The Effects of Acute Bisphenol A Toxicity on the Hematological Parameters, Hematopoiesis, and Kidney Histology of Zebrafish (Danio rerio)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Maintenance
2.2. Acute Toxicity Bioassay
2.3. Preparation of Peripheral Blood and Kidney Smears
2.4. Assessment of Hemopoiesis Activity and Composition of Peripheral Blood
2.5. Micronucleus Test
2.6. Histological Preparations, Histomorphometric Analysis and Semi-Quantitative Assessment
2.7. Statistical Analysis
3. Results
3.1. Determination of LC50
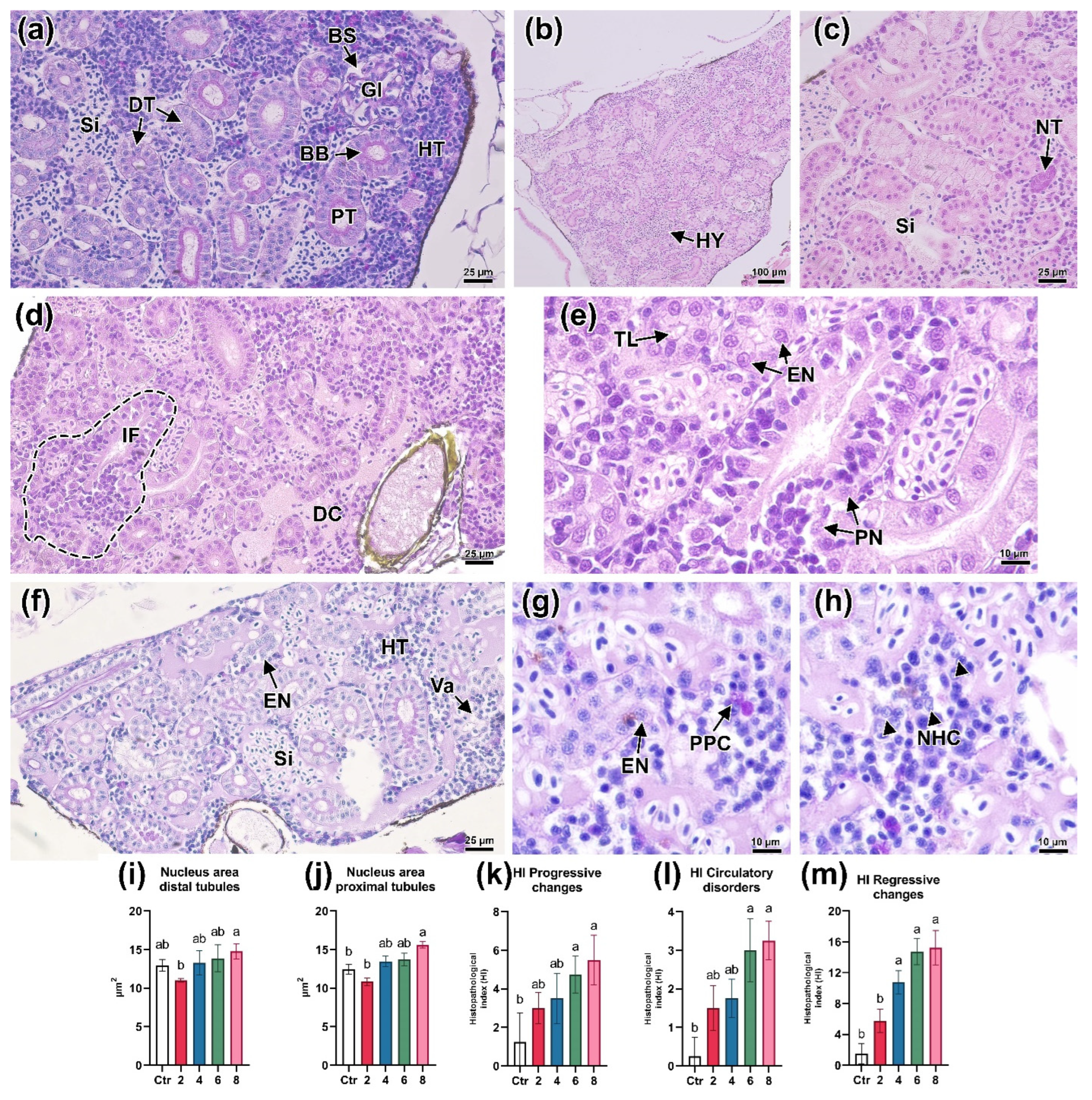
3.2. Hematopoiesis in the Head Kidney
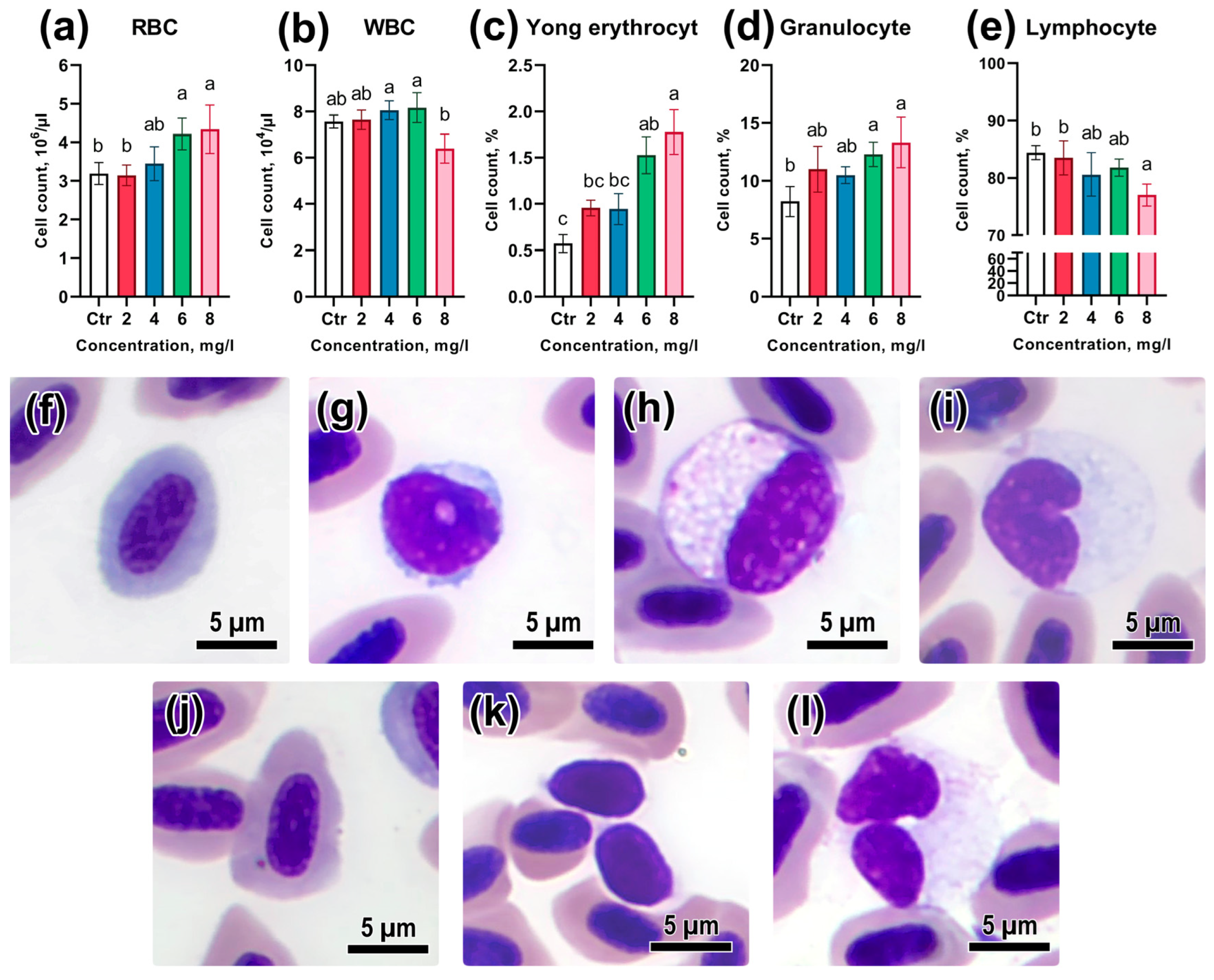
3.3. Peripheral Blood Composition
3.4. Assessment of Genotoxicity
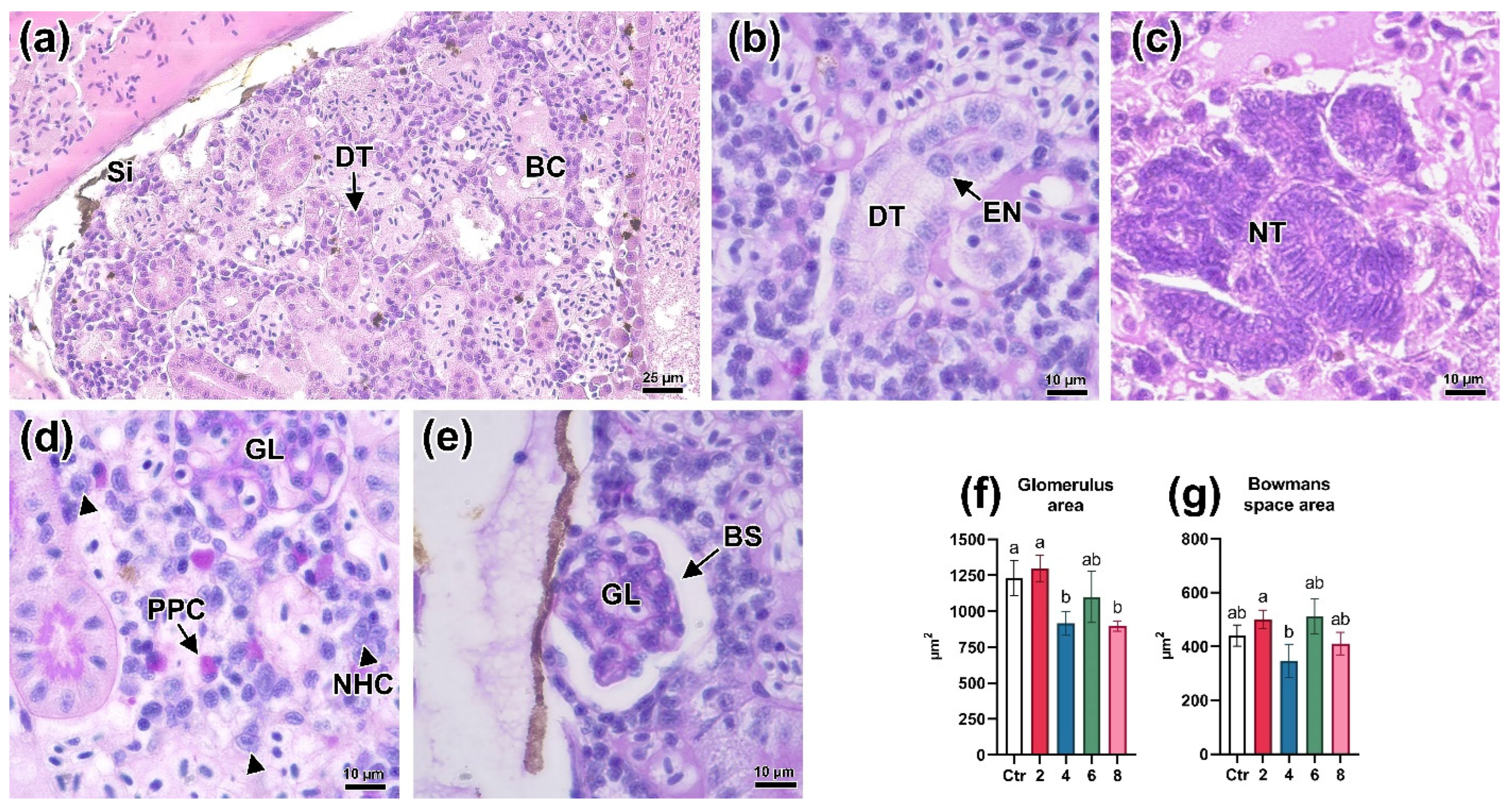
3.5. Histologic Parameters of the Kidneys
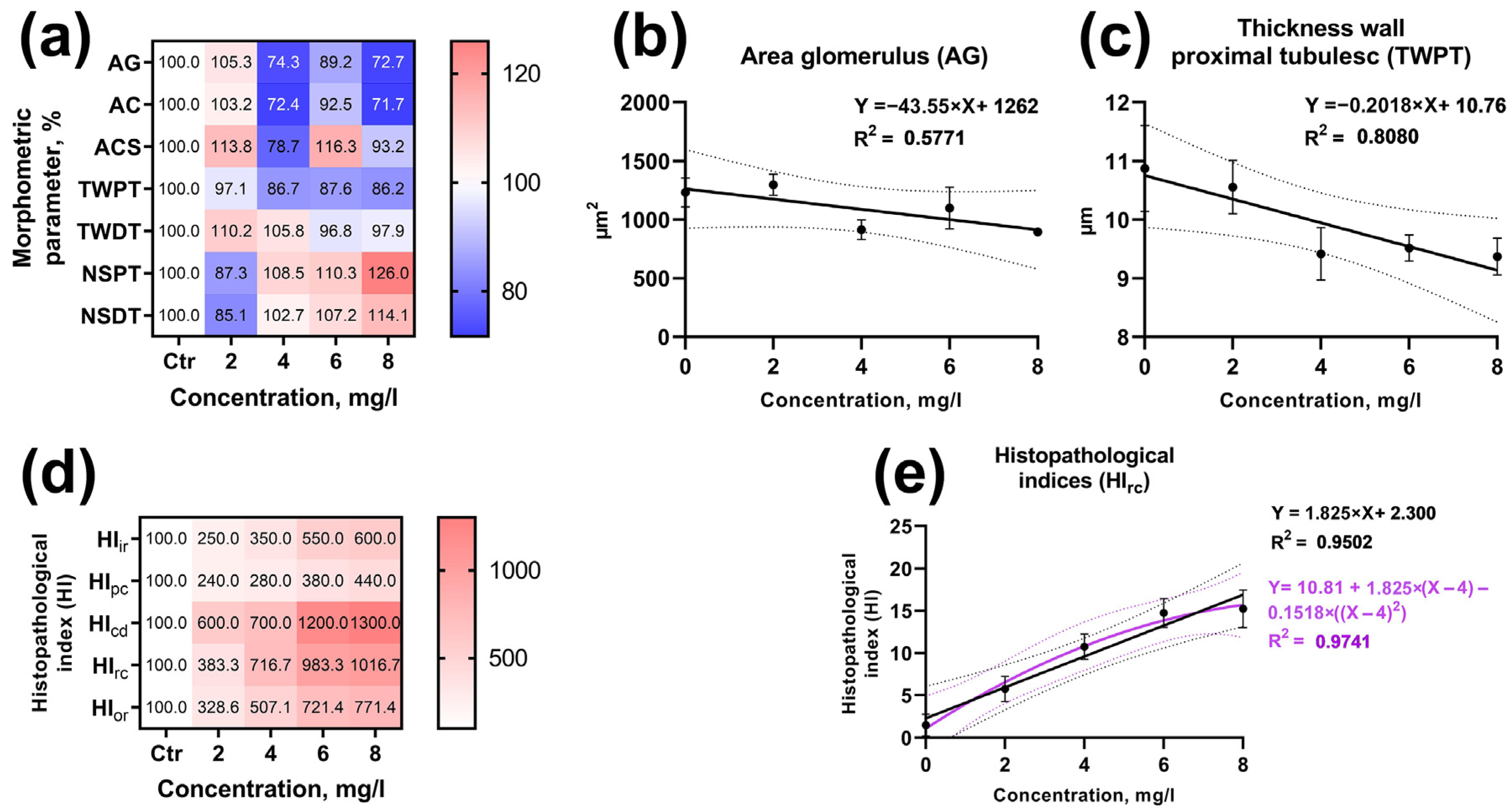
3.6. Identifying the Concentration–Effect Relationship
4. Discussion
4.1. Acute BPA Toxicity to Adult Zebrafish
4.2. Hematopoietic Parameters of the Head Kidney
4.3. Genotoxicity
4.4. Peripheral Blood Composition
4.5. Histological Parameters of Head and Trunk Kidney
4.6. Concentration–Effect Relationship
4.7. Hematological Parameters in the Evaluation of Endocrine-Disruptive Substances
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staples, C.A.; Dome, P.B.; Klecka, G.M.; Oblock, S.T.; Harris, L.R. A Review of the Environmental Fate, Effects, and Exposures of Bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, L.K.; Noonan, G.O.; Heiserman, W.M.; Roach, J.A.; Limm, W.; Begley, T.H. Determination of bisphenol A in US infant formulas: Updated methods and concentrations. J. Agric. Food Chem. 2010, 58, 2307–2313. [Google Scholar] [CrossRef] [PubMed]
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol a in the Environment. Dose-Response 2015, 13, 155932581559830. [Google Scholar] [CrossRef]
- Rezg, R.; El-Fazaa, S.; Gharbi, N.; Mornagui, B. Bisphenol a and Human Chronic Diseases: Current Evidences, Possible Mechanisms, and Future Perspectives. Environ. Int. 2014, 64, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Uadia, P.O. A survey of the level of bisphenol A (BPA) in effluents, soil leachates, food samples, drinking water and consumer products in south-western Nigeria. World Environ. 2015, 5, 135–139. [Google Scholar]
