Effects of Dietary Folic Acid Supplementation on Sex Differences in Oriental River Prawn, Macrobrachium nipponense
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Prawn and Feeding Protocol
2.2. Diet Preparation
2.3. Sample Collection and Biochemical Analysis
2.4. Resistance of Prawn to the Thermal Stress
2.5. Transcriptomic Profiling Analysis
2.6. Quantitative Real-Time PCR (qRT-PCR) Verification
2.7. Statistical Analysis
2.8. Ethics Approval
3. Results
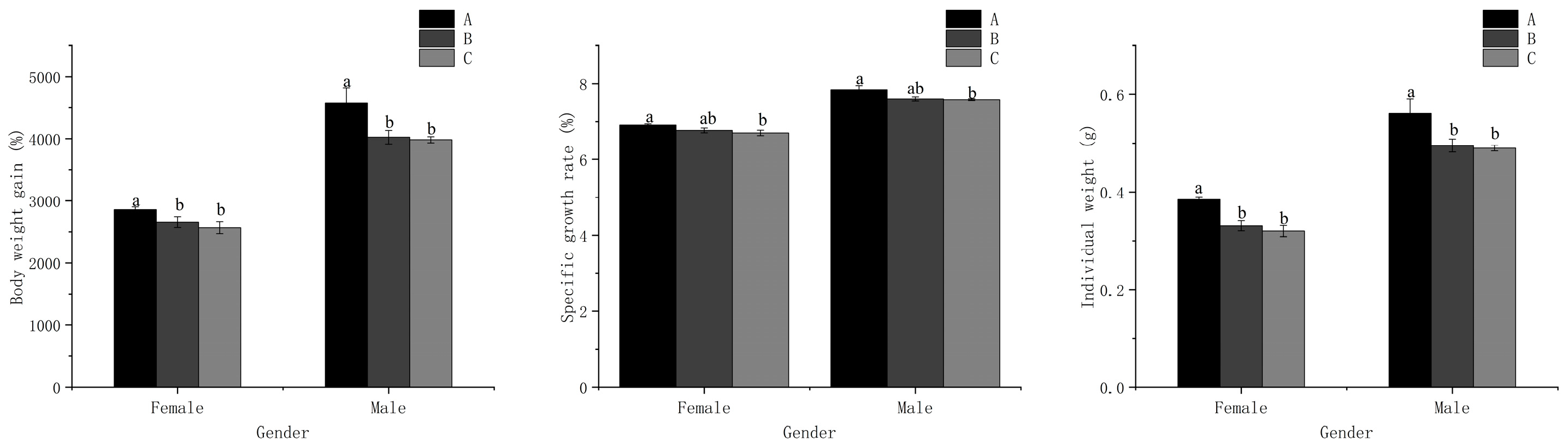
3.1. Growth Performance and Sex Differentiation
3.2. Biochemical Analysis of Prawn
3.3. Digestive Enzyme Activities of Hepatopancreas
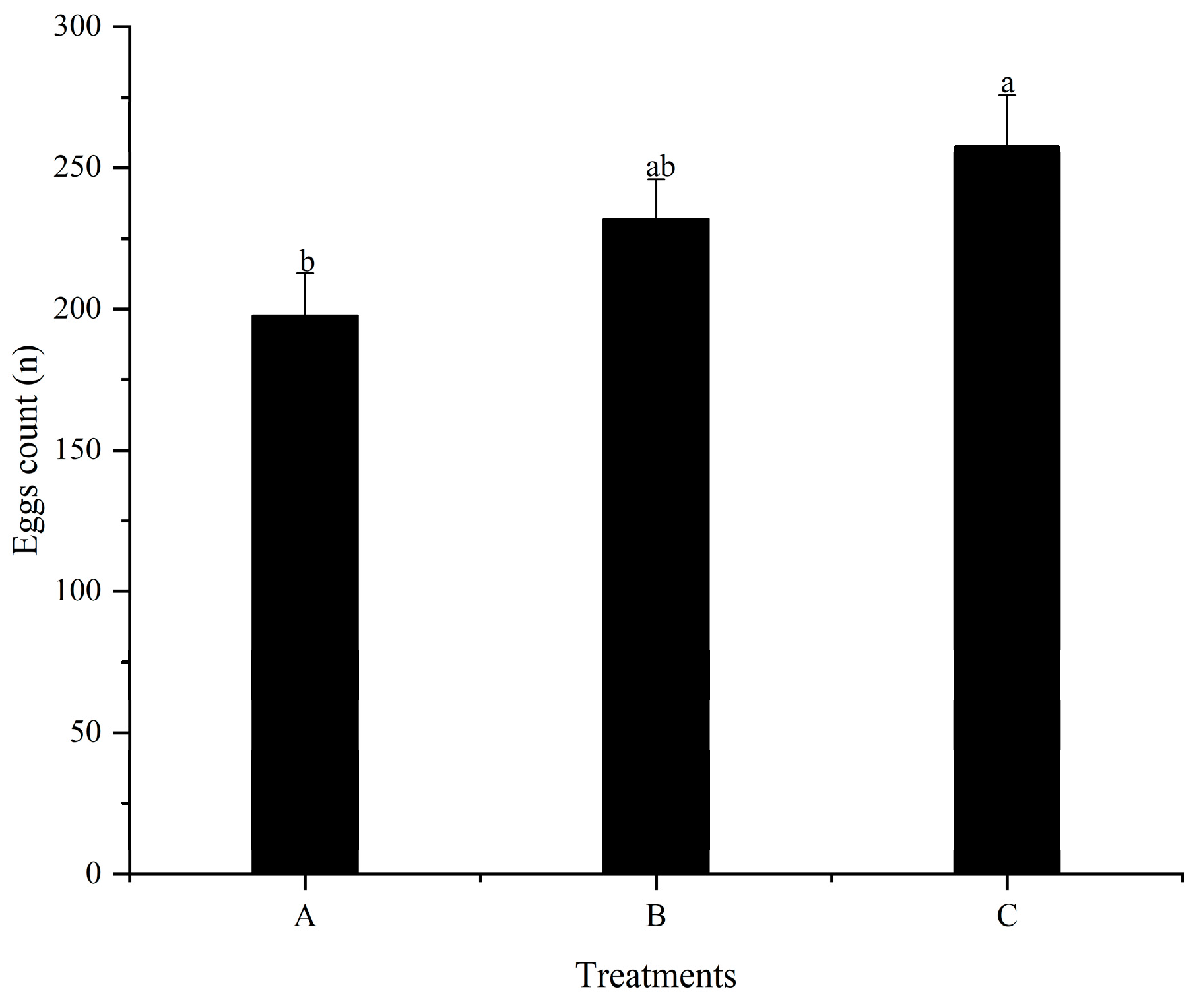
3.4. The First Spawning Time and Fecundity
3.5. Survival and Antioxidant Activity in Hepatopancreas upon a Challenge with Thermal Stress
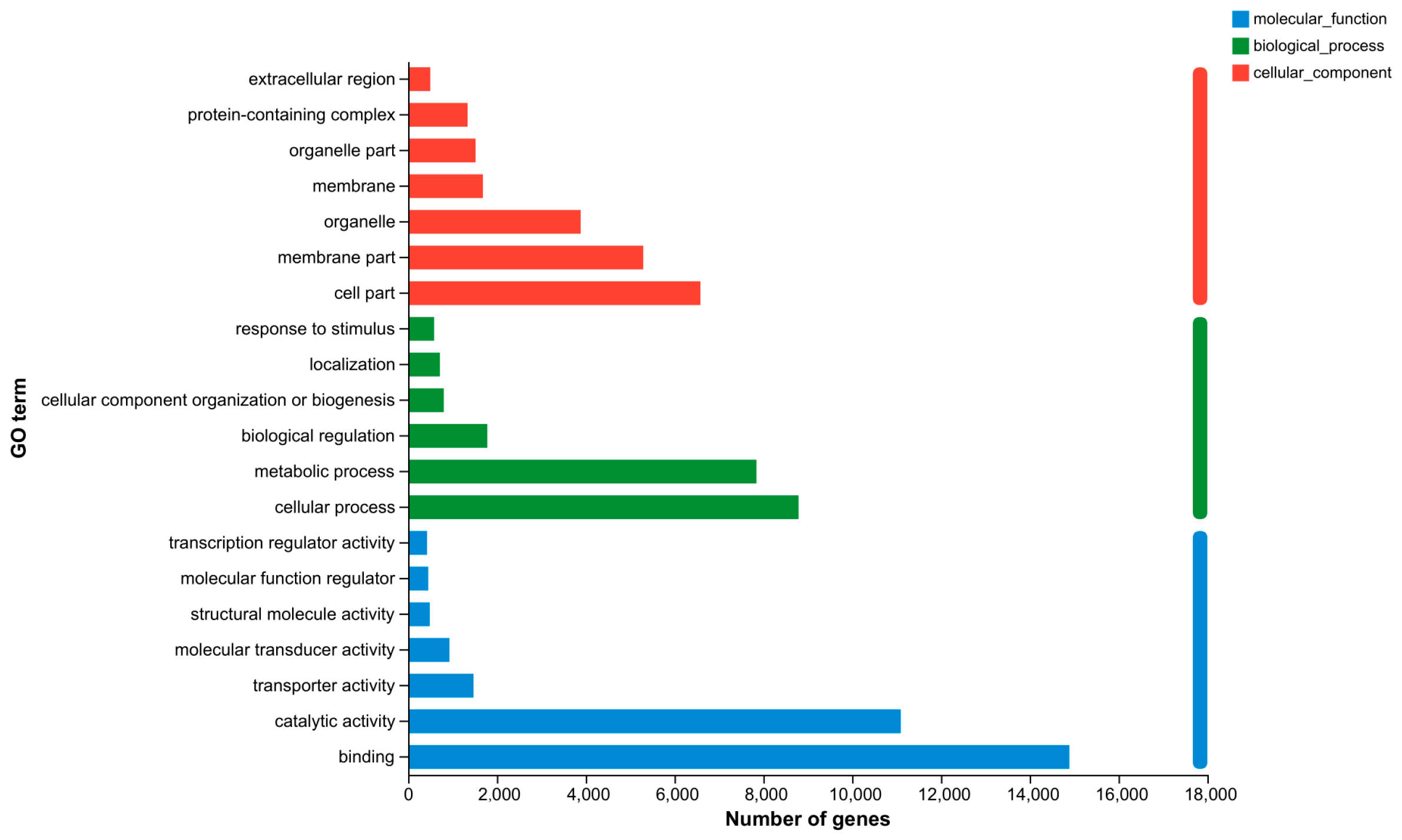
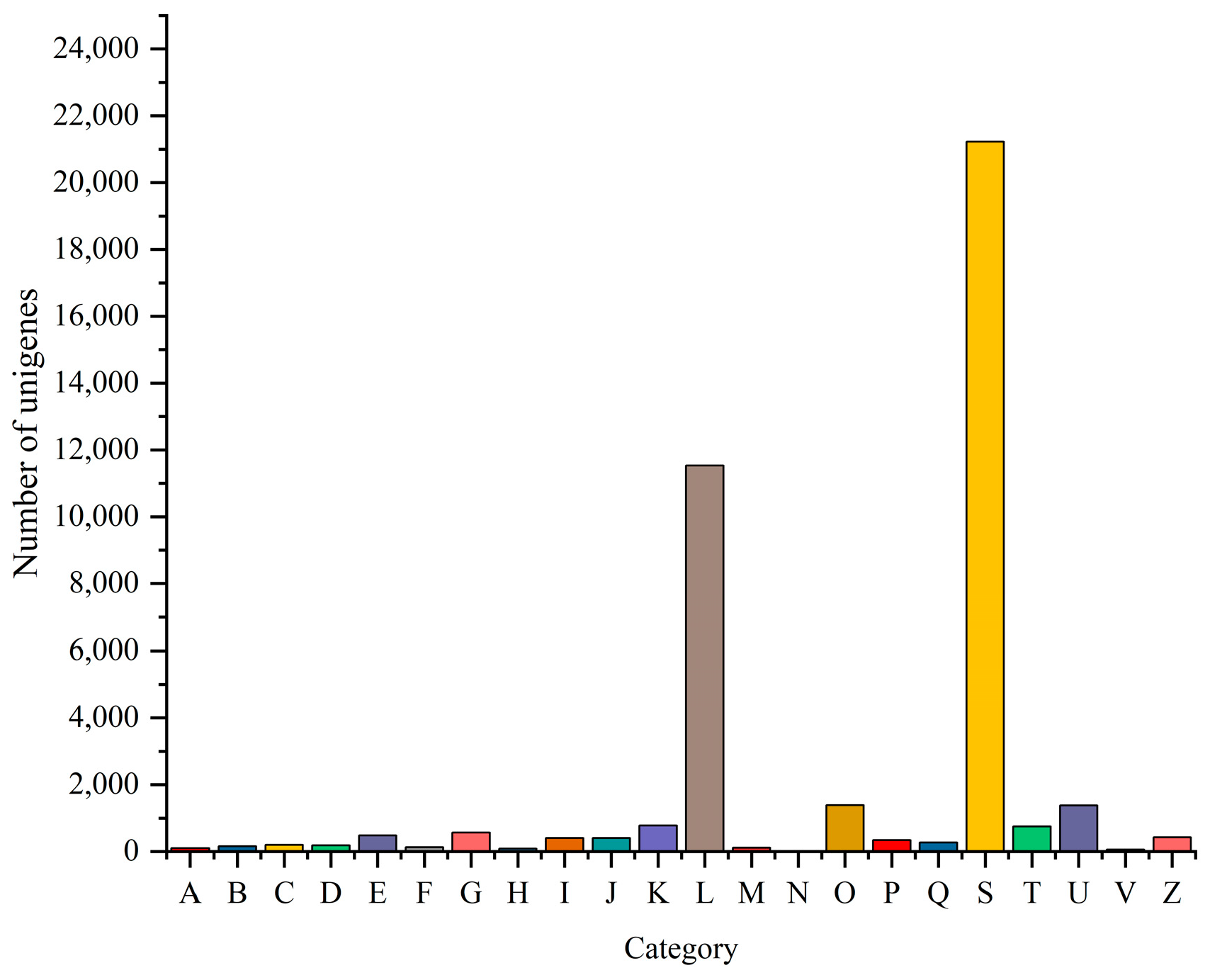
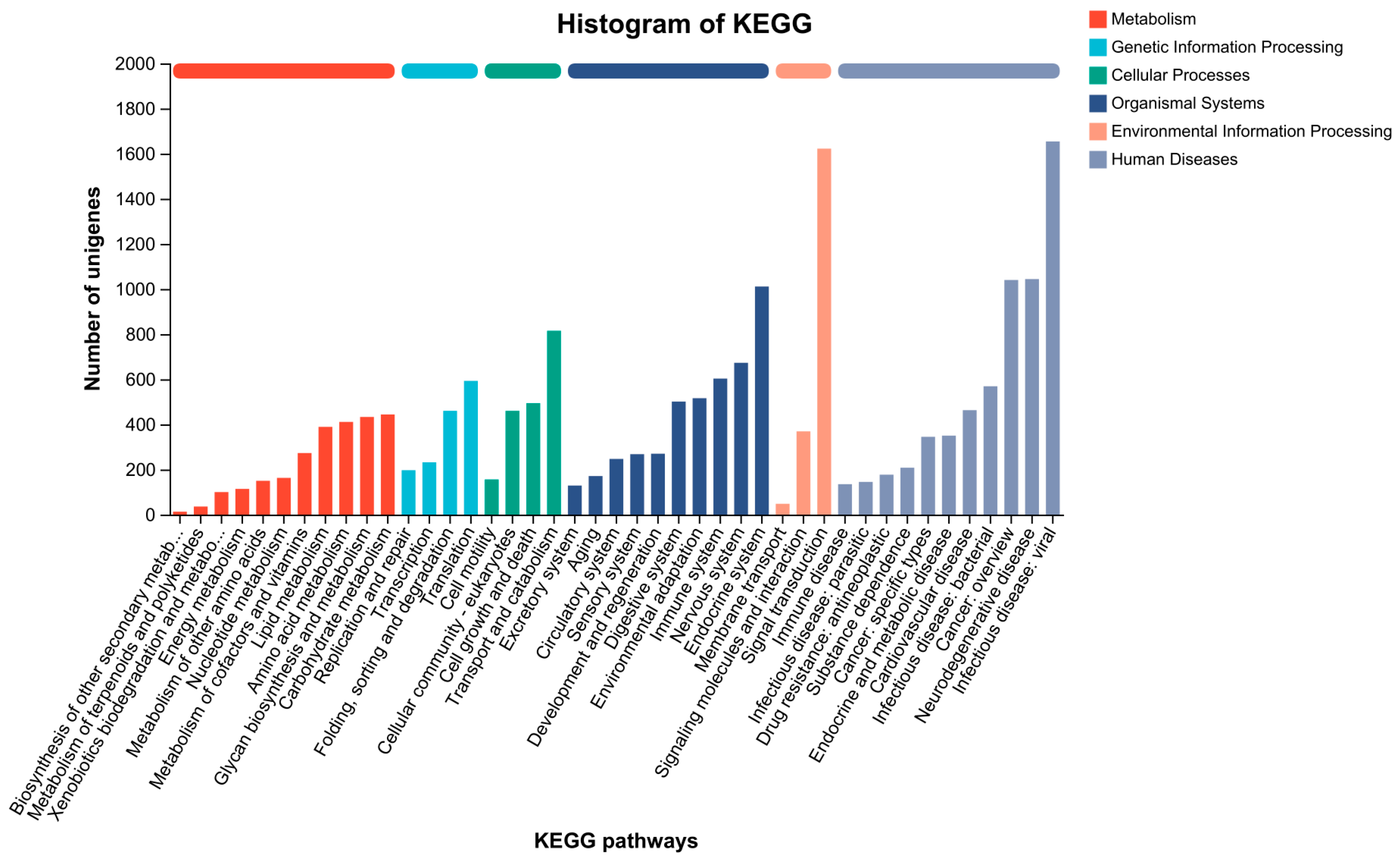
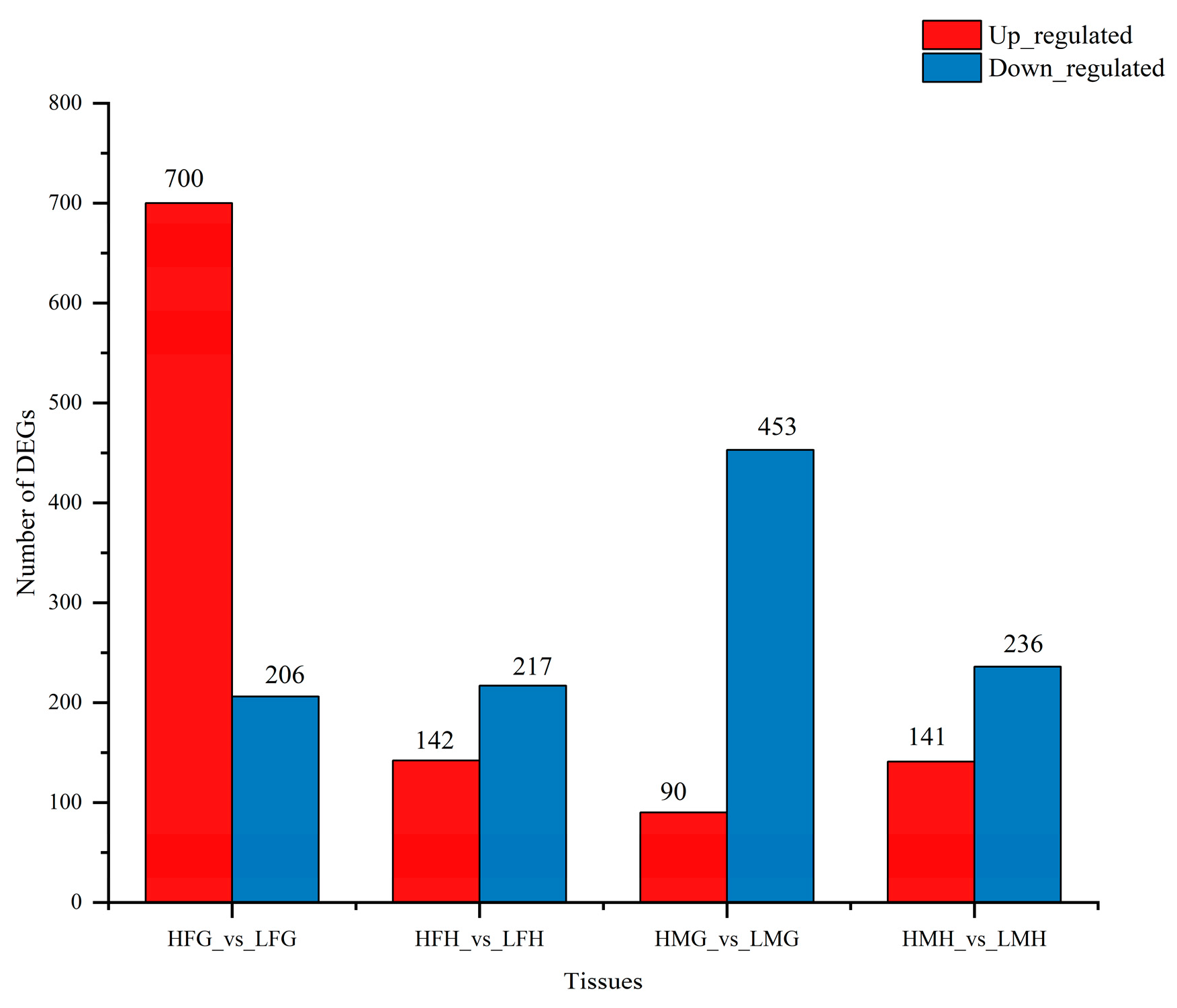
3.6. Transcriptome Analysis
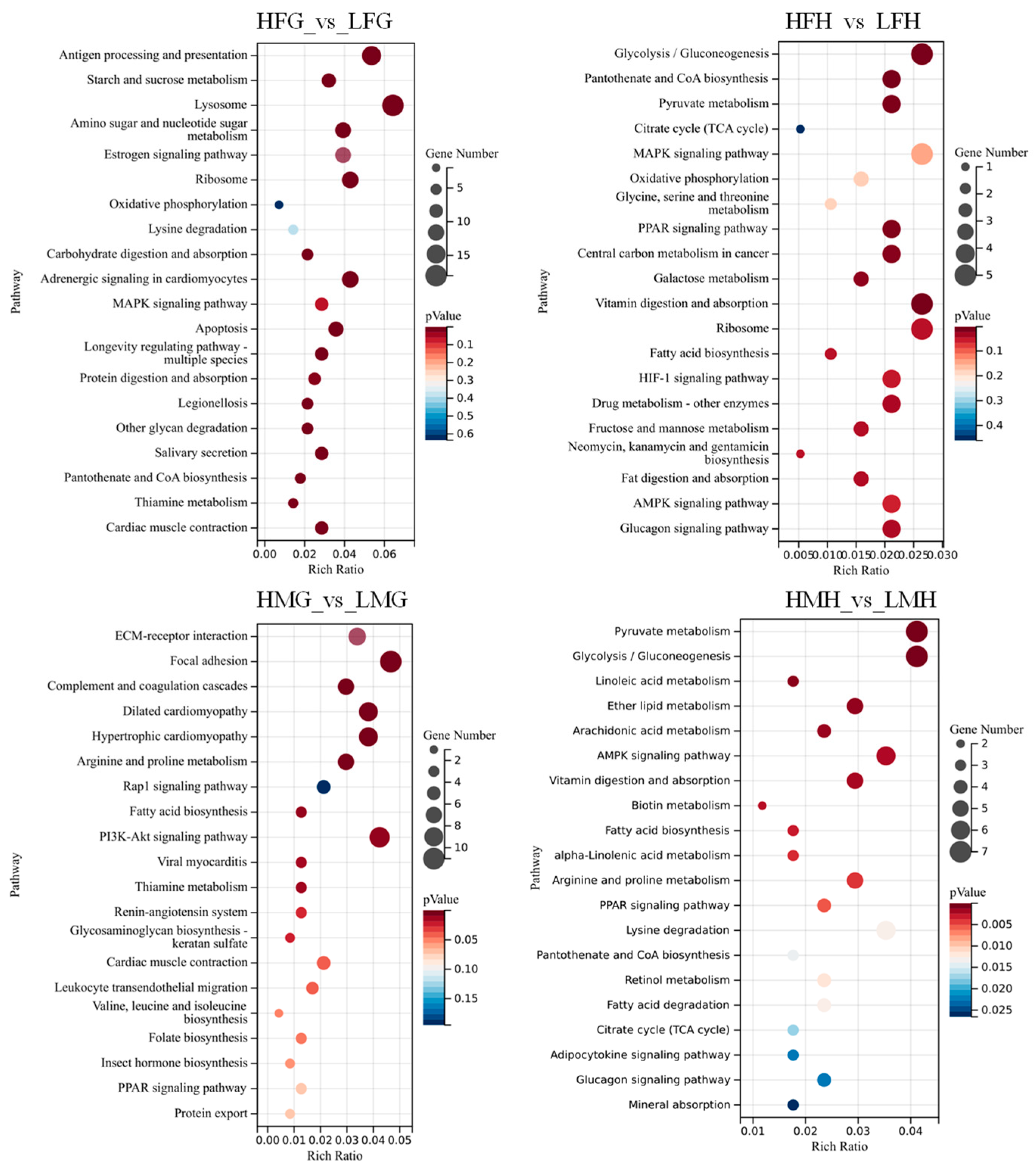
3.7. Analysis of the Intrinsic Regulatory Process of Folic Acid in Male and Female Prawns under Thermal Stress
3.8. qRT-PCR Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, Y.X.; Ng, P.K.L. The freshwater palaemonid prawns (Crustacea: Decapoda: Caridea) of Myanmar. Hydrobiologia 2002, 487, 59–83. [Google Scholar] [CrossRef]
- Fu, H.T.; Jiang, S.F.; Xiong, Y.W. Current status and prospects of farming the giant river prawn (Macrobrachium rosenbergii) and the oriental river prawn (Macrobrachium nipponense) in China. Aquac. Res. 2012, 43, 993–998. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Chen, L.Q.; Qin, J.G.; Zhao, W.H.; Wu, P.; Yu, N.; Ma, L.B. cDNA Cloning and Expression Analysis of Gustavus Gene in the Oriental River Prawn Macrobrachium nipponense. PLoS ONE 2011, 6, e17170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, S.; Qiao, H.; Xiong, Y.; Fu, H.; Zhang, W.; Gong, Y.; Jin, S.; Wu, Y. Transcriptome analysis of five ovarian stages reveals gonad maturation in female updates Macrobrachium nipponense. BMC Genom. 2021, 22, 510. [Google Scholar] [CrossRef]
- Ma, K.-Y.; Liu, Z.-Q.; Lin, J.-Y.; Li, J.-L.; Qiu, G.-F. Molecular characterization of a novel ovary-specific gene fem-1 homolog from the oriental river prawn, Macrobrachium nipponense. Gene 2016, 575, 244–252. [Google Scholar] [CrossRef]
- Ma, K.; Feng, J.; Lin, J.; Li, J. The complete mitochondrial genome of Macrobrachium nipponense. Gene 2011, 487, 160–165. [Google Scholar] [CrossRef]
- Tacon, A.G.J. Nutritional studies in crustaceans and the problems of applying research findings to practical farming systems. Aquac. Nutr. 1996, 2, 165–174. [Google Scholar] [CrossRef]
