japanese quails. Environ. Sci. Pollut. Res. 2018, 25, 11694–11702. [Google Scholar] [CrossRef] [PubMed]
- Conte, F.M.; Cestonaro, L.V.; Piton, Y.V.; Guimarães, N.; Garcia, S.C.; da Silva, D.D.; Arbo, M.D. Toxicity of pesticides widely applied on soybean cultivation: Synergistic effects of fipronil, glyphosate and imidacloprid in HepG2 cells. Toxicol. In Vitro 2022, 84, 105446. [Google Scholar] [CrossRef] [PubMed]
- Franzen-Klein, D.; Jankowski, M.; Roy, C.L.; Nguyen-Phuc, H.; Chen, D.; Neuman-Lee, L.; Redig, P.; Ponder, J. Evaluation of neurobehavioral abnormalities and immunotoxicity in response to oral imidacloprid exposure in domestic chickens (Gallus gallus domesticus). J. Toxicol. Environ. Health Part A. 2020, 83, 45–65. [Google Scholar] [CrossRef]
- Rios, F.M.; Wilcoxen, T.E.; Zimmerman, L.M. Effects of imidacloprid on Rana catesbeiana immune and nervous system. Chemosphere 2017, 188, 465–469. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Yang, Y.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martinez-Larranaga, M.-R.; Wang, X.; Anadón, A.; Martinez, M.-A. Neonicotinoids: Mechanisms of systemic toxicity based on oxidative stress-mitochondrial damage. Arch. Toxicol. 2022, 96, 1493–1520. [Google Scholar] [CrossRef]
- Naiel, M.A.; Shehata, A.M.; Negm, S.S.; Abd El-Hack, M.E.; Amer, M.S.; Khafaga, A.F.; Bin-Jumah, M.; Allam, A.A. The new aspects of using some safe feed additives on alleviated imidacloprid toxicity in farmed fish: A review. Rev. Aquac. 2020, 12, 2250–2267. [Google Scholar] [CrossRef]
- Osman, K.A.; Shaaban, M.M.; Ahmed, N.S. Biomarkers of imidacloprid toxicity in Japanese quail, Coturnix coturnix japonica. Environ. Sci. Pollut. Res. 2023, 30, 5662–5676. [Google Scholar] [CrossRef] [PubMed]
- Khandia, R.; Pathe, C.S.; Vishwakarma, P.; Dhama, K.; Munjal, A. Evaluation of the ameliorative effects of Phyllanthus niruri on the deleterious insecticide imidacloprid in the vital organs of chicken embryos. J. Ayurveda Integr. Med. 2020, 11, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.T.; Khan, R.L.; Saleemi, M.K.; Ahmad, M.; Hussain, R.; Khan, A. Amelioration of toxicopathological effects of thiamethoxam in broiler birds with vitamin E and selenium. Toxin Rev. 2022, 41, 218–228. [Google Scholar] [CrossRef]
- Ravikanth, V.; Lakshman, M.; Madhuri, D.; Kalakumar, B. Haematological alterations in broilers administered with imidacloprid and spinosad and its amelioration with Vitamin E and Silymarin. Int. J. Curr. Microbiol. App. Sci 2017, 6, 496–500. [Google Scholar]
- Sasidhar Babu, N.; Kumar, A.; Reddy, A.; Amaravathi, P.; Hemanth, I. Chronic experimental feeding of imidacloprid induced oxidative stress and amelioration with vitamin C and Withania somnifera in layer birds. Int. J. Sci. Environ. ISSN Technol. 2014, 3, 1679–1684. [Google Scholar]
- Hussein, M.; Singh, V. Effect on chick embryos development after exposure to neonicotinoid insecticide imidacloprid. J. Anat. Soc. India 2016, 65, 83–89. [Google Scholar] [CrossRef]
- Gobeli, A.; Crossley II, D.; Johnson, J.; Reyna, K. The effects of neonicotinoid exposure on embryonic development and organ mass in northern bobwhite quail (Colinus virginianus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 195, 9–15. [Google Scholar] [CrossRef]
- Gupta, M.; Singh, S.P.; Pankaj, N.K. Ameliorative effect of Spirulina platensis and Erythrina variegata on oxidative stress in imidacloprid intoxicated white leghorn cockerels. Toxicol. Int 2017, 24, 171–177. [Google Scholar] [CrossRef]
- Cheng, P.; Ishfaq, M.; Yu, H.; Yang, Y.; Li, S.; Li, X.; Fazlani, S.A.; Guo, W.; Zhang, X. Curcumin ameliorates duodenal toxicity of AFB1 in chicken through inducing P-glycoprotein and downregulating cytochrome P450 enzymes. Poult. Sci. 2020, 99, 7035–7045. [Google Scholar] [CrossRef]
- Ravikanth, V.; Lakshman, M.; Madhuri, D.; Kalakumar, B. Effect of spinosad and imidacloprid on Serum Biochemical alterations in male broilers and Its Amelioration with vitamin E and Silymarin. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 2186–2192. [Google Scholar] [CrossRef]
- Aviagen, W.R. 308: Broiler’s Management and Nutrition Specification; AOAC International Aviagen Inc.: Huntsville, AL, USA, 2018; Available online: http://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerHandbook2018-EN.pdf (accessed on 1 January 2018).
