Secretome Analysis of High- and Low-Virulent Bovine Pasteurella multocida Cultured in Different Media
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Cultural Conditions
2.2. Experimental Animals and Ethics Statement
2.3. Growth Curve Analysis
2.4. Virulence Analysis of Bacteria Cultured in Different Media
2.5. Sample Preparation
2.6. LC-MS/MS and DIA Quantitative Analysis
2.7. Bioinformatics Analysis
2.8. Protein Expression and Polyclonal Antibody Preparation
2.9. Immunohistochemistry
2.10. Immunoelectron Microscopy Observation
2.11. Immune Protection Assay
2.12. Serum Antibody Titer Detemination
2.13. rTuf Induces Inflammatory Factor Secretion in Macrophage
2.14. Statistical Analysis
3. Results
3.1. The Virulence and Growth Level of PmCQ2 and PmCQ6 Were Different in Different Media
3.2. Analysis of Differentially Putative Secretory Proteins between High- and Low-Virulent Strains
3.3. Analysis of Differentially Putative Secretory Proteins in Different Media
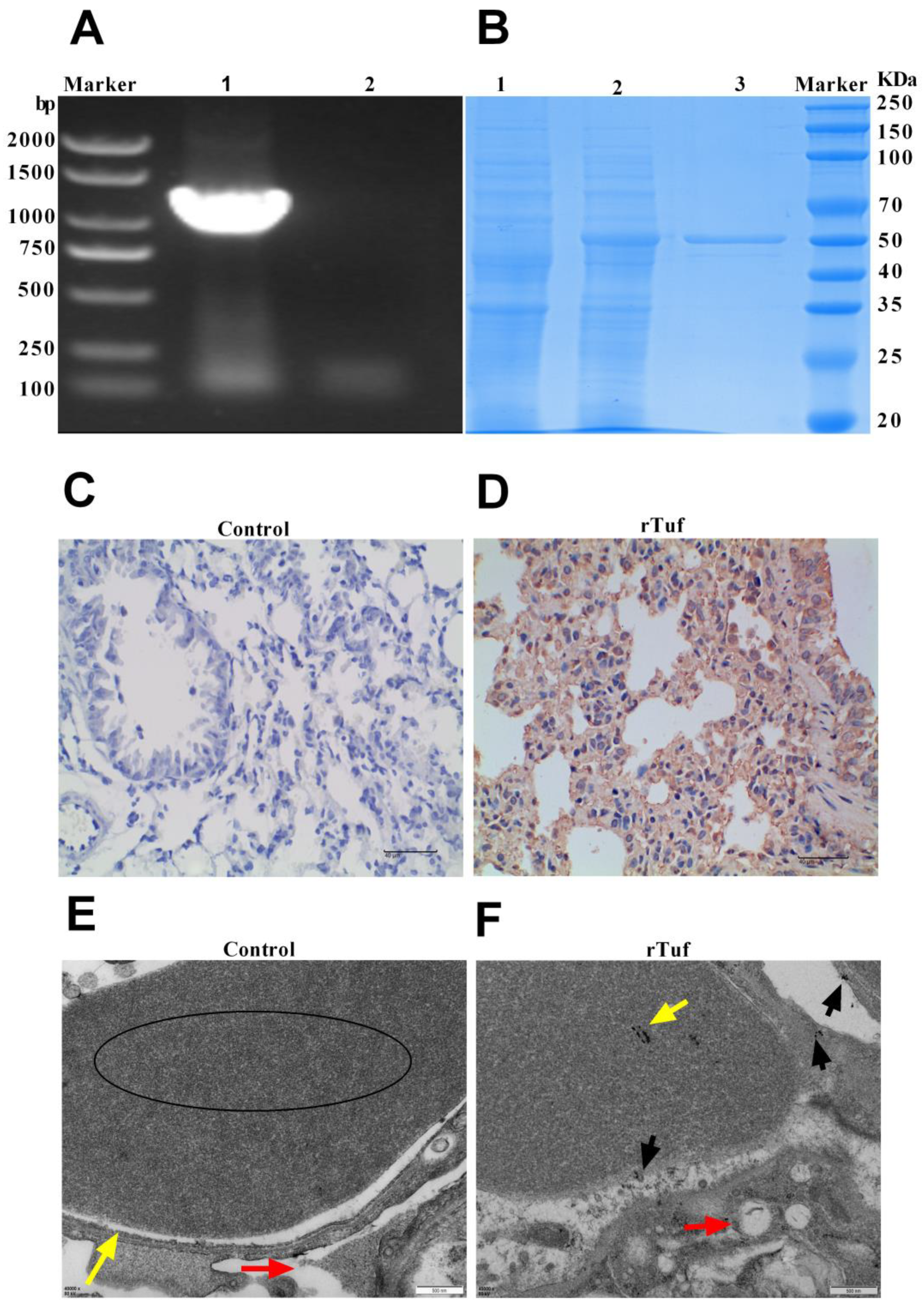
3.4. Validation of Secreted Proteins
3.5. Results of rTuf Protein Biological Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Alwis, M.C. Serological classification of Pasteurella multocida. Vet. Rec. 1987, 120, 351. [Google Scholar] [CrossRef] [PubMed]
- Dabo, S.M.; Taylor, J.D.; Confer, A.W. Pasteurella multocida and bovine respiratory disease. Anim. Health Res. Rev. 2007, 8, 129–150. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Long, Q.; Du, H.; Zhang, J.; Pan, T.; Wu, C.; Lei, G.; Peng, Y.; Hardwidge, P.R. High and low-virulent bovine Pasteurella multocida capsular type A isolates exhibit different virulence gene expression patterns in vitro and in vivo. Vet. Microbiol. 2016, 196, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Feng, T.; Wang, Y.; Li, P.; Yin, Y.; Zhao, Z.; Hardwidge, P.R.; Peng, Y.; He, F. A single point mutation in the hyaC gene affects Pasteurella multocida serovar A capsule production and virulence. Microb. Pathog. 2021, 159, 105145. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhao, Z.; Wu, X.; Duan, L.; Li, N.; Fang, R.; Li, P.; Peng, Y. Transcriptomic Analysis of High- and Low-Virulence Bovine Pasteurella multocida in vitro and in vivo. Front. Vet. Sci. 2021, 8, 616774. [Google Scholar] [CrossRef] [PubMed]
- Galán, J.E.; Waksman, G. Protein-Injection Machines in Bacteria. Cell 2018, 172, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Sörman, A.; Zhang, L.; Ding, Z.; Heyman, B. How antibodies use complement to regulate antibody responses. Mol. Immunol. 2014, 61, 79–88. [Google Scholar] [CrossRef]