- Conceicao, L.E.C.; Grasdalen, H.; Ronnestad, I. Amino acid requirements of fish larvae and post-larvae: New tools and recent findings. Aquaculture 2003, 227, 221–232. [Google Scholar] [CrossRef]
- Alhoshy, M.; Shehata, A.I.; Habib, Y.J.; Abdel-Latif, H.M.R.; Wang, Y.L.; Zhang, Z.P. Nutrigenomics in crustaceans: Current status and future prospects. Fish. Shellfish. Immun. 2022, 129, 1–12. [Google Scholar] [CrossRef]
- Wu, X.; Cheng, Y.; Sui, L.; Yang, X.; Nan, T.; Wang, J. Biochemical composition of pond-reared and lake-stocked Chinese mitten crab Eriocheir sinensis (H. Milne-Edwards) broodstock. Aquac. Res. 2007, 38, 1459–1467. [Google Scholar] [CrossRef]
- Alejandro, Y.J.; Gabriel, M.S.X.; Oscar, R.M.; Carrasco-Chavez, V.; Luis, O.J. Feeding habits of Paralabrax nebulifer (Serranidae) during reproductive and non-reproductive seasons in an adjacent area to Magdalena Bay, Baja California Sur, Mexico. Revista Biología Marina Oceanografía 2021, 56, 89–101. [Google Scholar] [CrossRef]
- Badran, M.F.; Ali, M. Effects of folic acid on growth performance and blood parameters of flathead grey mullet, Mugil cephalus. Aquaculture 2021, 536, 736459. [Google Scholar] [CrossRef]
- Bailey, L.B.; Gregory, J.F. Folate metabolism and requirements. J. Nutr. 1999, 129, 779–782. [Google Scholar] [CrossRef]
- Zehra, S.; Khan, M.A. Dietary folic acid requirement of fingerling Channa punctatus (Bloch) based on growth, protein productive value and liver folic acid concentrations-ScienceDirect. Anim. Feed. Sci. Technol. 2020, 262, 114397. [Google Scholar] [CrossRef]
- Stokstad, E.L.; Koch, J. Folic Acid Metabolism. Physiol. Rev. 1967, 47, 83–116. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Zhang, T.; Zhao, J.; Feng, J. Study on extraction conditions and antioxygenic activity of folic acid. Mod. Food Sci. Technol. 2011, 27, 1234–1237. [Google Scholar]
- Annamalai, A.; Saravana Bhavan, P.; Karuppaiya, V. Effects of different levels of dietary folic acid on the growth performance, muscle composition, immune response and antioxidant capacity of freshwater prawn, Macrobrachium rosenbergii. Aquaculture 2016, 464, 136–144. [Google Scholar] [CrossRef]
- Dietary folic acid requirement determined for grass shrimp, Penaeus monodon. Aquaculture. 2001, 200, 339–347. [CrossRef]
- Liu, T.Z.; Li, A.J. Studies on the optimal requirements of pantothenic acid, biotin, folic acid and vitamin B12 in the shrimp Panaeus chinensis. J. Fish. Sci. China 1995, 2, 48–55. [Google Scholar]