- Aviagen, W.R. 308: Broiler’s Management and Nutrition Specification; 308 Broiler Nutrition Specifications; Aviagen, W.R., Ed.; Aviagen: Huntsville, AL, USA, 2014. [Google Scholar]
- Horwitz, W.; Latimer, G. AOAC Official Methods of Analysis of AOAC International. AOAC Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Giambrone, J.; Clay, R.P. Vaccination of day-old broiler chicks against Newcastle disease and infectious bursal disease using commercial live and/or inactivated vaccines. Avian Dis. 1986, 30, 557–561. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Poultry: 1994; National Academies Press: Cambridge, MA, USA, 1994. [Google Scholar]
- AOAC. Official Method of Analysis: Association of Analytical Chemists, 19th ed.; AOAC: Washington, DC, USA, 2012; pp. 121–130. [Google Scholar]
- Kishawy, A.T.; Al-Khalaifah, H.S.; Nada, H.S.; Roushdy, E.M.; Zaglool, A.W.; Ahmed Ismail, T.; Ibrahim, S.M.; Ibrahim, D. Black pepper or radish seed oils in a new combination of essential oils modulated broiler chickens’ performance and expression of digestive enzymes, lipogenesis, immunity, and autophagy-related genes. Vet. Sci. 2022, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.; El-Sayed, H.I.; Mahmoud, E.R.; El-Rahman, G.I.A.; Bazeed, S.M.; Abdelwarith, A.A.; Elgamal, A.; Khalil, S.S.; Younis, E.M.; Kishawy, A.T. Impacts of Solid-State Fermented Barley with Fibrolytic Exogenous Enzymes on Feed Utilization, and Antioxidant Status of Broiler Chickens. Vet. Sci. 2023, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- Harrison, G.J.; Harrison, L.R. Clinical Avian Medicine and Surgery, Including Aviculture; W. B. Saunders co.: Philadelphia, WB, USA, 1986. [Google Scholar]
- Nengsih, R.F.; Mustika, A. Evaluasi Gambaran Darah dan Marker Stres (Rasio H/L) Ayam Pedaging yang Diberi Daun Bangun-Bangun selama 28 Hari. Acta Vet. Indones. 2020, 8, 9–15. [Google Scholar] [CrossRef]
- Bos, H.; de Souza, W. Phagocytosis of yeast: A method for concurrent quantification of binding and internalization using differential interference contrast microscopy. J. Immunol. Methods 2000, 238, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.E. Lysozyme assays. Tech. Fish Immunol. 1990, 1, 101–103. [Google Scholar]
- Doumas, B.T.; Biggs, H.G.; Arends, R.L.; Pinto, P.V. Determination of serum albumin. In Standard Methods of Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 1972; Volume 7, pp. 175–188. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018; ISBN 0702068861. [Google Scholar]
- Hsu, S.-M.; Cossman, J.; Jaffe, E.S. A comparison of ABC, unlabeled antibody and conjugated immunohistochemical methods with monoclonal and polyclonal antibodies—An examination of germinal center of tonsils. Am. J. Clin. Pathol. 1983, 80, 429–435. [Google Scholar] [CrossRef]
- Romero-Garay, M.G.; Montalvo-González, E.; Hernández-González, C.; Soto-Domínguez, A.; Becerra-Verdín, E.M.; García-Magaña, M.D.L. Bioactivity of peptides obtained from poultry by-products: A review. Food Chem. X 2022, 13, 100181. [Google Scholar] [CrossRef]
- Alandiyjany, M.N.; Kishawy, A.T.; Abdelfattah-Hassan, A.; Eldoumani, H.; Elazab, S.T.; El-Mandrawy, S.A.; Saleh, A.A.; ElSawy, N.A.; Attia, Y.A.; Arisha, A.H. Nano-silica and magnetized-silica mitigated lead toxicity: Their efficacy on bioaccumulation risk, performance, and apoptotic targeted genes in Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 2022, 242, 106054. [Google Scholar] [CrossRef]
- Adegoke, A.; Abimbola, M.; Sanwo, K.; Egbeyale, L.; Abiona, J.; Oso, A.; Iposu, S. Performance and blood biochemistry profile of broiler chickens fed dietary turmeric (Curcuma longa) powder and cayenne pepper (Capsicum frutescens) powders as antioxidants. Vet. Anim. Sci. 2018, 6, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, A.; AboSalem, M.; Elshewy, E.A.; Abdeen, A. Alleviation of imidacloprid-induced oxidative stress and immune damage by Spirulina platensis in broiler chickens. Benha Vet. Med. J. 2021, 40, 144–148. [Google Scholar] [CrossRef]
- Li, J.; Bi, D.; Pan, S.; Zhang, Y. Effect of diet with thiram on liver antioxidant capacity and tibial dyschondroplasia in broilers. Br. Poult. Sci. 2007, 48, 724–728. [Google Scholar] [CrossRef]
- Miao, Z.; Miao, Z.; Wang, S.; Wu, H.; Xu, S. Exposure to imidacloprid induce oxidative stress, mitochondrial dysfunction, inflammation, apoptosis and mitophagy via NF-kappaB/JNK pathway in grass carp hepatocytes. Fish Shellfish Immunol. 2022, 120, 674–685. [Google Scholar] [CrossRef]