- Bendtsen, J.D.; Nielsen, H.; Widdick, D.; Palmer, T.; Brunak, S. Prediction of twin-arginine signal peptides. BMC Bioinform. 2005, 6, 167. [Google Scholar] [CrossRef]
- Cianciotto, N.P. Type II secretion: A protein secretion system for all seasons. Trends Microbiol. 2005, 13, 581–588. [Google Scholar] [CrossRef]
- Kang, Q.; Zhang, D. Principle and potential applications of the non-classical protein secretory pathway in bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 953–965. [Google Scholar] [CrossRef]
- Ludwig, C.; Gillet, L.; Rosenberger, G.; Amon, S.; Collins, B.C.; Aebersold, R. Data-independent acquisition-based SWATH-MS for quantitative proteomics: A tutorial. Mol. Syst. Biol. 2018, 14, e8126. [Google Scholar] [CrossRef] [PubMed]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Yin, Z.; Wu, C.; Xia, Y.; Wu, M.; Li, P.; Zhang, H.; Yin, Y.; Li, N.; Zhu, G.; et al. l-Serine Lowers the Inflammatory Responses during Pasteurella multocida Infection. Infect. Immun. 2019, 87, e00677-19. [Google Scholar] [CrossRef] [PubMed]
- Samen, U.; Gottschalk, B.; Eikmanns, B.J.; Reinscheid, D.J. Relevance of peptide uptake systems to the physiology and virulence of Streptococcus agalactiae. J. Bacteriol. 2004, 186, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Antenucci, F.; Magnowska, Z.; Nimtz, M.; Roesch, C.; Jänsch, L.; Bojesen, A.M. Immunoproteomic characterization of outer membrane vesicles from hyper-vesiculating Actinobacillus pleuropneumoniae. Vet. Microbiol. 2019, 235, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Ihalin, R.; Eneslätt, K.; Asikainen, S. Peptidoglycan-associated lipoprotein of Aggregatibacter actinomycetemcomitans induces apoptosis and production of proinflammatory cytokines via TLR2 in murine macrophages RAW 264.7 in vitro. J. Oral Microbiol. 2018, 10, 1442079. [Google Scholar] [CrossRef]
- He, F.; Qin, X.; Xu, N.; Li, P.; Wu, X.; Duan, L.; Du, Y.; Fang, R.; Hardwidge, P.R.; Li, N.; et al. Pasteurella multocida Pm0442 Affects Virulence Gene Expression and Targets TLR2 to Induce Inflammatory Responses. Front. Microbiol. 2020, 11, 1972. [Google Scholar] [CrossRef]
- Röhm, M.; Lindemann, E.; Hiller, E.; Ermert, D.; Lemuth, K.; Trkulja, D.; Sogukpinar, O.; Brunner, H.; Rupp, S.; Urban, C.F.; et al. A family of secreted pathogenesis-related proteins in Candida albicans. Mol. Microbiol. 2013, 87, 132–151. [Google Scholar] [CrossRef]
- Reimann, S.; Poschmann, G.; Kanonenberg, K.; Stühler, K.; Smits, S.H.; Schmitt, L. Interdomain regulation of the ATPase activity of the ABC transporter haemolysin B from Escherichia coli. Biochem. J. 2016, 473, 2471–2483. [Google Scholar] [CrossRef]