- Wei, J.J.; Zhang, F.; Tian, W.J.; Kong, Y.Q.; Li, Q.; Yu, N.; Du, Z.Y.; Wu, Q.Q.; Qin, J.G.; Chen, L.Q. Effects of dietary folic acid on growth, antioxidant capacity, non-specific immune response and disease resistance of juvenile Chinese mitten crab Eriocheir sinensis (Milne-Edwards, 1853). Aquac. Nutr. 2016, 22, 567–574. [Google Scholar] [CrossRef]
- Jiang, G.; Zhu, Y.; Xue, Y.; Lu, Y.; Liu, Z.; Huang, X. Screening and Analyzing of Genes and Signaling Pathways Associated with Size Differentiation of Adult Male Prawn Macrobrachium nipponense. Aquac. Res. 2023, 2023, 3134932. [Google Scholar] [CrossRef]
- Shiau, S.Y. Nutrient requirements of penaeid shrimps. Aquaculture 1998, 164, 77–93. [Google Scholar] [CrossRef]
- New, M.; Valenti, W.; Tidwell, J.; D’Abramo, L.; Kutty, M. Freshwater Prawns: Biology and Farming; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 84, pp. 509–511. [Google Scholar]
- Cunniff, P. Official methods of analysis of AOAC international. Volume I agricultural chemicals, contaminants, drugs. Volume II Food composition, additives, natural contaminants. Gaithersburg Md Aoac Int. Append. D 1995, 6, 382. [Google Scholar]
- Cejas, J.R.; Almansa, E.; Jerez, S.; Bolanos, A.; Felipe, B.; Lorenzo, A. Changes in lipid class and fatty acid composition during development in white seabream (Diplodus sargus) eggs and larvae. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2004, 139, 209–216. [Google Scholar] [CrossRef]
- Huang, X.; Feng, L.; Wen, W.; Chen, Q.; Wei, L. The changes in lipid and fatty acid profiles of devil stinger Inimicus japonicas during the development of embryo and yolk-sac larvae. J. Fish. China 2013, 37, 526. [Google Scholar] [CrossRef]
- Laiqiang, L.; Weilong, W.; Abdullateef, Y.; Yongming, Z.; Yue, Z.; Peng, J.; Xuxiong, H. Effects of dietary lipid levels on the growth, fatty acid profile and fecundity in the oriental river prawn, Macrobrachium nipponense. Aquac. Res. 2020, 51, 1893–1902. [Google Scholar] [CrossRef]
- Ca, A.; Ww, A.; Hl, A.; Yue, Z.A.; Xhab, C. The effect of dietary supplementation of Astragalus membranaceus and Bupleurum chinense on the growth performance, immune-related enzyme activities and genes expression in white shrimp, Litopenaeus vannamei. Fish. Shellfish. Immun. 2020, 107, 379–384. [Google Scholar]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Jin, S.; Fu, H.; Zhou, Q.; Sun, S.; Jiang, S.; Xiong, Y.; Gong, Y.; Qiao, H.; Zhang, W. Transcriptome Analysis of Androgenic Gland for Discovery of Novel Genes from the Oriental River Prawn, Macrobrachium nipponense, Using Illumina Hiseq 2000. PLoS ONE 2013, 8, e76840. [Google Scholar] [CrossRef]