- Lopez-Antia, A.; Ortiz-Santaliestra, M.E.; Mougeot, F.; Mateo, R. Imidacloprid-treated seed ingestion has lethal effect on adult partridges and reduces both breeding investment and offspring immunity. Environ. Res. 2015, 136, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.H.; El-Kazaz, S.E.; Alharthi, B.; Ghamry, H.I.; Alshehri, M.A.; Sayed, S.; Shukry, M.; El-Sayed, Y.S. The impact of curcumin on growth performance, growth-related gene expression, oxidative stress, and immunological biomarkers in broiler chickens at different stocking densities. Animals 2022, 12, 958. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, M.; Sheikhi, N.; Nazarpak, H.H.; Brujeni, G.N. Effects of dietary turmeric (Curcuma longa) on innate and acquired immune responses in broiler chicken. Vet. Anim. Sci. 2021, 14, 100213. [Google Scholar] [CrossRef] [PubMed]
- Rajput, N.; Muhammad, N.; Yan, R.; Zhong, X.; Wang, T. Effect of dietary supplementation of curcumin on growth performance, intestinal morphology and nutrients utilization of broiler chicks. J. Poult. Sci. 2013, 50, 44–52. [Google Scholar] [CrossRef]
- Yadav, S.; Teng, P.-Y.; Dos Santos, T.S.; Gould, R.L.; Craig, S.W.; Fuller, A.L.; Pazdro, R.; Kim, W.K. The effects of different doses of curcumin compound on growth performance, antioxidant status, and gut health of broiler chickens challenged with Eimeria species. Poult. Sci. 2020, 99, 5936–5945. [Google Scholar] [CrossRef] [PubMed]
- Salah, A.S.; Mahmoud, M.A.; Ahmed-Farid, O.A.; El-Tarabany, M.S. Effects of dietary curcumin and acetylsalicylic acid supplements on performance, muscle amino acid and fatty acid profiles, antioxidant biomarkers and blood chemistry of heat-stressed broiler chickens. J. Therm. Biol. 2019, 84, 259–265. [Google Scholar] [CrossRef]
- Eid, Y.Z.; Omara, Y.; Ragab, A.; Ismail, A.; Zommara, M.; Dawood, M.A. Mitigation of Imidacloprid Toxicity in Poultry Chicken by Selenium Nanoparticles: Growth Performance, Lipid Peroxidation, and Blood Traits. Biol. Trace Elem. Res. 2023, 201, 5379–5388. [Google Scholar] [CrossRef] [PubMed]
- Sankhala, L.; Ranjan, A.; Suvidhi, L.K.; Tripathi, S. Evaluation of ameliorative effect of cow urine distillate on serum biochemical parameters in imidacloprid intoxicated white leghorn broilers. J. Pharm. Innov. 2022, 11, 263–266. [Google Scholar]
- Pang, S.; Lin, Z.; Zhang, Y.; Zhang, W.; Alansary, N.; Mishra, S.; Bhatt, P.; Chen, S. Insights into the toxicity and degradation mechanisms of imidacloprid via physicochemical and microbial approaches. Toxics 2020, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Abd EL-Samie, L. Comparative study on effect of turmeric powder, probiotic and prebiotic supplementation on broiler performance and immunity. Assiut Vet. Med. J. 2019, 65, 143–151. [Google Scholar]
- Kafi, A.; Uddin, M.N.; Uddin, M.J.; Khan, M.M.H.; Haque, M.E. Effect of Dietary Supplementation of Turmeric (Curcuma longa), Ginger (Zingiber officinale) and Their Combination as Feed Additives on Feed Intake, Growth Performance and Economics of Broiler. Int. J. Poult. Sci. 2017, 16, 257–265. [Google Scholar] [CrossRef]
- Shohe, A.; Vidyarthi, V.; Zuyie, R. Performance of broiler chicken on diet supplemented with Turmeric powder (Curcuma longa). Livest. Res. Int. 2019, 7, 77–82. [Google Scholar]
- Ali, A.; Ismoyowati, I.; Indrasanti, D. Jumlah eritrosit, kadar hemoglobin dan hematokrit pada berbagai jenis itik lokal terhadap penambahan probiotik dalam ransum. J. Ilm. Peternak. 2013, 1, 1001–1013. [Google Scholar]
- Yosi, F.; Sandi, S. Meat quality, blood profile, and fecal ammonia concentration of broiler supplemented with liquid smoke. Media Peternak. 2014, 37, 169–174. [Google Scholar] [CrossRef]
- Davis, A.; Maney, D.; Maerz, J. The use of leukocyte profiles to measure stress in vertebrates: A review for ecologists. Funct. Ecol. 2008, 22, 760–772. [Google Scholar] [CrossRef]
- Puvadolpirod, S.; Thaxton, J. Model of physiological stress in chickens 1. Response parameters. Poult. Sci. 2000, 79, 363–369. [Google Scholar] [CrossRef]
- Pimson, C.; Bakban, P.; Suwanrat, S.; Chanutsa, N. The effect of curcumin on growth performance, blood biochemistry and antioxidant activities in boiler chickens. Vet. Integr. Sci. 2018, 16, 95–107. [Google Scholar]
- Ma’rifah, B.; Isroli, I.; Sartono, T.A. Pengaruh air rebusan kunyit (Curcuma domestica) dalam air minum terhadap daya tahan dan perfromans karkas ayam broiler. J. Ris. Agribisnis Dan Peternak. 2020, 5, 7–12. [Google Scholar] [CrossRef]
- Agustanti, L. Gambaran sel Darah Putih dan Indeks Stres Ayam Broiler Yang Diberi Jamu Bagas Waras (jahe, kunyit, dan kencur) Melalui Air Minum. Skripsi. Institut Pertanian Bogor. Bogor. 2014. Available online: https://repository.ipb.ac.id/handle/123456789/72263 (accessed on 23 November 2023).