- Canesi, L.; Fabbri, E. Environmental effects of BPA: Focus on aquatic species. Dose-Response 2015, 13, 1559325815598304. [Google Scholar] [CrossRef] [PubMed]
- Afzal, G.; Ahmad, H.I.; Hussain, R.; Jamal, A.; Kiran, S.; Hussain, T.; Saeed, S.; Nisa, M.u. Bisphenol a Induces Histopathological, Hematobiochemical Alterations, Oxidative Stress, and Genotoxicity in Common Carp (Cyprinus carpio L.). Oxidative Med. Cell. Longev. 2022, 2022, 5450421. [Google Scholar] [CrossRef]
- Akram, R.; Iqbal, R.; Hussain, R.; Jabeen, F.; Ali, M. Evaluation of Oxidative Stress, Antioxidant Enzymes and Genotoxic Potential of Bisphenol a in Fresh Water Bighead Carp (Aristichthys nobils) Fish at Low Concentrations. Environ. Pollut. 2021, 268, 115896. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An Endocrine Disruptor with Widespread Exposure and Multiple Effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- Crain, D.A.; Eriksen, M.; Iguchi, T.; Jobling, S.; Laufer, H.; LeBlanc, G.A.; Guillette, L.J. An Ecological Assessment of Bisphenol-A: Evidence from Comparative Biology. Reprod. Toxicol. 2007, 24, 225–239. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.; Fan, X.; Dong, C.; Wang, G.; Wang, Z. Chronic Exposure to Bisphenol a Resulted in Alterations of Reproductive Functions via Immune Defense, Oxidative Damage and Disruption DNA/Histone Methylation in Male Rare Minnow Gobiocypris Rarus. Aquat. Toxicol. 2021, 236, 105849. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.C.; Seebacher, F. Effect of the Plastic Pollutant Bisphenol a on the Biology of Aquatic Organisms: A Meta-Analysis. Glob. Change Biol. 2020, 26, 3821–3833. [Google Scholar] [CrossRef] [PubMed]
- Megarani, D.V.; Hardian, A.B.; Arifianto, D.; Santosa, C.M.; Salasia, S.I.O. Comparative Morphology and Morphometry of Blood Cells in Zebrafish (Danio Rerio), Common Carp (Cyprinus carpio Carpio), and Tilapia (Oreochromis niloticus). J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 673–680. [Google Scholar] [CrossRef]
- Witeska, M. Anemia in Teleost Fishes. Bull. Eur. Assoc. Fish Pathol. 2015, 35, 148–160. [Google Scholar]
