Age Structure and Body Size of the Plateau Brown Frog (Rana kukunoris) in the Jiuzhaigou National Nature Reserve and Potential Climatic Impacts on Its Life History Variations
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
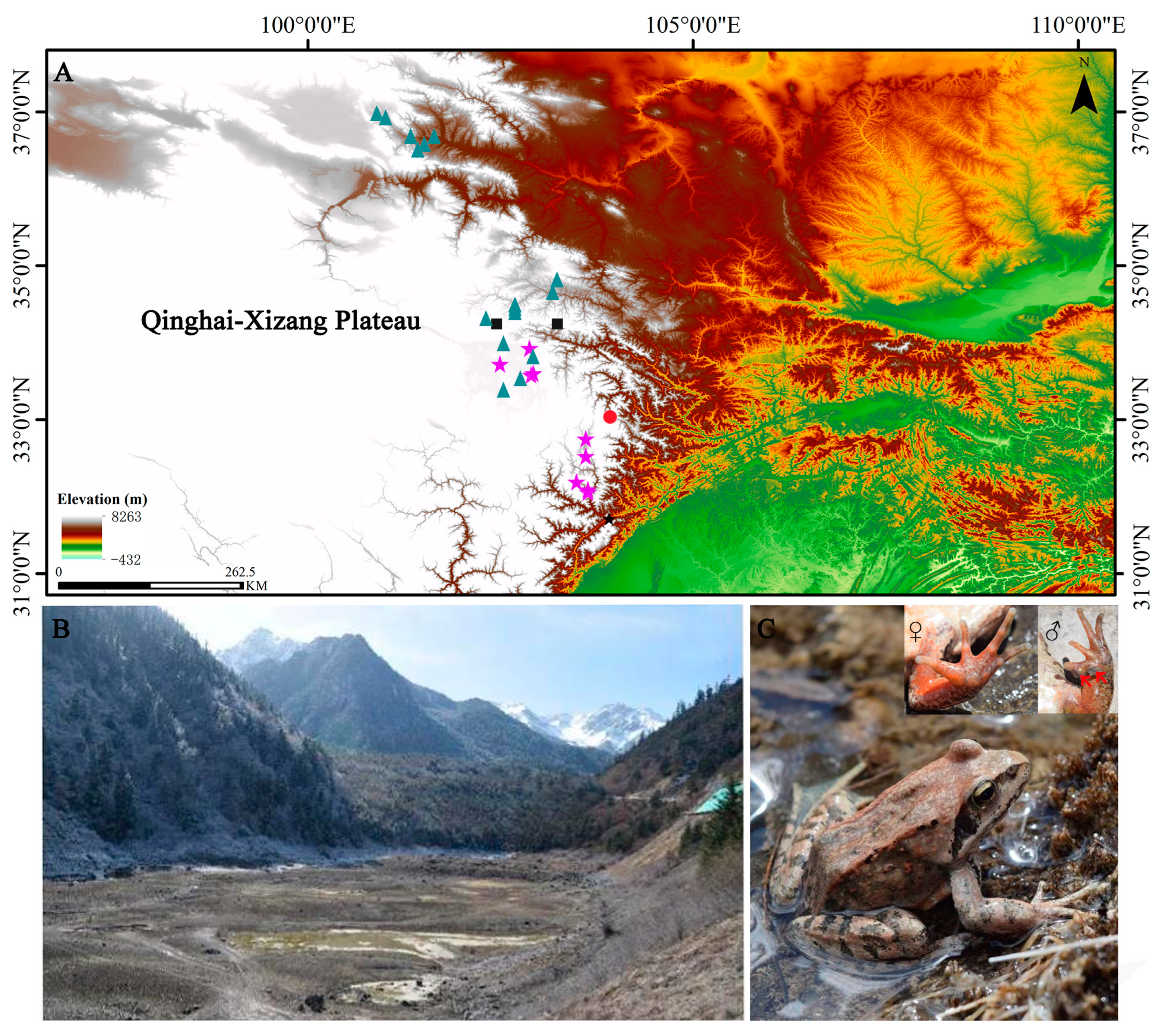
2.1. Sampling
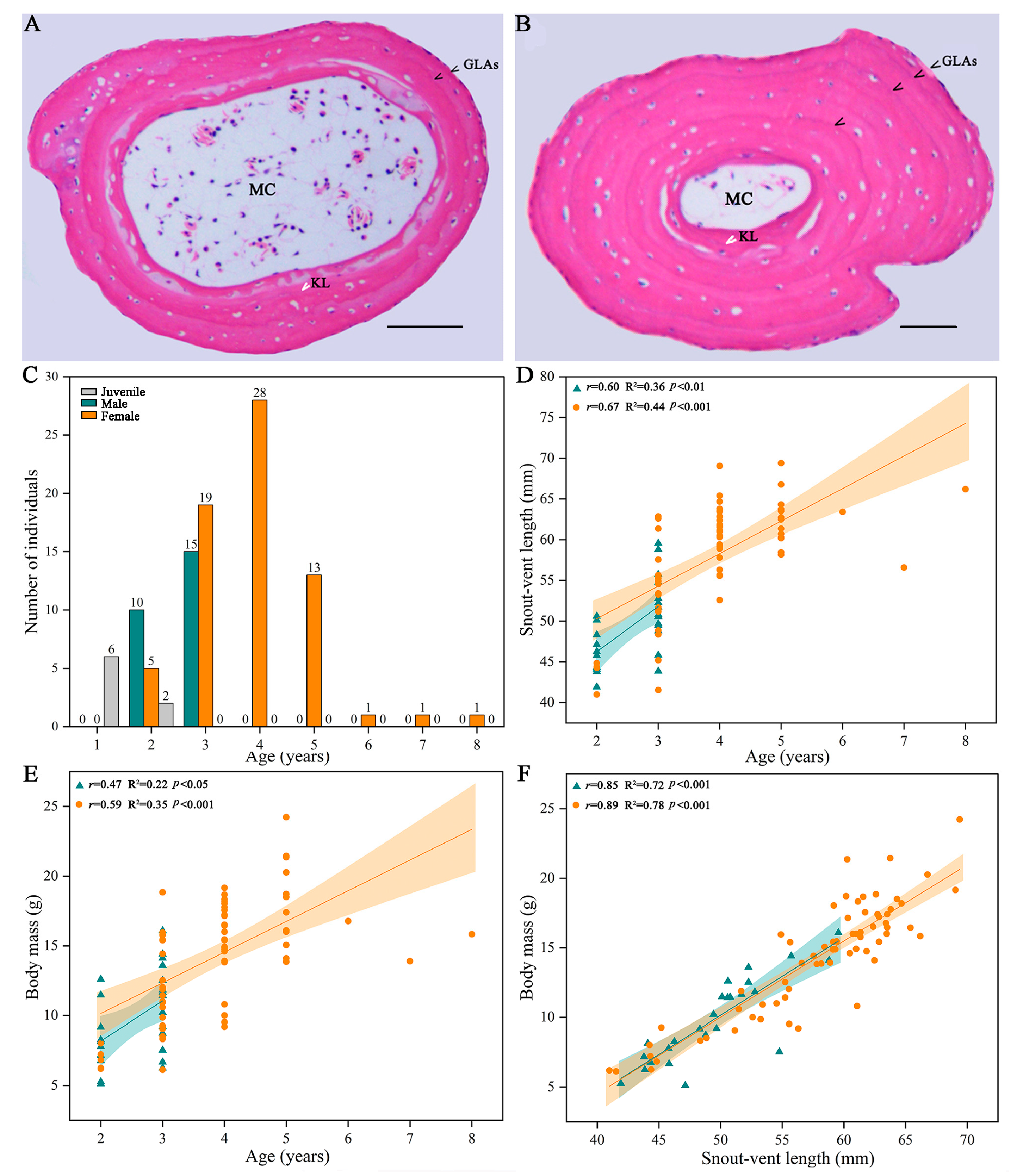
2.2. Skeletochronology
2.3. Climatic Variables
2.4. Statistical Analyses
3. Results
3.1. Age Structure and Body Size of R. kukunoris in JNNR
3.2. Comparisons of the Average Age, ASM, and Average SVL among 29 Populations of R. kukunoris
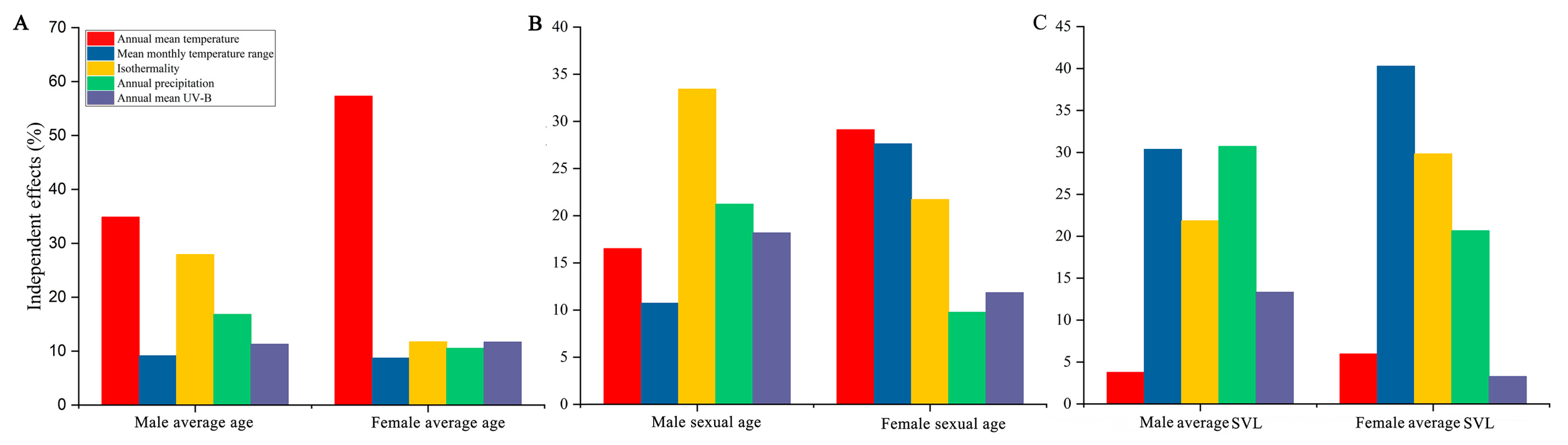
3.3. The Environmental Impacts on the Life History Variations of These Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gade, M.R.; Connette, G.M.; Crawford, J.A.; Hocking, D.J.; Maerz, J.C.; Milanovich, J.R.; Peterman, W.E. Predicted alteration of surface activity as a consequence of climate change. Ecology 2020, 101, e03154. [Google Scholar] [CrossRef]
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzée, A.; Hamidy, A.; Aowphol, A.; Jean, A.; et al. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.L.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [PubMed]
- Hof, C.; Araújo, M.B.; Jetz, W.; Rahbek, C. Additive threats from pathogens, climate and land-use change for global amphibian diversity. Nature 2011, 480, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.E.; DeVault, T.L.; Belant, J.L. Cause-specific mortality of the world’s terrestrial vertebrates. Glob. Ecol. Biogeogr. 2019, 28, 680–689. [Google Scholar] [CrossRef]
- Powers, R.P.; Jetz, W. Global habitat loss and extinction risk of terrestrial vertebrates under future land-use-change scenarios. Nat. Clim. Chang. 2019, 9, 323–329. [Google Scholar] [CrossRef]
- Selwood, K.E.; McGeoch, M.A.; Mac Nally, R. The effects of climate change and land-use change on demographic rates and population viability. Biol. Rev. 2015, 90, 837–853. [Google Scholar] [CrossRef] [PubMed]
- Cogălniceanu, D.; Stănescu, F.; Székely, D.; Topliceanu, T.S.; Iosif, R.; Székely, P. Age, size and body condition do not equally reflect population response to habitat change in the common spadefoot toad Pelobates fuscus. PeerJ 2021, 9, e11678. [Google Scholar] [CrossRef]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ecology: Individuals, Populations and Communities; Blackwell Scientific Publications: Oxford, UK, 1986. [Google Scholar]