- Ramadan, A.; Mansour, U. Cytotoxic Effect of Some Commercial Neonicotinoids on Sheep Phagocytic Cells Activity In vitro. Res. Rev. J. Vet. Sci. 2018, 4, 2. [Google Scholar]
- Abou-Zeid, S.M.; Aljuaydi, S.H.; AbuBakr, H.O.; Tahoun, E.A.; Di Cerbo, A.; Alagawany, M.; Khalil, S.R.; Farag, M.R. Astaxanthin mitigates thiacloprid-induced liver injury and immunotoxicity in male rats. Mar. Drugs 2021, 19, 525. [Google Scholar] [CrossRef]
- Mohany, M.; Badr, G.; Refaat, I.; El-Feki, M. Immunological and histological effects of exposure to imidacloprid insecticide in male albino rats. Afr. J. Pharm. Pharmacol. 2011, 5, 2106–2114. [Google Scholar] [CrossRef]
- Walderdorff, L.; Laval-Gilly, P.; Wechtler, L.; Bonnefoy, A.; Falla-Angel, J. Phagocytic activity of human macrophages and Drosophila hemocytes after exposure to the neonicotinoid imidacloprid. Pestic. Biochem. Physiol. 2019, 160, 95–101. [Google Scholar] [CrossRef]
- Julius, A.; Abernathy, L.; Yung, R. Defective Dendritic Cell Phagocytic Function in Aging (134.36). J. Immunol. 2009, 182, 134–136. [Google Scholar] [CrossRef]
- Gul, S.T.; Khan, A.; Ahmad, M.; Ahmad, H.; Saleemi, M.K.; Naseem, M.N.; Bilal, M. Immuno-toxicological effects of different sub-lethal doses of thiamethoxam (TMX) in broiler birds. Toxin Rev. 2018, 38, 200–205. [Google Scholar] [CrossRef]
- Gawade, L.; Dadarkar, S.S.; Husain, R.; Gatne, M. A detailed study of developmental immunotoxicity of imidacloprid in Wistar rats. Food Chem. Toxicol. 2013, 51, 61–70. [Google Scholar] [CrossRef]
- Lopez-Antia, A.; Ortiz-Santaliestra, M.E.; Mougeot, F.; Mateo, R. Experimental exposure of red-legged partridges (Alectoris rufa) to seeds coated with imidacloprid, thiram and difenoconazole. Ecotoxicology 2013, 22, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Khayal, E.E.-S.; Alabiad, M.A.; Elkholy, M.R.; Shalaby, A.M.; Nosery, Y.; El-Sheikh, A.A. The immune modulatory role of marjoram extract on imidacloprid induced toxic effects in thymus and spleen of adult rats. Toxicology 2022, 471, 153174. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.R.; Alagawany, M.M.; Dhama, K. Antidotal effect of turmeric (Curcuma longa) against endosulfan-induced cytogenotoxicity and immunotoxicity in broiler chicks. Int. J. Pharmacol. 2014, 10, 429–439. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Aggarwal, B.B. “Spicing up” of the immune system by curcumin. J. Clin. Immunol. 2007, 27, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Kuo, J.; Jiang, H.; Deeb, D.; Liu, Y.; Divine, G.; Chapman, R.A.; Dulchavsky, S.A.; Gautam, S.C. Immunomodulatory activity of curcumin: Suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production in vitro. Biochem. Pharmacol. 2004, 68, 51–61. [Google Scholar] [CrossRef]
- Attia, A.; El-Saadawy, H.; El-Belbasi, H.; Abd El-Hameed, S. Ameliorative effect of azolla pinnata on imidacloprid induced hepatorenal toxicity, oxidative stress and immunosuppression in nile tilapia. J. Anim. Health Prod. 2021, 9, 1–6. [Google Scholar] [CrossRef]
- Rahman, A.N.A.; Mohamed, A.A.-R.; Mohammed, H.H.; Elseddawy, N.M.; Salem, G.A.; El-Ghareeb, W.R. The ameliorative role of geranium (Pelargonium graveolens) essential oil against hepato-renal toxicity, immunosuppression, and oxidative stress of profenofos in common carp, Cyprinus carpio (L.). Aquaculture 2020, 517, 734777. [Google Scholar] [CrossRef]
- Isroli, I.; Yudiarti, T.; Widiastuti, E.; Sugiharto, S. Effect of decocted turmeric on performance, hematological parameters and carcass traits of broiler chickens. J. Indones. Trop. Anim. Agric. 2017, 42, 263–269. [Google Scholar] [CrossRef]