- Yoshida, K.; Toyofuku, M.; Obana, N.; Nomura, N. Biofilm formation by Paracoccus denitrificans requires a type I secretion system-dependent adhesin BapA. FEMS Microbiol. Lett. 2017, 364, 4. [Google Scholar] [CrossRef]
- Luo, Q.; Kong, L.; Dong, J.; Zhang, T.; Wang, H.; Zhang, R.; Lu, Q.; Chen, H.; Shao, H.; Jin, M. Protection of chickens against fowl cholera by supernatant proteins of Pasteurella multocida cultured in an iron-restricted medium. Avian Pathol. 2019, 48, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Wang, X.; Han, X.; Zhu, C.; Yu, Y.; Liu, X.; Xu, B. Effects of Different Bacterial Culture Condition on the Expression of Avian Salmonella Fimbrial Genes. Acta. Ve. Et Zootechnica. Sinica. 2014, 45, 116–122. [Google Scholar]
- Starck, J.; Källenius, G.; Marklund, B.I.; Andersson, D.I.; Åkerlund, T. Comparative proteome analysis of Mycobacterium tuberculosis grown under aerobic and anaerobic conditions. Microbiology 2004, 150, 3821–3829. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tian, M.; Hu, H.; Yin, Y.; Guan, X.; Ding, C.; Wang, S.; Yu, S. Lable-free based comparative proteomic analysis of secretory proteins of rough Brucella mutants. J. Proteomics. 2019, 195, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Venable, J.D.; Dong, M.Q.; Wohlschlegel, J.; Dillin, A.; Yates, J.R. Automated approach for quantitative analysis of complex peptide mixtures from tandem mass spectra. Nat. Methods. 2004, 1, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.; Arora, K.; Gupta, A.; Guptasarma, P. The DNA-binding protein HU is a molecular glue that attaches bacteria to extracellular DNA in biofilms. J. Biol. Chem. 2021, 296, 100532. [Google Scholar] [CrossRef]
- Asanuma, N.; Kanada, K.; Arai, Y.; Yoshizawa, K.; Ichikawa, T.; Hino, T. Molecular characterization and significance of phosphoenolpyruvate carboxykinase in a ruminal bacterium, Streptococcus bovis. J. Gen. Appl. Microbiol. 2010, 56, 121–122. [Google Scholar] [CrossRef]
- Kiliç, N.K.; Stensballe, A.; Otzen, D.E.; Dönmez, G. Proteomic changes in response to chromium (VI) toxicity in Pseudomonas aeruginosa. Bioresour. Technol. 2010, 101, 2134–2140. [Google Scholar] [CrossRef]
- Ma, J.; Du, H.; Ji, J.; Li, C.; Feng, S.; Peng, Y. Expression of the fusion gene pm0979-ompH of Pasteurella multocida and its immunoprotection. Chin. J. Vet. Sci. 2015, 45, 1053–1057. [Google Scholar]
- Kunze, G.; Zipfel, C.; Robatzek, S.; Niehaus, K.; Boller, T.; Felix, G. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 2004, 16, 3496–3507. [Google Scholar] [CrossRef]