- Liao, W.B.; Luo, Y.; Lou, S.L.; Lu, D.; Jehle, R. Geographic variation in life-history traits: Growth season affects age structure, egg size and clutch size in Andrew’s toad (Bufo andrewsi). Front. Zool. 2016, 13, 6. [Google Scholar] [CrossRef]
- Cabezas, C.F.; Boretto, J.M.; Ibargüengoytía, N.R. Effects of climate and latitude on age at maturity and longevity of lizards studied by skeletochronology. Integr. Comp. Biol. 2018, 58, 1086–1097. [Google Scholar] [CrossRef]
- Sinclair, A.R.E.; Fryxell, J.M.; Caughley, G. Wildlife Ecology, Conservation, and Management, 2nd ed.; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- IUCN. Guidelines for Application of IUCN Red List Criteria at Regional and National Levels: Version 4.0.; IUCN: Gland, Switzerland, 2012. [Google Scholar]
- Jiang, J.P.; Xie, F.; Li, C.; Wang, B. China’s Red List of Biodiversity: Vertebrates Volume IV, Amphibians; Science Press: Beijing, China, 2021. [Google Scholar]
- Bidau, C.; Martí, D.; Baldo, D. Inter- and intraspecific geographic variation of body size in South American redbelly toads of the genus Melanophryniscus Gallardo, 1961 (Anura: Bufonidae). J. Herpetol. 2011, 45, 66–74. [Google Scholar] [CrossRef]
- Feijó, A.; Karlsson, C.M.; Gray, R.; Yang, Q.S.; Hughes, A.C. Extreme-sized anurans are more prone to climate-driven extinctions. Clim. Chang. Ecol. 2022, 4, 100062. [Google Scholar] [CrossRef]
- Weil, S.S.; Gallien, L.; Nicolaï, M.P.J.; Lavergne, S.; Börger, L.; Allen, W.L. Body size and life history shape the historical biogeography of tetrapods. Nat. Ecol. Evol. 2023, 7, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, Z.Y.; Cai, Y.S.; Liu, S.Y.; Ran, J.H.; Liu, Z.J.; Wang, Y.Z. The herpetofaunal diversity in Jiuzhaigou National Nature Reserve, China. Chin. J. Zool. 2004, 39, 74–77. [Google Scholar]
- Liu, S.Y.; Sun, Z.Y.; Ran, J.H.; Liu, Y.; Fu, J.R.; Cai, Y.S.; Lei, K.M. Mammalian survey of Jiuzhaigou National Nature Reserve, Sichuan Province. Acta Theriol. Sin. 2005, 25, 273–281. [Google Scholar]
- Qiao, X.; Du, J.; Lugli, S.; Ren, J.H.; Xiao, W.Y.; Chen, P.; Tang, Y. Are climate warming and enhanced atmospheric deposition of sulfur and nitrogen threatening tufa landscapes in Jiuzhaigou National Nature Reserve, Sichuan, China? Sci. Total Environ. 2016, 562, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Wake, D.B.; Vredenburg, V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA 2008, 105, 11466–11473. [Google Scholar] [CrossRef]