- Shawky, S.; Fathalla, S.; Orabi, S.; El-Mosalhi, H.; Abu-Alya, I. Turmeric Powder Supplementation in Broiler Diet Improves Growth Performance and Immunity via Increasing mRNA Expression of Growth Hormone, Insulin Like Growth Factor-1, Interferon Gamma and Interleukin12. Res. Sq. 2022, 1–13. [Google Scholar] [CrossRef]
- Arshami, J.; Pilevar, M.; Azghadi, M.A.; Raji, A.R. Hypolipidemic and antioxidative effects of curcumin on blood parameters, humoral immunity, and jejunum histology in Hy-line hens. Avicenna J. Phytomed. 2013, 3, 178. [Google Scholar]
- Akter, L.; Kobir, M.A.; Nasrin, M.; Siddiqi, M.N.H.; Pervin, M.; Karim, M.R. Effects of exposure to imidacloprid contaminated feed on the visceral organs of adult male rabbits (Oryctolagus cuniculus). Saudi J. Biol. Sci. 2023, 30, 103684. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, W.; Gao, M.; Lin, H. Mechanism of evodiamine blocking Nrf2/MAPK pathway to inhibit apoptosis of grass carp hepatocytes induced by DEHP. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 263, 109506. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.R.d.J.S.; Bizerra, P.F.V.; Miranda, C.A.; Mingatto, F.E. Effects of imidacloprid on viability and increase of reactive oxygen and nitrogen species in HepG2 cell line. Toxicol. Mech. Methods 2022, 32, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Laganá, C.; Saldanha, E.S.B.; Sartori, J.R.; Turco, P.H.N.; Gonzales, E.; Luciano, R.L.; Zanatta, G.; Fascina, V.B. Turmeric on poultry production: A Review. Agric. Sci. 2019, 10, 1592. [Google Scholar] [CrossRef]
- Vettorazzi, A.; van Delft, J.; de Cerain, A.L. A review on ochratoxin A transcriptomic studies. Food Chem. Toxicol. 2013, 59, 766–783. [Google Scholar] [CrossRef] [PubMed]
- Nebbia, C. Biotransformation enzymes as determinants of xenobiotic toxicity in domestic animals. Vet. J. 2001, 161, 238–252. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y. Hypocholesterolemic effects of curcumin via up-regulation of cholesterol 7a-hydroxylase in rats fed a high fat diet. Nutr. Res. Pract. 2010, 4, 191–195. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, C.; Ba, D.; Wang, N.; Wang, Y.; Li, X.; Li, Q.; Zhao, G. Ferroptosis contribute to neonicotinoid imidacloprid-evoked pyroptosis by activating the HMGB1-RAGE/TLR4-NF-κB signaling pathway. Ecotoxicol. Environ. Saf. 2023, 253, 114655. [Google Scholar] [CrossRef]
- Subha, G. Long term exposure to chemicals, insecticides and heavy metals causing toxicity: A review. Int. J. Pharm. Res. Bio-Sci. 2013, 2, 333–342. [Google Scholar]
- Li, S.; Muhammad, I.; Yu, H.; Sun, X.; Zhang, X. Detection of Aflatoxin adducts as potential markers and the role of curcumin in alleviating AFB1-induced liver damage in chickens. Ecotoxicol. Environ. Saf. 2019, 176, 137–145. [Google Scholar] [CrossRef]
- El-Bahr, S. Effect of curcumin on hepatic antioxidant enzymes activities and gene expressions in rats intoxicated with aflatoxin B1. Phytother. Res. 2015, 29, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Sashidhar, R. Metabolic intervention of aflatoxin B1 toxicity by curcumin. J. Ethnopharmacol. 2010, 127, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.-Y.; Qi, M.; Zhao, L.; Zhu, M.-K.; Guo, J.; Liu, J.; Gu, C.-Q.; Rajput, S.A.; Krumm, C.S.; Qi, D.-S. Curcumin prevents aflatoxin B1 hepatoxicity by inhibition of cytochrome P450 isozymes in chick liver. Toxins 2016, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Careem, M.; Hunter, D.; Lambourne, M.; Barta, J.; Sharif, S. Ontogeny of cytokine gene expression in the chicken spleen. Poult. Sci. 2007, 86, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef]