- Kwong, K.S.; Holland, B.; Cheung, S.H. A modified Benjamini-Hochberg multiple comparisons procedure for controlling the false discovery rate. J. Stat. Plan. Inference 2002, 104, 351–362. [Google Scholar] [CrossRef]
- Jin, S.; Fu, Y.; Hu, Y.; Fu, H.; Jiang, S.; Xiong, Y.; Qiao, H.; Zhang, W.; Gong, Y.; Wu, Y. Identification of candidate genes from androgenic gland in Macrobrachium nipponense regulated by eyestalk ablation. Sci. Rep. 2021, 11, 19855. [Google Scholar] [CrossRef] [PubMed]
- Sesay, D.F.; Habte-Tsion, H.M.; Zhou, Q.L.; Ren, M.C.; Xie, J.; Liu, B.; Chen, R.L.; Pan, L.K. Effects of dietary folic acid on the growth, digestive enzyme activity, immune response and antioxidant enzyme activity of blunt snout bream (Megalobrama amblycephala) fingerling. Aquaculture 2016, 452, 142–150. [Google Scholar] [CrossRef]
- Jing, M.; Munyaka, P.M.; Tactacan, G.B.; Rodriguez-Lecompte, J. Performance, serum biochemical responses, and gene expression of intestinal folate transporters of young and older laying hens in response to dietary folic acid supplementation and challenge with Escherichia coli lipopolysaccharide. Poult. Sci. 2014, 93, 122–131. [Google Scholar] [CrossRef]
- Gómez-Requeni, P.; Bedolla-Cázares, F.; Montecchia, C.; Zorrilla, J.; Villian, M.; Toledo-Cuevas, E.M.; Canosa, F. Effects of increasing the dietary lipid levels on the growth performance, body composition and digestive enzyme activities of the teleost pejerrey (Odontesthes bonariensis). Aquaculture 2013, 416, 15–22. [Google Scholar] [CrossRef]
- Wu, X.G.; Wang, Z.K.; Cheng, Y.X.; Zeng, C.S.; Yang, X.Z.; Lu, J.F. Effects of dietary phospholipids and highly unsaturated fatty acids on the precocity, survival, growth and hepatic lipid composition of juvenile Chinese mitten crab, Eriocheir sinensis (H.Milne-Edwards). Aquac. Res. 2011, 42, 457–468. [Google Scholar] [CrossRef]
- Stryer, L. Biochemistry, 3rd ed.; WH Freeman: New York, NY, USA, 1988. [Google Scholar]
- Yamashita, M.; Konagaya, S. The elevation of catheptic activity in muscle and liver of Ayu Plecoglossus altivelis during maturation. Nippon. Suisan Gakkaishi 1990, 56, 1157. [Google Scholar] [CrossRef]
- Matte, J.J.; Girard, C.L.; Brisson, G.J. Folic acid and reproductive performances of sows. J. Anim. Sci. 1984, 59, 1020–1025. [Google Scholar] [CrossRef]
- Tremblay, G.F.; Matte, J.J.; Dufour, J.J.; Brisson, G.J. Survival rate and development of fetuses during the first 30 days of gestation after folic acid addition to a swine diet. J. Anim. Sci. 1989, 67, 724–732. [Google Scholar] [CrossRef]