- Schmidt, K.P. New Reptiles and a New Salamander from China. Amer. Mus. Novit. 1925, 157, 1–5. [Google Scholar]
- Cope, E.D. On the primary divisions of the Salamandridae, with descriptions of two new species. Proc. Acad. Nat. Sci. USA 1859, 11, 122–128. [Google Scholar]
- Bedriaga, J.V. Amphibien und Reptilien. Wissenschaftliche Resultate der von N. M. Przewalski nach Central-Asien unternommenen Reisen, & c.-Nauchnuie Rezul’tatui puteshestvii N. M. Przheval’skagho po tzentral’noi Azii, & c. Volume 3, Zoologischer Theil, Part 1; Akadamie der Wissenschaften: St. Petersburg, Russia, 1898. [Google Scholar]
- Bonaparte, C.L. Conspectus Systematum. Herpetologiae et Amphibiologiae; Editio Altera Reformata; E. J. Brill: Leyden, The Netherlands, 1850. [Google Scholar]
- Cantor, T. General features of Chusan, with remarks on the flora and fauna of that island. Ann. Mag. Nat. Hist. 1842, 9, 481–493. [Google Scholar] [CrossRef]
- Gray, J.E. A synopsis of the genera of reptiles and amphibians with a description of some new species. Ann. Phi. 1825, 10, 193–217. [Google Scholar]
- Nikolskii, A.M. Fauna rossii i sopredel’nykh stran. In Zemnovodnye; Russian Academy of Sciences: Petrograd, Russia, 1918. [Google Scholar]
- Batsch, A.J.G.K. Umriß der gesammten Naturgeschichte: Ein Auszug aus den frühern Handbüchern des Verfassers für seine Vorfesungen; Christian Ernst Gabler: Jena, Germany, 1796. [Google Scholar]
- David, A. Rapport adressé à MM les Professeurs-Administrateurs du Muséum d’Histoire Naturelle. Nouv. Arch. Muséum D’histoire Nat. 1872, 7, 75–100. [Google Scholar]
- Sinsch, U.; Leskovar, C.; Drobig, A.; König, A.; Grosse, W.R. Life-history traits in green toad (Bufo viridis) populations: Indicators of habitat quality. Can. J. Zool. 2007, 85, 665–673. [Google Scholar] [CrossRef]
- Kissel, A.M.; Palen, W.J.; Ryan, M.E.; Adams, M.J. Compounding effects of climate change reduce population viability of a montane amphibian. Ecol. Appl. 2019, 29, e01832. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Ye, C.Y.; Jiang, J.P. Colored Atlas of Chinese Amphibians and Their Distributions; Sichuan Publishing House of Science & Technology: Chengdu, China, 2012. [Google Scholar]
- Fei, L.; Hu, S.Q.; Ye, C.Y.; Huang, Y.Z. Fauna Sinica. Amphibia, Vol. 3. Anura; Science Press: Beijing, China, 2009. [Google Scholar]