- Zipfel, C.; Kunze, G.; Chinchilla, D.; Caniard, A.; Jones, J.D.; Boller, T.; Felix, G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 2006, 125, 749–760. [Google Scholar] [CrossRef] [PubMed]



| Protein | Description | Secretory Pathway Prediction | Co-Expressed or Not |
|---|---|---|---|
| CQ2GL000012 | 50S ribosomal protein L33 | Non-classical secretory pathway | Yes |
| CQ2GL000017 | 50S ribosomal protein L31 | Non-classical secretory pathway | Yes |
| CQ2GL000368 | 50S ribosomal protein L20 | Non-classical secretory pathway | No |
| CQ2GL001658 | 30S ribosomal protein S11 | Non-classical secretory pathway | No |
| CQ2GL001677 | 50S ribosomal protein L2 | Non-classical secretory pathway | No |
| CQ2GL001678 | 50S ribosomal protein L23 | Non-classical secretory pathway | No |
| CQ2GL002041 | 50S ribosomal protein L1 | Non-classical secretory pathway | Yes |
| CQ2GL000089 | hypothetical protein | Non-classical secretory pathway | Yes |
| CQ2GL000090 | hypothetical protein | Non-classical secretory pathway | No |
| CQ2GL000092 | hypothetical protein | Non-classical secretory pathway | No |
| CQ2GL000104 | hypothetical protein | Non-classical secretory pathway | Yes |
| CQ2GL001414 | hypothetical protein | Non-classical secretory pathway | No |
| CQ2GL000675 | type I glyceraldehyde-3-phosphate dehydrogenase, GAPDH | Non-classical secretory pathway | No |
| CQ2GL000697 | peptidyl-prolyl cis-trans isomerase, ppiB | Non-classical secretory pathway | No |
| CQ2GL000950 | pyruvate dehydrogenase complex dihydrolipoyllysine-residue acetyltransferase, PdhC | Non-classical secretory pathway | No |
| CQ2GL001229 | peptidase C58 | Non-classical secretory pathway | Yes |
| CQ2GL001642 | pirin family protein | Non-classical secretory pathway | No |
| CQ2GL001744 | NAD(P)H-dependent oxidoreductase | Non-classical secretory pathway | Yes |
| CQ2GL001815 | phosphoenolpyruvate carboxykinase, PCK | Non-classical secretory pathway | Yes |
| CQ2GL002027 | glutathione amide-dependent peroxidase | Non-classical secretory pathway | Yes |
| CQ2GL002051 | DNA-binding protein HU, HupA | Non-classical secretory pathway | Yes |
| CQ2GL000177 | Outer-membrane protein A, OmpA | Sec/cleaved by SPI | Yes |
| CQ2GL000187 | nucleotide sugar dehydrogenase, UGDH | Sec/cleaved by SPI | No |
| CQ2GL000236 | htrA protein | Sec/cleaved by SPI | Yes |
| CQ2GL000270 | phosphate acetyltransferase, pta | Sec/cleaved by SPI | No |
| CQ2GL000380 | dipeptide transporter substrate-binding protein, dppA | Sec/cleaved by SPI | Yes |
| CQ2GL000424 | malate dehydrogenase, mdh | Sec/cleaved by SPI | No |
| CQ2GL000495 | C4-dicarboxylate ABC transporter substrate-binding protein | Sec/cleaved by SPI | No |