- Xu, X.L.; Ji, W.J.; Castell, J.D.; O’Dor, R.K. Influence of dietary lipid sources on fecundity, egg hatchability and fatty acid composition of Chinese prawn (Penaeus chinensis) broodstock. Aquaculture 1994, 119, 359–370. [Google Scholar] [CrossRef]
- Wouters, R.; Lavens, P.; Nieto, J.; Sorgeloos, P. Penaeid shrimp broodstock nutrition: An updated review on research and development. Aquaculture 2001, 202, 1–21. [Google Scholar] [CrossRef]
- Palacios, E.; Perez-Rostro, C.I.; Ramirez, J.L.; Ibarra, A.M.; Racotta, I.S. Reproductive exhaustion in shrimp (Penaeus vannamei) reflected in larval biochemical composition, survival and growth. Aquaculture 1999, 171, 309–321. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lin, H.Y.; Shiau, S.Y. Dietary folic acid requirement of grouper, Epinephelus malabaricus, and its effects on non-specific immune responses. Aquaculture 2012, 317, 133–137. [Google Scholar] [CrossRef]
- Shi, L.D.; Zhai, H.J.; Wei, L.B.; Zeng, F.S.; Ren, T.J.; Han, Y.Z. Effects of dietary folic acid on growth performance, body wall folate content, immune and antioxidant enzyme activity of juvenile sea cucumber, Apostichopus japonicus. Aquac. Res. 2022, 53, 6219–6226. [Google Scholar] [CrossRef]
- Hang, Y.; Hua, X.M.; Li, X.; Yi, W.T.; Cong, X.M. Effect of dietary levels of folic acid on growth performance, blood biochemistry, antioxidant capacity, and immunity of juvenile largemouth bass, Micropterus salmoides. J. World Aquac. Soc. 2022, 53, 836–847. [Google Scholar] [CrossRef]
- Wu, J.H.; Chen, X.; Shan, C.Y.; Tan, R.X. Antioxidant properties and PC12 cell protective effects of APS-1, a polysaccharide from Aloe vera var. chinensis. Life Sci. 2006, 78, 622–630. [Google Scholar] [CrossRef]
- Liang, X.-P.; Li, Y.; Hou, Y.-M.; Qiu, H.; Zhou, Q.-C. Effect of dietary vitamin C on the growth performance, antioxidant ability and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco Richardson). Aquac. Res. 2015, 48, 11–19. [Google Scholar]
- To, V.A.; Liou, C.H.; Yang, S.D. Can dietary with a taurine supplement improve lipid utilization, growth performance, haemolymph parameters and immune responses of white shrimp (Litopenaeus vannamei)? Aquac. Res. 2021, 52, 6612–6625. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Yao, R.; Hu, Y.; Li, S. Dietary supplementary glutamine and L-carnitine enhanced the anti-cold stress of Arbor Acres broilers. Arch. Anim. Breed. 2021, 64, 231–243. [Google Scholar] [CrossRef]