- Yu, T.L.; Li, H.J.; Lu, X. Mating patterns of Rana kukunoris from three populations along an altitudinal gradient on the Tibetan Plateau. Anim. Biol. 2013, 63, 131–138. [Google Scholar] [CrossRef]
- Qi, Y.; Felix, Z.; Dai, Q.; Wang, Y.; Wang, B.; Wang, Y.Z. Post-breeding movements, home range, and microhabitat use of plateau brown frog Rana kukunoris in Zoige Alpine Wetland. Curr. Zool. 2007, 6, 974–981. [Google Scholar]
- Qi, Y.; Felix, Z.; Dai, Q.; Wang, Y.; Liu, L.; Zhang, Q.; Wang, Y.Z. Activities of Rana kukunoris in summer and autumn around the seasonal pond in Zoige alpine peat land. Zool. Res. 2007, 28, 526–530. [Google Scholar]
- Qi, Y.; Felix, Z.; Wang, Y.; Gu, H.; Wang, Y. Postbreeding movement and habitat use of the plateau brown frog, Rana kukunoris, in a high-elevation Wetland. J. Herpetol. 2011, 45, 421–427. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, F.; Fu, J.; Wu, S.; Murphy, R.W.; Che, J.; Zhang, Y. River islands, refugia and genetic structuring in the endemic brown frog Rana kukunoris (Anura, Ranidae) of the Qinghai-Tibetan Plateau. Mol. Ecol. 2013, 22, 130–142. [Google Scholar] [CrossRef]
- Shen, H.J.; Xu, M.Y.; Yang, X.Y.; Chen, Z.; Xiao, N.W.; Chen, X.H. A new brown frog of the genus Rana (Anura, Ranidae) from North China, with a taxonomic revision of the R. chensinensis species group. Asian Herpetol. Res. 2022, 13, 145–158. [Google Scholar]
- Qi, Y.; Lu, B.; Gao, H.Y.; Hu, P.; Fu, J.Z. Hybridization and mitochondrial genome introgression between Rana chensinensis and R. kukunoris. Mol. Ecol. 2014, 23, 5575–5588. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yu, T.L.; Lu, X. Age and body size of Rana kukunoris, a high-elevation frog native to the Tibetan plateau. J. Herpetol. 2011, 21, 149–151. [Google Scholar]
- Feng, X.Y.; Chen, W.; Hu, J.H.; Jiang, J.P. Variation and sexual dimorphism of body size in the plateau brown frog along an altitudinal gradient. Asian Herpetol. Res. 2015, 6, 291–297. [Google Scholar]
- Yu, T.L.; Jia, G.; Sun, H.Q.; Shi, W.H.; Li, X.L.; Wang, H.B.; Huang, M.R.; Ding, S.Y.; Chen, J.P.; Zhang, M. Altitudinal body size variation in Rana kukunoris: The effects of age and growth rate on the plateau brown frog from the eastern Tibetan Plateau. Ethol. Ecol. Evol. 2021, 34, 120–132. [Google Scholar] [CrossRef]