- Kondera, E.; Witeska, M. Cadmium and Copper Reduce Hematopoietic Potential in Common Carp (Cyprinus carpio L.) Head Kidney. Fish Physiol. Biochem. 2012, 39, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.; Rohani, M.F.; Zabed, S.A.; Islam, M.T.; Jannat, R.; Akter, Y.; Shahjahan, M. Acute Effects of Chromium on Hemato-Biochemical Parameters and Morphology of Erythrocytes in Striped Catfish Pangasianodon Hypophthalmus. Toxicol. Rep. 2020, 7, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, E.S.H.; Abdu, S.B.; Ali, T.E.S.; Fouad, H.F. Haemopoiesis in the Head Kidney of Tilapia, Oreochromis niloticus (Teleostei: Cichlidae): A Morphological (Optical and Ultrastructural) Study. Fish Physiol. Biochem. 2010, 36, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Carradice, D.; Lieschke, G.J. Zebrafish in Hematology: Sushi or Science? Blood 2008, 111, 3331–3342. [Google Scholar] [CrossRef]
- Kobayashi, I.; Sekiya, M.; Moritomo, T.; Ototake, M.; Nakanishi, T. Demonstration of Hematopoietic Stem Cells in Ginbuna Carp (Carassius auratus Langsdorfii) Kidney. Dev. Comp. Immunol. 2006, 30, 1034–1046. [Google Scholar] [CrossRef]
- de Souza Anselmo, C.; Sardela, V.F.; de Sousa, V.P.; Pereira, H.M.G. Zebrafish (Danio Rerio): A Valuable Tool for Predicting the Metabolism of Xenobiotics in Humans? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 212, 34–46. [Google Scholar] [CrossRef]
- Gjini, E.; Jing, C.-B.; Nguyen, A.T.; Reyon, D.; Gans, E.; Kesarsing, M.; Peterson, J.; Pozdnyakova, O.; Rodig, S.J.; Mansour, M.R.; et al. Disruption of Asxl1 Results in Myeloproliferative Neoplasms in Zebrafish. Dis. Models Mech. 2019, 12, dmm035790. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Q.; Huang, Z.; Ding, L.; Xu, Z. Immunoglobulins, Mucosal Immunity and Vaccination in Teleost Fish. Front. Immunol. 2020, 11, 567941. [Google Scholar] [CrossRef] [PubMed]
- Gökalp Muranli, F.D.; Güner, U. Induction of Micronuclei and Nuclear Abnormalities in Erythrocytes of Mosquito Fish (Gambusia affinis) Following Exposure to the Pyrethroid Insecticide Lambda-Cyhalothrin. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 726, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Bagdonas, E.; Vosylienė, M.Z. A study of toxicity and genotoxicity of copper, zinc and their mixture to rainbow trout (Oncorhynchus mykiss). Biologija 2006, 52, 8–13. [Google Scholar]