- Xu, Z.; Gan, L.; Li, T.; Xu, C.; Chen, K.; Wang, X.; Qin, J.G.; Chen, L.; Li, E. Transcriptome Profiling and Molecular Pathway Analysis of Genes in Association with Salinity Adaptation in Nile Tilapia Oreochromis niloticus. PLoS ONE 2015, 10, e0136506. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Dong, Y.; Ma, S.; Zhou, Y.; Ma, Z. Effects of dietary supplementation with freeze-dried powder of Ampithoe sp on the growth performance, energy metabolism, and ammonia-nitrogen tolerance of the Pacific white shrimp, Litopenaeus vannamei. Aquac. Res. 2018, 49, 2633–2643. [Google Scholar] [CrossRef]
- Cerenius, L.; Kawabata, S.-i.; Lee, B.L.; Nonaka, M.; Soderhall, K. Proteolytic cascades and their involvement in invertebrate immunity. Trends Biochem. Sci. 2010, 35, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Xu, S.; Chai, S.; Yin, D.; Zakon, H.; Yang, G. Stronger selective constraint on downstream genes in the oxidative phosphorylation pathway of cetaceans. J. Evol. Biol. 2018, 31, 217–228. [Google Scholar] [CrossRef]
- Ma, T.; Liu, T.; Xie, P.; Jiang, S.; Yi, W.; Dai, P.; Guo, X. UPLC-MS-based urine nontargeted metabolic profiling identifies dysregulation of pantothenate and CoA biosynthesis pathway in diabetic kidney disease. Life Sci. 2020, 258, 118160. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.L.; Hopkins, W.A.; Zehnder, C.; Congdon, J.D. Metabolic costs incurred by crayfish (Procambarus acutus) in a trace element-polluted habitat: Further evidence of similar responses among diverse taxonomic groups. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2001, 129, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Rokitta, S.D.; Von Dassow, P.; Rost, B.; John, U. Emiliania huxleyi endures N-limitation with an efficient metabolic budgeting and effective ATP synthesis. BMC Genom. 2014, 15, 1051. [Google Scholar] [CrossRef]
- Lu, Y.; Wohlrab, S.; Groth, M.; Gloeckner, G.; Guillou, L.; John, U. Transcriptomic profiling of Alexandrium fundyense during physical interaction with or exposure to chemical signals from the parasite Amoebophrya. Mol. Ecol. 2016, 25, 1294–1307. [Google Scholar] [CrossRef]




| Ingredients (%) | Contents |
|---|---|
| Flour a | 20.9 |
| Soybean meal a | 19 |
| Brown fish meal a | 19 |
| α-starch a | 11.9 |
| Peanut meal a | 7.6 |
| Chicken meal a | 3.8 |
| Corn protein powder a | 3.8 |
| Spray dried blood powder a | 2.8 |
| Beer yeast cell a | 2.0 |
| Monocalcium phosphate a | 2 |
| Squid paste a | 2 |
| Fish oil a | 1.5 |
| Mineral premix b | 1.5 |
| Vitamin premix c | 1 |
| Cellulose b | 0.5 |
| Soybean lecithin a | 0.4 |
| Choline chloride a | 0.3 |
| Total | 100 |
| Proximate compositions (%) | |
| Crude protein | 39.04 |
| Crude lipid | 6.37 |
| Ash | 9.35 |
| Moisture | 10.24 |
| Parameters | Treatments | ANOVA p-Value | ||
|---|---|---|---|---|
| A (1.22 mg/kg) | B (5.44 mg/kg) | C (10.09 mg/kg) | ||