| CQ2GL000577 | OmpH | Sec/cleaved by SPI | Yes |
| CQ2GL000589 | thiamine ABC transporter substrate binding subunit, tbpA | Sec/cleaved by SPI | No |
| CQ2GL000696 | peptidyl-prolyl cis-trans isomerase (cyclophilin B, ppiB) | Sec/cleaved by SPI | No |
| CQ2GL000737 | translocation protein TolB | Sec/cleaved by SPI | No |
| CQ2GL000770 | MipA/OmpV | Sec/cleaved by SPI | Yes |
| CQ2GL000846 | membrane protein, long-chain fatty acid transport protein, fadL | Sec/cleaved by SPI | No |
| CQ2GL001241 | iron ABC transporter substrate-binding protein, FbpA | Sec/cleaved by SPI | No |
| CQ2GL001440 | RlpA-like protein | Sec/cleaved by SPI | No |
| CQ2GL002029 | FKBP-type peptidyl-prolyl cis-trans isomerase FkpA, fkpA | Sec/cleaved by SPI | No |
| CQ2GL002076 | sialic acid-binding protein, SABP | Sec/cleaved by SPI | No |
| CQ2GL002078 | N-acetylneuraminate epimerase, nanM | Sec/cleaved by SPI | Yes |
| CQ2GL002179 | TonB-dependent hemoglobin/transferrin/lactoferrin family receptor | Sec/cleaved by SPI | Yes |
| CQ2GL000185 | sugar ABC transporter substrate-binding protein | Sec/cleaved by SPII | Yes |
| CQ2GL000321 | osmotically-inducible protein OsmY | Sec/cleaved by SPII | Yes |
| CQ2GL000324 | penicillin-binding protein activator, LpoA | Sec/cleaved by SPII | Yes |
| CQ2GL000420 | glycine zipper 2TM domain-containing protein, slyB | Sec/cleaved by SPII | Yes |
| CQ2GL000736 | peptidoglycan-associated lipoprotein, pal | Sec/cleaved by SPII | No |
| CQ2GL000898 | pilus assembly protein TadD | Sec/cleaved by SPII | No |
| CQ2GL001790 | hypothetical protein | Sec/cleaved by SPII | Yes |
| CQ2GL000999 | DUF882 domain-containing protein | Tat secretory pathway | No |
| CQ2GL001655 | 50S ribosomal protein L17 | Tat secretory pathway | No |
| CQ2GL001673 | 50S ribosomal protein L16 | Tat secretory pathway | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Wang, J.; He, F.; Wu, X.; Dan, R.; Hardwidge, P.R.; Li, N.; Peng, Y. Secretome Analysis of High- and Low-Virulent Bovine Pasteurella multocida Cultured in Different Media. Animals 2023, 13, 3683. https://doi.org/10.3390/ani13233683
Qiu Y, Wang J, He F, Wu X, Dan R, Hardwidge PR, Li N, Peng Y. Secretome Analysis of High- and Low-Virulent Bovine Pasteurella multocida Cultured in Different Media. Animals. 2023; 13(23):3683. https://doi.org/10.3390/ani13233683
Chicago/Turabian StyleQiu, Yangyang, Jianan Wang, Fang He, Xiaoyan Wu, Ruitong Dan, Philip R. Hardwidge, Nengzhang Li, and Yuanyi Peng. 2023. "Secretome Analysis of High- and Low-Virulent Bovine Pasteurella multocida Cultured in Different Media" Animals 13, no. 23: 3683. https://doi.org/10.3390/ani13233683