- Canedo, A.; de Jesus, L.W.O.; Bailão, E.F.L.C.; Rocha, T.L. Micronucleus Test and Nuclear Abnormality Assay in Zebrafish (Danio Rerio): Past, Present, and Future Trends. Environ. Pollut. 2021, 290, 118019. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio); University of Oregon Press: Eugene, OR, USA, 1995. [Google Scholar]
- Mathieu-Denoncourt, J.; Wallace, S.J.; de Solla, S.R.; Langlois, V.S. Influence of Lipophilicity on the Toxicity of Bisphenol a and Phthalates to Aquatic Organisms. Bull. Environ. Contam. Toxicol. 2016, 97, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.N.; Xiao, H.; Ke, W.B.; Zhan, Y.; Sun, Y. Acute and Subacute Toxicity of Bisphenol a on Zebrafish (Danio rerio). Adv. Mater. Res. 2011, 356–360, 138–141. [Google Scholar] [CrossRef]
- OECD. Test no. 203: Fish, acute toxicity test. In OECD Guidelines for the Testing of Chemicals; OECD: Paris, France, 1992; Volume 1, pp. 1–10. [Google Scholar]
- Di Paola, D.; Capparucci, F.; Lanteri, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Combined Toxicity of Xenobiotics Bisphenol a and Heavy Metals on Zebrafish Embryos (Danio Rerio). Toxics 2021, 9, 344. [Google Scholar] [CrossRef]
- Kochetkov, N.; Smorodinskaya, S.; Nikiforov-Nikishin, D.; Klimov, V.; Golovacheva, N.; Nikiforov-Nikishin, A.; Grozesku, Y. Evaluating possible genotoxicity of three feed additives recommended for aquaculture by using micronucleus test on danio rerio erythrocytes. Vestn. Astrakhan State Tech. Univ. Ser. Fish. Ind. 2022, 3, 48–59. [Google Scholar] [CrossRef]
- Rusia, V.; Sood Routine, S.K. Haematological tests. In Medical Laboratory Technology; Kanai, L., Mukerjee, I., Eds.; McGraw Hill: New Delhi, India, 1992; pp. 252–258. [Google Scholar]
- Ivanovski, O.; Kulkeaw, K.; Nakagawa, M.; Sasaki, T.; Mizuochi, C.; Horio, Y.; Ishitani, T.; Sugiyama, D. Characterization of Kidney Marrow in Zebrafish (Danio rerio) by Using a New Surgical Technique. Contrib. Maced. Acad. Sci. Arts 2009, 30, 71–80. [Google Scholar]
- Sula, E.; Aliko, V.; Pagano, M.; Faggio, C. Digital Light Microscopy as a Tool in Toxicological Evaluation of Fish Erythrocyte Morphological Abnormalities. Microsc. Res. Tech. 2020, 83, 362–369. [Google Scholar] [CrossRef] [PubMed]