- Leung, K.W.; Yang, S.N.; Wang, X.Y.; Tang, K.; Hu, J.H. Ecogeographical adaptation revisited: Morphological variations in the plateau brown frog along an elevation gradient on the Qinghai-Tibetan Plateau. Biology 2021, 10, 1081. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Z.; Qi, Y.; Bi, K.; Fu, J.Z. Toward understanding the genetic basis of adaptation to high-elevation life in poikilothermic species: A comparative transcriptomic analysis of two ranid frogs, Rana chensinensis and R. kukunoris. BMC Genom. 2012, 13, 588. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chen, H.Z.; Liao, J.H.; Tang, M.; Qin, H.F.; Zhao, Z.K.; Liu, X.Y.; Wu, Y.F.; Jiang, L.C.; Zhang, L.X.; et al. Chromosome-level genome assembly of a high-altitude-adapted frog (Rana kukunoris) from the Tibetan plateau provides insight into amphibian genome evolution and adaptation. Front. Zool. 2023, 20, 1–12. [Google Scholar] [CrossRef]
- Liao, W.B.; Lu, X. Age and growth of a subtropical high-elevation torrent frog, Amolops mantzorum, in Western China. J. Herpetol. 2010, 44, 172–176. [Google Scholar] [CrossRef]
- Hemelaar, A. An improved method to estimate the number of year rings resorbed in phalanges of Bufo bufo (L.) and its application to populations from different latitudes and altitudes. Amphibia-Reptilia 1985, 6, 323–341. [Google Scholar] [CrossRef]
- Guarino, F.M.; Erismis, U.C. Age determination and growth by skeletochronology of Rana holtzi, an endemic frog from Turkey. Ital. J. Zool. 2008, 75, 237–242. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Beckmann, M.; Václavík, T.; Manceur, A.M.; Šprtová, L.; von Wehrden, H.; Welk, E.; Cord, A.F. glUV: A global UV-B radiation data set for macroecological studies. Methods Ecol. Evol. 2014, 5, 372–383. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Blach-Overgaard, A.; Svenning, J.C.; Dransfield, J.; Greve, M.; Balslev, H. Determinants of palm species distributions across Africa: The relative roles of climate, non-climatic environmental factors, and spatial constraints. Ecography 2010, 33, 380–391. [Google Scholar] [CrossRef]
- Chevan, A.; Sutherland, M. Hierarchical partitioning. Am. Stat. 1991, 45, 90–96. [Google Scholar]
- Walsh, C.; MacNally, R. The Hier. Part Package. Hierarchical Partitioning. R Package Version 1.0-6. 2020. Available online: https://github.com/cran/hier.part (accessed on 29 March 2023).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: http://www.R-project.org/ (accessed on 26 March 2023).