- Farag, M.R.; Abou-EL Fotoh, M.F.; EL-Sayed, G.G.; EL-Sayed, E.W. Modulatory effect of ginger aqueous extract against imidacloprid-induced neurotoxicity in rats. Zagazig Vet. J. 2019, 47, 432–446. [Google Scholar] [CrossRef]
- Alves-Filho, J.C.; Freitas, A.; Souto, F.O.; Spiller, F.; Paula-Neto, H.; Silva, J.S.; Gazzinelli, R.T.; Teixeira, M.M.; Ferreira, S.H.; Cunha, F.Q. Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc. Natl. Acad. Sci. USA 2009, 106, 4018–4023. [Google Scholar] [CrossRef]
- Sugiyama, K.-I.; Muroi, M.; Tanamoto, K.-I. A novel TLR4-binding peptide that inhibits LPS-induced activation of NF-κB and in vivo toxicity. Eur. J. Pharmacol. 2008, 594, 152–156. [Google Scholar] [CrossRef]
- Singh, A.; Jiang, Y. Lipopolysaccharide (LPS) induced activation of the immune system in control rats and rats chronically exposed to a low level of the organothiophosphate insecticide, acephate. Toxicol. Ind. Health 2003, 19, 93–108. [Google Scholar] [CrossRef]
- Nemati, M.; Larussa, T.; Khorramdelazad, H.; Mahmoodi, M.; Jafarzadeh, A. Toll-like receptor 2: An important immunomodulatory molecule during Helicobacter pylori infection. Life Sci. 2017, 178, 17–29. [Google Scholar] [CrossRef]
- Cui, J.; Chen, Y.; Wang, H.Y.; Wang, R.-F. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum. Vaccines Immunother. 2014, 10, 3270–3285. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.; Tsung, A.; Billiar, T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic. Biol. Med. 2010, 48, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Pandit, A.A.; Choudhary, S.; Singh, B.; Sethi, R. Imidacloprid induced histomorphological changes and expression of TLR-4 and TNFα in lung. Pestic. Biochem. Physiol. 2016, 131, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.R.; Khalil, S.R.; Zaglool, A.W.; Hendam, B.M.; Moustafa, A.A.; Cocco, R.; Di Cerbo, A.; Alagawany, M. Thiacloprid induced developmental neurotoxicity via ROS-oxidative injury and inflammation in chicken embryo: The possible attenuating role of chicoric and rosmarinic acids. Biology 2021, 10, 1100. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, M.; Che, T.; Lee, J.; Bravo, D.; Maddox, C.; Pettigrew, J. Dietary plant extracts modulate gene expression profiles in ileal mucosa of weaned pigs after an Escherichia coli infection. J. Anim. Sci. 2014, 92, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Che, T.; Song, M.; Lee, J.; Almeida, J.; Bravo, D.; Van Alstine, W.; Pettigrew, J. Dietary plant extracts improve immune responses and growth efficiency of pigs experimentally infected with porcine reproductive and respiratory syndrome virus. J. Anim. Sci. 2013, 91, 5668–5679. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Deepika, L. Clinico-pathological studies of imidacloprid toxicity in broiler chickens. Haryana Vet. 2016, 55, 163–165. [Google Scholar]
- Kammon, A.M.; Brar, R.S.; Banga, H.S.; Sodhi, S. Patho-biochemical studies on hepatotoxicity and nephrotoxicity on exposure to chlorpyrifos and imidacloprid in layer chickens. Vet. Arh. 2010, 80, 663–672. [Google Scholar]
- Alhusaini, A.; Fadda, L.M.; Ali, H.M.; Hasan, I.H.; Ali, R.A.; Zakaria, E.A. Mitigation of acetamiprid—Induced renotoxicity by natural antioxidants via the regulation of ICAM, NF-kB and TLR 4 pathways. Pharmacol. Rep. 2019, 71, 1088–1094. [Google Scholar] [CrossRef]