- IIvanova, N.T. Atlas Kletok Krovi ryb (Sravnitel’naya Morfologiya i Klassifikatsiya Formennykh Elementov Krovi ryb) (Atlas of the Fish Blood Cells: Comparative Morphology and Classification of the Fish Blood Elements); Legkaya Pishch. Prom-st: Moscow, Russia, 1983; p. 325. [Google Scholar]
- Fijan, N. Composition of Main Haematopoietic Compartments in Normal and Bled Channel Catfish. J. Fish Biol. 2002, 60, 1142–1154. [Google Scholar] [CrossRef]
- Kondera, E. Haematopoiesis in the Head Kidney of Common Carp (Cyprinus carpio L.): A Morphological Study. Fish Physiol. Biochem. 2010, 37, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Wlasow, T.; Dabrowska, H. Cellular Changes in the Blood and Haemopoietic Tissues of Common Carp Exposed to Sublethal Concentration of Ammonia. Aquat. Living Resour. 1989, 2, 169–174. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, C.; Hayashi, M. Micronucleus Assay in Aquatic Animals. Mutagenesis 2010, 26, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: Oxford, UK, 2019. [Google Scholar]
- Kochetkov, N.; Smorodinskaya, S.; Vatlin, A.; Nikiforov-Nikishin, D.; Nikiforov-Nikishin, A.; Danilenko, V.; Anastasia, K.; Reznikova, D.; Grishina, Y.; Antipov, S.; et al. Ability of Lactobacillus brevis 47f to Alleviate the Toxic Effects of Imidacloprid Low Concentration on the Histological Parameters and Cytokine Profile of Zebrafish (Danio rerio). Int. J. Mol. Sci. 2023, 24, 12290. [Google Scholar] [CrossRef]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in Fish: Proposal for a Protocol to Assess Aquatic Pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Antunes, A.M.; Rocha, T.L.; Pires, F.L.; Alves, M.; Leite, V.S.; Arana, S.; Moreira, P.; Maria, S. Gender-Specific Histopathological Response in GuppiesPoecilia ReticulataExposed to Glyphosate or Its Metabolite Aminomethylphosphonic Acid. J. Appl. Toxicol. 2017, 37, 1098–1107. [Google Scholar] [CrossRef]
- Healy, M.J.R.; Finney, D.J. Statistical Method in Biological Assay. J. R. Stat. Society. Ser. A Gen. 1979, 142, 507. [Google Scholar] [CrossRef]
- R Core Team. R: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 2 September 2023).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 2 August 2023).