- Yetman, C.A.; Mokonoto, P.; Ferguson, J.W.H. Conservation implications of the age/size distribution of Giant Bullfrogs (Pyxicephalus adspersus) at three peri-urban breeding sites. Herpetol. J. 2012, 22, 23–32. [Google Scholar]
- Johnson, M.D.; Sherry, T.W.; Holmes, R.T.; Marra, P.P. Assessing habitat quality for a migratory songbird wintering in natural and agricultural habitats. Conserv. Biol. 2006, 20, 1433–1444. [Google Scholar] [CrossRef]
- Patrelle, C.; Hjernquist, M.B.; Laurila, A.; Söderman, F.; Merilä, J. Sex differences in age structure, growth rate and body size of common frogs Rana temporaria in the subarctic. Polar Biol. 2012, 35, 1505–1513. [Google Scholar] [CrossRef]
- Mi, C.R.; Ma, L.; Yang, M.Y.; Li, X.H.; Meiri, S.; Roll, U.; Oskyrko, O.; Pincheira-Donoso, D.; Harvey, L.P.; Jablonski, D.; et al. Global Protected Areas as refuges for amphibians and reptiles under climate change. Nat. Commun. 2023, 14, 1389. [Google Scholar] [CrossRef]
- Chen, W.; Lu, X. Sex recognition and mate choice in male Rana Kukunoris. Herpetol. J. 2011, 21, 141–144. [Google Scholar]
- Pincheira-Donoso, D.; Hunt, J. Fecundity selection theory: Concepts and evidence. Biol. Rev. 2017, 92, 341–356. [Google Scholar] [CrossRef]
- Monnet, J.M.; Cherry, M.I. Sexual size dimorphism in anurans. Proc. R. Soc. Lond. B. 2002, 269, 2301–2307. [Google Scholar] [CrossRef]
- Sinsch, U.; Pelster, B.; Ludwig, G. Large-scale variation of size- and age-related life-history traits in the common frog: A sensitive test case for macroecological rules. J. Zool. 2015, 297, 32–43. [Google Scholar] [CrossRef]
- Dai, Q.; Dai, J.H.; Zhang, J.D.; Yang, Y.; Zhang, M.; Li, C.; Liu, Z.J.; Gu, H.J.; Wang, Y.Z. Terrestrial core habitat of three anurans in Zoige Wetland Nature Reserve. Acta Eco. Sin. 2005, 25, 2256–2262. [Google Scholar]
- Sohal, R.S. The rate of living theory: A contemporary interpretation; Collatz, K.G., Sohal, R.S., Eds.; Springer: Berlin, Germany, 1986. [Google Scholar]
- Brys, K.; Vanfleteren, J.R.; Braeckman, B.P. Testing the rate-of-living/oxidative damage theory of aging in the nematode model Caenorhabditis elegans. Exp. Gerontol. 2007, 42, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Turbill, C.; Bieber, C.; Ruf, T. Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc. R. Soc. Lond. B. 2011, 278, 3355–3363. [Google Scholar] [CrossRef] [PubMed]