| SR (%) | 96.25 ± 2.165 | 93.75 ± 3.608 | 92.50 ± 1.767 | 0.609 |
| Female/male ratio | 2.30 ± 0.229 | 2.15 ± 0.134 | 2.17 ± 0.225 | 0.798 |
| Parameters | Treatments | ANOVA p-Value | |||
|---|---|---|---|---|---|
| A (1.22 mg/kg) | B (5.44 mg/kg) | C (10.09 mg/kg) | |||
| Male | Moisture | 74.02 ± 0.057 | 74.04 ± 0.165 | 75.49 ± 1.564 | 0.356 |
| Ash | 15.71 ± 0.870 | 16.23 ± 0.794 | 16.61 ± 0.144 | 0.661 | |
| Crude protein | 67.11 ± 0.754 a | 64.21 ± 0.497 b | 64.53 ± 0.666 b | 0.037 | |
| Total lipid | 10.40 ± 0.180 a | 9.63 ± 0.385 a | 8.69 ± 0.633 b | 0.023 | |
| Female | Moisture | 73.27 ± 0.130 | 73.90 ± 0.465 | 73.94 ± 0.793 | 0.566 |
| Ash | 16.48 ± 0.487 | 15.33 ± 1.053 | 16.05 ± 0.748 | 0.612 | |
| Crude protein | 65.28 ± 1.484 | 62.79 ± 1.057 | 62.91 ± 1.262 | 0.364 | |
| Total lipid | 11.67 ± 0.534 a | 10.13 ± 1.892 b | 10.78 ± 0.187 b | 0.045 | |
| Parameters | Treatments | ANOVA p-Value | |||
|---|---|---|---|---|---|
| A (1.22 mg/kg) | B (5.44 mg/kg) | C (10.09 mg/kg) | |||
| Amylase | 0.96 ± 0.015 a | 0.69 ± 0.045 b | 0.52 ± 0.013 c | <0.001 | |
| Male | Lipase | 327.49 ± 5.663 a | 284.83 ± 2.414 b | 291.79 ± 2.702 b | 0.001 |
| Protease | 27.45 ± 1.068 c | 45.66 ± 1.627 a | 32.53 ± 0.204 b | <0.001 | |
| Amylase | 0.54 ± 0.011 | 0.48 ± 0.028 | 0.54 ± 0.010 | 0.079 | |
| Female | Lipase | 269.42 ± 4.453 a | 244.88 ± 2.247 b | 235.28 ± 2.241 b | 0.001 |
| Protease | 22.84 c ± 1.428 c | 26.98 ± 0.165 b | 33.54 ± 1.010 a | 0.001 | |
| Treatments | ANOVA p-Value | |||
|---|---|---|---|---|
| Parameters | A (1.22 mg/kg) | B (5.44 mg/kg) | C (10.09 mg/kg) | |
| Moisture | 722.46 ± 2.766 | 728.39 ± 9.229 | 713.77 ± 3.691 | 0.292 |
| Total protein | 35.81 ± 0.316 c | 36.86 ± 0.128 b | 38.69 ± 0.089 a | <0.001 |
| Total lipid | 51.19 ± 0.597 c | 53.66 ± 0.970 b | 56.48 ± 0.500 a | 0.006 |
| Parameters | Treatments | ANOVA p-Value | |||
|---|---|---|---|---|---|
| A (1.22 mg/kg) | B (5.44 mg/kg) | C (10.09 mg/kg) | |||
| Male | T-AOC (U/mL) | 0.32 ± 0.010 b | 0.28 ± 0.012 c | 0.37 ± 0.011 a | <0.001 |
| SOD (U/mL) | 98.63 ± 2.98 b | 106.89 ± 0.052 a | 107.27 ± 0.333 a | 0.02 | |
| CAT (U/mL) | 5.56 ± 0.241 b | 6.90 ± 0.526 a | 6.42 ± 0.207 ab | 0.045 | |
| MDA (mmol/mL) | 152.81 ± 3.510 a | 148.07 ± 1.384 b | 126.38 ± 0.642 c | <0.001 | |
| Female | T-AOC (U/mL) | 0.24 ± 0.013 c | 0.32 ± 0.014 b | 0.40 ± 0.012 a | <0.001 |
| SOD (U/mL) | 111.31 ± 2.16 b | 121.21 ± 1.40 a | 117.14 ± 0.882 a | 0.012 | |
| CAT (U/mL) | 7.10 ± 0.193 | 7.74 ± 0.285 | 7.31 ± 0.316 | 0.307 | |
| MDA (mmol/mL) | 134.91 ± 4.577 a | 131.80 ± 0.936 a | 117.31 ± 3.187 b | 0.019 | |
| Database | Number of Annotated Unigenes | % |
|---|---|---|