- Li, D.; Bi, R.; Chen, H.; Mu, L.; Zhang, L.; Chen, Q.; Xie, H.; Luo, Y.; Xie, L. The Acute Toxicity of Bisphenol a and Lignin-Derived Bisphenol in Algae, Daphnids, and Japanese Medaka. Environ. Sci. Pollut. Res. 2017, 24, 23872–23879. [Google Scholar] [CrossRef] [PubMed]
- Asifa, K.P.; Chitra, K.C. Evaluation of LC50 and behavioural responses of bisphenol A in the cichlid fish, Etroplus maculatus. Int. J. Curr. Res. 2015, 7, 16725–16729. [Google Scholar]
- Alexander, H.C.; Dill, D.C.; Smith, L.W.; Guiney, P.D.; Dorn, P. Bisphenol A: Acute aquatic toxicity. Environ. Toxicol. Chem. Int. J. 1988, 7, 19–26. [Google Scholar] [CrossRef]
- Sharma, P.; Chadha, P. Bisphenol a Induced Toxicity in Blood Cells of Freshwater Fish Channa Punctatus after Acute Exposure. Saudi J. Biol. Sci. 2021, 28, 4738–4750. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, Z. Effects of Environmental Pollution on Fish: A Short Review. Transylv. Rev. Syst. Ecol. Res. 2017, 19, 49–60. [Google Scholar] [CrossRef]
- Soldatov, A.A.; Kukhareva, T.A. Erythropoiesis and the contents of abnormal erythroid forms in the blood of the round goby, Neogobius melanostomus Pallas, 1811 (Osteichthyes: Gobiidae). Russ. J. Mar. Biol. 2015, 41, 315–320. [Google Scholar] [CrossRef]
- Fijan, N. Morphogenesis of Blood Cell Lineages in Channel Catfish. J. Fish Biol. 2002, 60, 999–1014. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Q.; Yang, S.; Zhao, L.; Fu, H.; Du, J.; Du, Z.; Yan, T.; Wu, H. Characterization of Hematopoiesis in Dabry’s Sturgeon (Acipenser dabryanus). Aquac. Fish. 2017, 2, 262–268. [Google Scholar] [CrossRef]
- Kondera, E.; Bojarski, B.; Ługowska, K.; Kot, B.; Witeska, M. Effects of Oxytetracycline and Gentamicin Therapeutic Doses on Hematological, Biochemical and Hematopoietic Parameters in Cyprinus carpio Juveniles. Animals 2020, 10, 2278. [Google Scholar] [CrossRef]
- Kondera, E.; Teodorczuk, B.; Ługowska, K.; Witeska, M. Effect of Glyphosate-Based Herbicide on Hematological and Hemopoietic Parameters in Common Carp (Cyprinus carpio L.). Fish Physiol. Biochem. 2018, 44, 1011–1018. [Google Scholar] [CrossRef]
- Suljević, D.; Islamagić, E.; Alijagić, A.; Fočak, M.; Mitrašinović-Brulić, M. Morphological identification of haematopoietic cells in pronephros of common carp (Cyprinus carpio Linnaeus, 1758). Acta Biol. Szeged. 2016, 60, 113–118. [Google Scholar]
- Murad, A.; Houston, A.H.; Samson, L.D. Haematological Response to Reduced Oxygen-Carrying Capacity, Increased Temperature and Hypoxia in Goldfish, Carassius auratus L. J. Fish Biol. 1990, 36, 289–305. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; Ran, Y.; Chan, K. Distribution and Bioconcentration of Endocrine Disrupting Chemicals in Surface Water and Fish Bile of the Pearl River Delta, South China. Chemosphere 2014, 107, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Valero, Y.; Cuesta, A.; Cammarata, M.; Cuesta, A.; Chaves-Pozo, E. Immune-Endocrine Interactions in the Fish Gonad during Infection: An Open Door to Vertical Transmission. Fishes 2018, 3, 24. [Google Scholar] [CrossRef]
- Casanova-Nakayama, A.; Wenger, M.; Burki, R.; Eppler, E.; Krasnov, A.; Segner, H. Endocrine Disrupting Compounds: Can They Target the Immune System of Fish? Mar. Pollut. Bull. 2011, 63, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef]
- Dangre, A.J.; Manning, S.; Brouwer, M. Effects of Cadmium on Hypoxia-Induced Expression of Hemoglobin and Erythropoietin in Larval Sheepshead Minnow, Cyprinodon Variegatus. Aquat. Toxicol. 2010, 99, 168–175. [Google Scholar] [CrossRef]
- Cui, B.; Ren, L.; Xu, Q.-H.; Yin, L.-Y.; Zhou, X.-Y.; Liu, J.-X. Silver_Nanoparticles Inhibited Erythrogenesis during Zebrafish Embryogenesis. Aquat. Toxicol. 2016, 177, 295–305. [Google Scholar] [CrossRef]
- Belair, C.D.; Peterson, R.E.; Heideman, W. Disruption of Erythropoiesis by Dioxin in the Zebrafish. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2001, 222, 581–594. [Google Scholar] [CrossRef]
- Lombó, M.; Getino-Álvarez, L.; Depincé, A.; Labbé, C.; Herráez, M.P. Embryonic Exposure to Bisphenol a Impairs Primordial Germ Cell Migration without Jeopardizing Male Breeding Capacity. Biomolecules 2019, 9, 307. [Google Scholar] [CrossRef]