- Stark, G.; Meiri, S. Cold and dark captivity: Drivers of amphibian longevity. Glob. Ecol. Biogeogr. 2018, 27, 1384–1397. [Google Scholar] [CrossRef]
- Healy, K.; Guillerme, T.; Finlay, S.; Kane, A.; Kelly, S.B.A.; McClean, D.; Kelly, D.J.; Donohue, I.; Jackson, A.L.; Cooper, N. Ecology and mode-of-life explain lifespan variation in birds and mammals. Proc. R. Soc. Lond. B 2014, 281, 20140298. [Google Scholar] [CrossRef] [PubMed]
- Laugen, A.T.; Laurila, A.; Jönsson, K.I.; Söderman, F.; Merilä, J. Do common frogs (Rana temporaria) follow Bergmann’s rule? Evol. Ecol. Res. 2005, 7, 717. [Google Scholar]
- Bergmann, C. Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Gött. Stud. 1847, 1, 595–708. [Google Scholar]
- Zhao, J.Y. Larval survival, growth and development of the alpine frog (Rana kukunoris) and associated ecological factors in Northwestern Sichuan, China. PhD. Thesis, Nanjing University, Nanjing, China, 2018. [Google Scholar]
- Verschooren, E.; Brown, R.K.; Vercammen, F.; Pereboom, J. Ultraviolet B radiation (UV-B) and the growth and skeletal development of the Amazonian milk frog (Trachycephalus resinifictrix) from metamorphis. J. Physiol. Pathophysiol. 2011, 2, 34–42. [Google Scholar]
- Zhou, S.; Yu, B.F.; Zhang, Y. Global concurrent climate extremes exacerbated by anthropogenic climate change. Sci. Adv. 2023, 9, eabo1638. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, Y.M.; Ji, P.; Wu, P.L.; Sheffield, J.; Otkin, J.A. A global transition to flash droughts under climate change. Science 2023, 380, 187–191. [Google Scholar] [CrossRef] [PubMed]

| Age | Male | Female | Z | P | Z | P | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | SVL (mm) (Mean ± SD) | BM (g) (Mean ± SD) | n | SVL (mm) (Mean ± SD) | BM (g) (Mean ± SD) | SVL | BM | |||
| 2 | 10 | 46.2 ± 2.8 (41.9–50.6) | 8.2 ± 2.4 (5.1–12.6) | 5 | 43.8 ± 1.6 (41.0–44.8) | 6.9 ± 0.8 (6.2–8.0) | −1.35 | 0.18 | −1.10 | 0.27 |
| 3 | 15 | 51.8 ± 4.3 (43.9–59.6) | 11.0 ± 2.9 (6.3–16.1) | 19 | 53.7 ± 5.5 (41.5–62.9) | 12.0 ± 3.3 (6.1–18.8) | −1.23 | 0.22 | −0.64 | 0.52 |
| 4 | 0 | / | / | 28 | 60.7 ± 3.4 (52.6–69.1) | 15.4 ± 2.7 (9.2–19.2) | / | / | / | / |
| 5 | 0 | / | / | 13 | 62.5 ± 3.2 (58.2–69.4) | 18.0 ± 3.1 (13.9–24.2) | / | / | / | / |
| 6 | 0 | / | / | 1 | 63.4 | 16.8 | / | / | / | / |