| Ingredient, % | Starter (1–10 Days) | Grower (11–20 Days) | Finisher (21–35 Days) |
|---|---|---|---|
| Yellow corn | 57.8 | 61 | 65.2 |
| Soybean meal, 48% | 34.8 | 30.3 | 25.6 |
| 1.2 | 1.2 | 1.2 | |
| Soybean oil | 0 | 0 | 0 |
| Calcium carbonate | 1.8 | 3.2 | 3.7 |
| Calcium diphasic phosphate | 1.2 | 1.2 | 1.2 |
| Common salt | 1.2 | 1.2 | 1.2 |
| Premix 1 | 0.3 | 0.3 | 0.3 |
| L-Lysine HCL, 78% | 0.8 | 0.8 | 0.8 |
| DL-Methionine, 99% | 0.35 | 0.3 | 0.3 |
| Choline chloride | 0.20 | 0.20 | 0.20 |
| Anti-mycotoxin | 0.10 | 0.10 | 0.10 |
| Calculated composition | |||
| Metabolizable energy (Kcal/Kg) | 3013 | 3130 | 3200 |
| Crude protein, % | 23.02 | 21.00 | 19.22 |
| Ether extract, % | 4.31 | 5.76 | 6.35 |
| Crude fiber, % | 2.64 | 2.54 | 2.47 |
| Calcium, % | 1.09 | 1.08 | 1.07 |
| Available phosphorous, % | 0.47 | 0.44 | 0.48 |
| Lysine, % | 1.45 | 1.29 | 1.17 |
| Methionine, % | 0.60 | 0.53 | 0.51 |
| Ingredient | Nutrient (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Moisture | CP | EE | * CF | * Calcium | * AP 1 | * Lysine | * Methionine | * ME 2 | |
| yellow corn | 8 | 8.9 | 3.7 | 2.3 | 0.05 | 0.08 | 0.26 | 0.18 | 3350 |
| Soybean meal, 48% | 10.6 | 47.8 | 1.1 | 3.7 | 0.25 | 0.27 | 2.92 | 0.67 | 2440 |
| Corn gluten, 60% | 11 | 59.4 | 2.4 | 1.8 | 0.07 | 0.14 | 1.03 | 1.78 | 3720 |
| Soybean oil | - | - | 98 | - | - | - | - | - | 8800 |
| Calcium carbonate | - | - | - | - | 38 | - | - | - | - |
| Calcium dibasic phosphate | - | - | - | - | 26 | 18 | - | - | - |
| premix | - | - | - | - | 26 | - | - | - | - |
| Lysine, HcL, 78% | - | 118 | - | - | - | - | 78 | - | 4600 |
| DL-Methionine, 98% | - | 58 | - | - | - | - | - | 98 | 3600 |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Gene Bank Accession No. | Product Size |
|---|---|---|---|---|
| TLR-4 | GTTCTTCTGTGACCCGTGAGA | GTGAGGAGCGTTGCGCTTT | FJ915520.1 | 129 |
| IL-1β | TGCCTGCAGAAGAAGCCTCG | CTCAGGTCGCTGTCAGCAAAG | NM_204524.2 | 173 |
| IL-10 | TTGGGGTGGCATTTCTCCTTG | GTTAGACTGCCTCAAACAGCG | EU999771.1 | 89 |
| actin-b | GTGGATCAGCAAGCAGGAGT | ATCCTGAGTCAAGCGCCAAA | NM_205518.2 | 182 |
| Parameter | IBW (g) | TFI (g) | TBWG (g) | TFCR | FBW (g) |
|---|---|---|---|---|---|
| Group | |||||
| 1st group C | 45.20 ± 0.80 | 4300 ± 8.097 a | 2349 ± 8.84 b | 1.83 ± 0.01 b | 2394 ± 8.79 b |
| 2nd group CUR | 46.00 ± 0.84 | 4133 ± 18.25 b | 2423 ± 9.67 a | 1.71 ± 0.01 c | 2468 ± 9.50 a |
| 3rd group IMI-treated | 44.00 ± 1.30 | 4267 ± 11.40 a | 1986 ± 36.46 d | 2.15 ± 0.04 a | 2033 ± 36.17 e |
| 4th group CUR+IMI Co-treated | 44.20 ± 0.86 | 4013 ± 23.74 c | 2196 ± 2.88 c | 1.83 ± 0.01 b | 2242 ± 2.59 d |
| 5th group CUR+IMI pro/co-treated | 45.40 ± 0.51 | 4089 ± 12.20 b | 2259 ± 5.51 c | 1.81 ± 0.01 b | 2303 ± 5.24 c |
| Parameters | RBCs (×106 mL3) | Hb (g/dL) | Hct (%) | TLC (×109/L) | Heterophils % | Lymphocytes % |
|---|---|---|---|---|---|---|
| Group | ||||||
| 1st group C | 3.51 ± 0.13 a | 10.13 ± 0.34 a | 31.00 ± 0.73 a | 10.20 ± 1.62 abc | 31.83 ± 1.14 ab | 68.17 ± 1.14 a |
| 2nd group CUR | 3.12 ± 0.02 b | 8.99 ± 0.04 b | 27.17 ± 0.11 b | 12.80 ± 0.42 a | 32.00 ± 0.58 a | 68.00 ± 0.58 a |
| 3rd group IMI-treated | 2.74 ± 0.14 d | 7.98 ± 0.29 b | 24.00 ± 0.84 c | 5.96 ± 0.63 d | 37.66 ± 1.02 c | 62.33 ± 1.02 b |
| 4th group CUR+IMI Co-treated | 2.96 ± 0.02 bc | 8.43 ± 0.05 b | 25.50 ± 0.18 ab | 8.50 ± 0.55 bcd | 35.00 ± 0.68 bc | 65.00 ± 0.68 ab |