- Ferreira, L.; Couto, R.C.; Oliveira, P.J. Bisphenol a as Epigenetic Modulator: Setting the Stage for Carcinogenesis? Eur. J. Clin. Investig. 2015, 45, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Palhares, D.; Grisolia, C.K. Comparison between the Micronucleus Frequencies of Kidney and Gill Erythrocytes in Tilapia Fish, Following Mitomycin c Treatment. Genet. Mol. Biol. 2002, 25, 281–284. [Google Scholar] [CrossRef]
- Cavas, T. In Vivo Genotoxicity Evaluation of Atrazine and Atrazine–Based Herbicide on Fish Carassius auratus Using the Micronucleus Test and the Comet Assay. Food Chem. Toxicol. 2011, 49, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ni, J.; Liang, Z.; Xue, J.; Fenech, M.F.; Wang, X. The molecular origins and pathophysiological consequences of micronuclei: New insights into an age-old problem. Mutat. Res. 2019, 779, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; Kulmann-Leal, B.; Ziliotto, M.; Chies, J.A.B. HIV Infection, Chromosome Instability, and Micronucleus Formation. Viruses 2023, 15, 155. [Google Scholar] [CrossRef]
- Fenech, M.; Knasmueller, S.; Bolognesi, C.; Holland, N.; Bonassi, S.; Kirsch-Volders, M. Micronuclei as biomarkers of DNA damage, aneuploidy, inducers of chromosomal hypermutation and as sources of pro-inflammatory DNA in humans. Mutat. Res. Rev. Mutat. Res. 2020, 786, 108342. [Google Scholar] [CrossRef]
- Nwani, C.D.; Somdare, P.O.; Ogueji, E.O.; Nwani, J.C.; Ukonze, J.A.; Nwadinigwe, A.O. Genotoxicity Assessment and Oxidative Stress Responses in Freshwater African Catfish Clarias gariepinus Exposed to Fenthion Formulations. Drug Chem. Toxicol. 2016, 40, 273–280. [Google Scholar] [CrossRef]
- Zaki, M.S.; Fawzi, O.M.; Shalaby, S.I. Phenol toxicity affecting hematological changes in cat fish (Clarius lazera). Life Sci. J. 2011, 8, 244–248. [Google Scholar]
- Clauss, T.M.; Dove, A.D.; Arnold, J.E. Hematologic disorders of fish. Vet. Clin. N. Am. Exot. Anim. Pract. 2008, 11, 445–462. [Google Scholar] [CrossRef]
- Minaz, M.; Er, A.; Ak, K.; Nane, İ.D.; İpek, Z.Z.; Kurtoğlu, İ.Z.; Kayış, Ş. Short-Term Exposure to Bisphenol a (BPA) as a Plastic Precursor: Hematological and Behavioral Effects on Oncorhynchus Mykiss and Vimba Vimba. Water Air Soil Pollut. 2022, 233, 122. [Google Scholar] [CrossRef]
- Shanthanagouda Admane Holeyappa; Kaur, A.; Bansal, N.; Ansal, M.D.; Patil, J.G.; Naveenkumar Billekallu Thammegowda; Vaneet Inder Kaur; Sethi, R.S. Biomarker-Assisted Assessment of Aquatic Health Using the Cosmopolitan Common Carp, Cyprinus carpio (L.): A Case Study of Bisphenol-A Exposures. Environ. Sci. Pollut. Res. 2021, 29, 14206–14218. [Google Scholar] [CrossRef]
- Witeska, M. Stress in fish-hematological and immunological effects of heavy metals. Electron. J. Ichthyol. 2005, 1, 35–41. [Google Scholar]
- Faheem, M.; Jahan, N.; Lone, K.P. Histopathological Effects of Bisphenol-A on Liver, Kidneys and Gills of Indian Major Carp, Catla Catla (Hamilton, 1822). J. Anim. Plant Sci. 2016, 26, 514–522. [Google Scholar]
- Lindholst, C.; Wynne, P.; Marriott, P.J.; Pedersen, S.N.; Bjerregaard, P. Metabolism of Bisphenol a in Zebrafish (Danio Rerio) and Rainbow Trout (Oncorhynchus Mykiss) in Relation to Estrogenic Response. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 135, 169–177. [Google Scholar] [CrossRef]
- Ike, M.; Chen, M.-Y.; Jin, C.-S.; Fujita, M. Acute Toxicity, Mutagenicity, and Estrogenicity of Biodegradation Products of Bisphenol-A. Environ. Toxicol. 2002, 17, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Hammam, A.M.; Zaki, M.S.; Yousef, R.A.; Fawzi, O. Toxicity, Mutagenicity and carcinogenicity of phenols and phenolic compounds on human and living organisms [A Review]. Adv. Environ. Biol. 2015, 9, 38–49. [Google Scholar]
- Cengiz, E.I. Gill and Kidney Histopathology in the Freshwater Fish Cyprinus carpio after Acute Exposure to Deltamethrin. Environ. Toxicol. Pharmacol. 2006, 22, 200–204. [Google Scholar] [CrossRef]
- Sanz, A.B.; Santamaría, B.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. Mechanisms of Renal Apoptosis in Health and Disease. J. Am. Soc. Nephrol. 2008, 19, 1634–1642. [Google Scholar] [CrossRef]
- McCampbell, K.K.; Springer, K.N.; Wingert, R.A. Atlas of Cellular Dynamics during Zebrafish Adult Kidney Regeneration. Stem Cells Int. 2015, 2015, 547636. [Google Scholar] [CrossRef]