| Annotated in NR | 39,753 (0.922) | 92.20 |
| Annotated in SwissProt | 23,772 (0.5514) | 55.14 |
| Annotated in KEGG | 13,145 (0.3049) | 30.49 |
| Annotated in COG | 33,514 (0.7773) | 77.73 |
| Annotated in PFAM | 28,185 (0.6537) | 65.37 |
| Annotated in GO | 23,093 (0.5356) | 53.56 |
| Total annotation | 40,085 (0.9297) | 92.97 |
| Total unigenes | 43,115 | 100 |
| DEGs | Annotation | Tissues | Log2Foldchange |
|---|---|---|---|
| CTSA | cathepsin A (carboxypeptidase C) | HFG_vs._LFG | 3.11 |
| GLB1 | beta-galactosidase | HFG_vs._LFG | 3.68 |
| NPC2 | Niemann-Pick C2 protein | HFG_vs._LFG | 6.22 |
| HSP90A | molecular chaperone HtpG | HFG_vs._LFG | 0.81 |
| HSPA1 | heat shock 70 kDa protein | HFG_vs._LFG | 2.03 |
| ALDO | fructose-bisphosphate aldolase, class I | HFH_vs._LFH | −0.48 |
| PGAM | 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase | HFH_vs._LFH | −0.47 |
| HK | Hexokinase | HFH_vs._LFH | −1.26 |
| ENO | Enolase | HFH_vs._LFH | −0.46 |
| CACNA1B | voltage-dependent calcium channel N type alpha-1B | HFH_vs._LFH | −1.37 |
| HSPA1/6/8 | heat shock 70 kDa protein 1/6/8 | HFH_vs._LFH | 3.17 |
| VEGFB | vascular endothelial growth factor B | HMH_vs._LMH | −0.52 |
| LAMC1 | laminin, gamma 1 | HMH_vs._LMH | −3.15 |
| VEGFB | vascular endothelial growth factor B | HMH_vs._LMH | −0.52 |
| ITGA8 | integrin alpha 8 | HMH_vs._LMH | 0.03 |
| ACTB/G1 | integrin beta 5 (ITGB5) actin beta/gamma 1 | HMH_vs._LMH | −1.3 |
| RAC1 | Ras-related C3 botulinum toxin substrate 1 | HMH_vs._LMH | 0.12 |
| ATP5J | F-type H+-transporting ATPase subunit 6 | HFG_vs._LFG | 2.68 |
| ALDH | aldehyde dehydrogenase (NAD+) | HFG_vs._LFG | 1.28 |
| VNN | pantetheine hydrolase | HFG_vs._LFG | 5.97 |
| FASN | fatty acid synthase, animal type | HMG_vs._LMG | −3.71 |
| ACACA | acetyl-CoA carboxylase/biotin carboxylase 1 | HMG_vs._LMG | −2.63 |
| ACSBG | long-chain-fatty-acid-CoA ligase ACSBG | HMG_vs._LMG | −0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, G.; Xue, Y.; Huang, X. Effects of Dietary Folic Acid Supplementation on Sex Differences in Oriental River Prawn, Macrobrachium nipponense. Animals 2023, 13, 3677. https://doi.org/10.3390/ani13233677
Jiang G, Xue Y, Huang X. Effects of Dietary Folic Acid Supplementation on Sex Differences in Oriental River Prawn, Macrobrachium nipponense. Animals. 2023; 13(23):3677. https://doi.org/10.3390/ani13233677
Chicago/Turabian StyleJiang, Gang, Yucai Xue, and Xuxiong Huang. 2023. "Effects of Dietary Folic Acid Supplementation on Sex Differences in Oriental River Prawn, Macrobrachium nipponense" Animals 13, no. 23: 3677. https://doi.org/10.3390/ani13233677