| 5th group CUR+IMI Pro/co-treated | 2.76 ± 0.10 bc | 8.20 ± 0.12 b | 24.33 ± 0.59 c | 9.50 ± 0.37 bc | 33.83 ± 1.11 b | 66.17 ± 1.11 a |
| Parameter | ALT (U/L) | AST (U/L) | TP (g/dL) | Albumin (g/dL) | Globulin (g/dL) | A/G Ratio | TG (mg/dL) | TC (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | VLDL (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | |||||||||||
| 1st group C | 20.67 ±0.92 b | 215.33 ±24.58 b | 2.79 ±0.16 ab | 1.54 ±0.09 a | 1.25 ±0.07 a | 1.23 ±0.01 | 43.00 ±1.32 b | 115.33 ±3.75 c | 78.33 ±1.12 ab | 34.60 ±1.27 c | 10.07± 0.22 b |
| 2nd group CUR | 17.83 ±0.60 c | 174.00 ±20.10 b | 2.92 ±0.17 a | 1.66 ±0.07 a | 1.26 ±0.11 a | 1.35 ±0.10 | 24.00 ±0.73 c | 101.33 ±2.74 d | 82.67 ±1.65 a | 11.07 ±1.00 d | 7.60± 0.48 b |
| 3rd group IMI-treated | 25.66 ±0.56 a | 438.33 ±55.56 a | 1.75 ±0.09 b | 0.93 ±0.04 d | 0.82 ±0.05 b | 1.16 ±0.05 | 77.04 ±2.01 a | 147.00 ±4.21 a | 55.00 ±2.39 c | 76.57 ±1.44 a | 15.43± 0.39 a |
| 4th group CUR+IMI Co-treated | 23.16 ±0.70 ab | 229.33 ±8.83 b | 2.03 ±0.11 b | 1.17 ±0.08 cd | 0.86 ±0.05 b | 1.33 ±0.08 | 49.50 ±1.78 b | 128.33 ±2.79 b | 71.67 ±4.59 b | 46.00 ±4.05 b | 10.67± 0.22 b |
| 5th group CUR+IMI Pro/co-treated | 21.00 ±0.73 b | 285.67 ±16.61 b | 2.09 ±0.16 bc | 1.21 ±0.09 bcd | 0.88 ±0.08 b | 1.42 ±0.11 | 44.33 ±2.76 b | 121.00 ±1.32 bc | 76.00 ±2.03 ab | 34.67 ±3.17 c | 10.33± 0.28 b |
| Groups | 1st Group C | 2nd Group CUR | 3th Group IMI-treated | 4th Group CUR+IMI Co-Treated | 5th Group IMI+CUR Pro/Co-Treated |
|---|---|---|---|---|---|
| Parameters | |||||
| Lymphoid depletion | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 32.00 ± 2.91 a | 8.00 ± 2.00 b | 9.00 ± 1.79 b |
| Lymphoid necrosis | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 6.00 ± 1.63 a | 2.00 ± 1.33 ab | 4.00 ± 1.63 ab |
| Vascular congestion | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 16.00 ± 1.63 a | 5.00 ± 1.66 bc | 7.00 ± 1.52 b |
| Vascular thrombosis | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 2.00 ± 1.33 a | 0.00 ± 0.00 a | 1.00 ± 0.10 a |
| Endothelial hypertrophy | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 5.00 ± 1.66 a | 2.00 ± 1.33 a | 3.00 ± 2.13 a |
| TNF-α Immuno-positive area fraction | 0.48 ± 0.06 c | 0.47 ± 0.04 c | 7.49 ± 0.43 a | 3.63 ± 0.13 b | 4.38 ± 0.22 b |
| TLR4 immuno-positive area fraction | 0.43 ± 0.07 d | 0.46 ± 0.08 d | 8.18 ± 0.44 a | 4.01 ± 0.11 bc | 4.62 ± 0.14 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eleiwa, N.Z.; El-Shabrawi, A.A.; Ibrahim, D.; Abdelwarith, A.A.; Younis, E.M.; Davies, S.J.; Metwally, M.M.M.; Abu-Zeid, E.H. Dietary Curcumin Modulating Effect on Performance, Antioxidant Status, and Immune-Related Response of Broiler Chickens Exposed to Imidacloprid Insecticide. Animals 2023, 13, 3650. https://doi.org/10.3390/ani13233650
Eleiwa NZ, El-Shabrawi AA, Ibrahim D, Abdelwarith AA, Younis EM, Davies SJ, Metwally MMM, Abu-Zeid EH. Dietary Curcumin Modulating Effect on Performance, Antioxidant Status, and Immune-Related Response of Broiler Chickens Exposed to Imidacloprid Insecticide. Animals. 2023; 13(23):3650. https://doi.org/10.3390/ani13233650
Chicago/Turabian StyleEleiwa, Naglaa Z., Ahmed A. El-Shabrawi, Doaa Ibrahim, Abdelwahab A. Abdelwarith, Elsayed M. Younis, Simon J. Davies, Mohamed M. M. Metwally, and Ehsan H. Abu-Zeid. 2023. "Dietary Curcumin Modulating Effect on Performance, Antioxidant Status, and Immune-Related Response of Broiler Chickens Exposed to Imidacloprid Insecticide" Animals 13, no. 23: 3650. https://doi.org/10.3390/ani13233650