| 7 | 0 | / | / | 1 | 56.6 | 13.9 | / | / | / | / |
| 8 | 0 | / | / | 1 | 66.2 | 15.8 | / | / | / | / |
| Total | 25 | 49.6 ± 4.6 (41.9–59.6) | 9.9 ± 3.0 (5.1–16.1) | 68 | 57.9 ± 6.6 (41.0–69.4) | 14.3 ± 4.1 (6.1–24.2) | −5.10 | 0.00 | −4.49 | 0.00 |
| Environmental Variables | Average Age | ASM | Average SVL | |||
|---|---|---|---|---|---|---|
| Male (n = 24) | Female (n = 22) | Male (n = 24) | Female (n = 21) | Male (n = 29) | Female (n = 27) | |
| Elevation | 0.54 ** | 0.17 | 0.68 *** | 0.60 ** | −0.25 | −0.34 |
| Annual mean temperature | −0.45 * | −0.38 | −0.48 * | −0.68 ** | 0.07 | 0.06 |
| Mean monthly temperature range | 0.20 | 0.16 | 0.30 | 0.72 *** | −0.22 | −0.40 * |
| Isothermality | 0.47 * | −0.05 | 0.65 ** | 0.61 ** | −0.26 | −0.43 * |
| Annual precipitation | 0.23 | −0.21 | 0.32 | −0.37 | −0.20 | −0.11 |
| Annual mean UV-B | 0.35 | 0.20 | 0.54 ** | 0.52 * | 0.09 | −0.17 |
| Sex | Life History Traits | Full Model (r2) | Annual Mean Temperature | Mean Monthly Temperature Range | Isothermality | Annual Precipitation | Annual Mean UV-B |
|---|---|---|---|---|---|---|---|
| Male | Average age | 0.44 | 34.87 | 9.15 | 27.91 | 16.80 | 11.28 |
| ASM | 0.64 ** | 16.51 | 10.71 | 33.40 | 21.21 | 18.17 | |
| SVL | 0.57 ** | 3.77 | 30.36 | 21.84 | 30.71 | 13.32 | |
| Average age | 0.21 | 57.32 | 8.72 | 11.73 | 10.54 | 11.68 | |
| Female | ASM | 0.69 ** | 29.11 | 27.61 | 21.71 | 9.75 | 11.83 |
| SVL | 0.45 * | 5.97 | 40.28 | 29.82 | 20.66 | 3.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Li, C.; Yan, P.; Dong, B.; Jiang, J. Age Structure and Body Size of the Plateau Brown Frog (Rana kukunoris) in the Jiuzhaigou National Nature Reserve and Potential Climatic Impacts on Its Life History Variations. Animals 2023, 13, 3654. https://doi.org/10.3390/ani13233654
Zhang M, Li C, Yan P, Dong B, Jiang J. Age Structure and Body Size of the Plateau Brown Frog (Rana kukunoris) in the Jiuzhaigou National Nature Reserve and Potential Climatic Impacts on Its Life History Variations. Animals. 2023; 13(23):3654. https://doi.org/10.3390/ani13233654
Chicago/Turabian StyleZhang, Meihua, Cheng Li, Peng Yan, Bingjun Dong, and Jianping Jiang. 2023. "Age Structure and Body Size of the Plateau Brown Frog (Rana kukunoris) in the Jiuzhaigou National Nature Reserve and Potential Climatic Impacts on Its Life History Variations" Animals 13, no. 23: 3654. https://doi.org/10.3390/ani13233654