- Mecozzi, M.; Conti, M.E. A new approach based on polynomial functions for measuring the acute toxicity of environmental samples. Int. J. Environ. Health 2007, 1, 87–97. [Google Scholar] [CrossRef]
- Sanyal, T.; Kaviraj, A.; Saha, S. Toxicity and Bioaccumulation of Chromium in Some Freshwater Fish. Hum. Ecol. Risk Assess. Int. J. 2017, 23, 1655–1667. [Google Scholar] [CrossRef]
- Yossa, R.; Verdegem, M. Misuse of Multiple Comparison Tests and Underuse of Contrast Procedures in Aquaculture Publications. Aquaculture 2015, 437, 344–350. [Google Scholar] [CrossRef]
- EFSA Scientific Committee (SC); More, S.J.; Bampidis, V.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; Machera, K.; et al. Guidance on aneugenicity assessment. EFSA J. 2021, 19, e06770. [Google Scholar]
- Vera-Candioti, J.; Soloneski, S.; Larramendy, M.L. Evaluation of the Genotoxic and Cytotoxic Effects of Glyphosate-Based Herbicides in the Ten Spotted Live-Bearer Fish Cnesterodon Decemmaculatus (Jenyns, 1842). Ecotoxicol. Environ. Saf. 2013, 89, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Lu, G.; Jiang, R.; Yan, Z.; Li, Y. Occurrence, toxicity and ecological risk of Bisphenol A analogues in aquatic environment–A review. Ecotoxicol. Environ. Saf. 2021, 208, 111481. [Google Scholar] [CrossRef]
- Wahby, M.M.; Abdallah, Z.M.; Abdou, H.M.; Yousef, M.I.; Newairy, A.S.A. Mitigating potential of Ginkgo biloba extract and melatonin against hepatic and nephrotoxicity induced by Bisphenol A in male rats. Egypt. J. Basic Appl. Sci. 2017, 4, 350–357. [Google Scholar]
- Sirasanagandla, S.R.; Al-Huseini, I.; Sakr, H.; Moqadass, M.; Das, S.; Juliana, N.; Abu, I.F. Natural products in mitigation of Bisphenol a toxicity: Future therapeutic use. Molecules 2022, 27, 5384. [Google Scholar] [CrossRef]
- Giommi, C.; Habibi, H.R.; Candelma, M.; Carnevali, O.; Maradonna, F. Probiotic administration mitigates bisphenol A reproductive toxicity in zebrafish. Int. J. Mol. Sci. 2021, 22, 9314. [Google Scholar] [CrossRef]





| Concentration, mg/L | Survival Rate, % | LC50 Value (CI), mg/L | |||
|---|---|---|---|---|---|
| 12 h | 24 h | 48 h | 96 h | ||
| Control | 100 | 100 | 100 | 100 | LC5012 = 18.04 (17.81–18.28); LC5024 = 7.55 (7.38–7.72); LC5048/96 = 6.22 (6.11–6.33) |
| 0.5 | 100 | 100 | 100 | 100 | |
| 1 | 100 | 100 | 100 | 100 | |
| 2 | 100 | 100 | 100 | 100 | |
| 4 | 100 | 100 | 96.6 | 96.6 | |
| 6 | 100 | 83.3 | 56.6 | 56.6 | |
| 8 | 100 | 40 | 13.3 | 13.3 | |
| 12 | 100 | 0 | 0 | 0 | |
| 16 | 76.6 | 0 | 0 | 0 | |
| 20 | 26.6 | 0 | 0 | 0 | |
| Hematopoetic Cell, % | BPA Concentration, mg/L | ||||
|---|---|---|---|---|---|
| Ctr | 2 | 4 | 6 | 8 | |
| Blast cells (BC) | 1.55 ± 0.15 b | 1.57 ± 0.26 ab | 2.06 ± 0.86 ab | 2.2 ± 0.39 a | 2.13 ± 0.32 a |
| Basophilic erythroblast (BEb) | 14.7 ± 1.66 b | 14.02 ± 1.7 b | 13.19 ± 1.8 b | 17.29 ± 1.7 ab | 23.7 ± 1.79 a |
| Polychromatic erythroblast (PEb) | 13.58 ± 1.7 b | 26.9 ± 2.88 a | 22.7 ± 1.07 ab | 27.35 ± 0.92 a | 25.65 ± 3.8 a |
| Orthochromatic erythroblast (OEb) | 14.58 ± 3.1 ab | 17.49 ± 2.5 a | 20.04 ± 1.09 a | 9.22 ± 1.01 b | 10.19 ± 2.2 b |
| Erythrocyte (ER) | 7.34 ± 1.45 | 7.56 ± 0.55 | 6.66 ± 0.81 | 6.61 ± 0.91 | 6.45 ± 1.13 |
| Promyelocyte (PMy) | 1.74 ± 0.39 | 2.46 ± 0.96 | 2.43 ± 0.71 | 1.99 ± 0.08 | 2.02 ± 0.45 |
| Myelocyte (My) | 6.37 ± 1.11 | 6.91 ± 1.63 | 6.31 ± 0.69 | 6.38 ± 1.04 | 5.22 ± 0.68 |
| Metamyelocyte (MMy) | 17.4 ± 1.78 a | 10.76 ± 2.2 b | 9.83 ± 0.58 b | 11.5 ± 1.28 ab | 9.49 ± 2.4 b |
| Granulocyte-band form (GBF) | 8.36 ± 1.48 a | 4.97 ± 0.9 c | 6.2 ± 0.42 abc | 7.38 ± 1.26 ab | 5.66 ± 0.63 c |
| Granulocyte-segmented form (GSF) | 11 ± 1.38 a | 4.78 ± 0.97 c | 7.83 ± 0.65 ab | 7.11 ± 0.71 bc | 6.71 ± 0.94 bc |
| Unclassified cells | 3.14 ± 0.18 | 2.5 ± 0.5 | 2.54 ± 1.17 | 2.87 ± 0.79 | 2.68 ± 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smorodinskaya, S.; Kochetkov, N.; Gavrilin, K.; Nikiforov-Nikishin, D.; Reznikova, D.; Vatlin, A.; Klimuk, A.; Odorskaya, M.; Nikiforov-Nikishin, A.; Ponomarev, A.; et al. The Effects of Acute Bisphenol A Toxicity on the Hematological Parameters, Hematopoiesis, and Kidney Histology of Zebrafish (Danio rerio). Animals 2023, 13, 3685. https://doi.org/10.3390/ani13233685
Smorodinskaya S, Kochetkov N, Gavrilin K, Nikiforov-Nikishin D, Reznikova D, Vatlin A, Klimuk A, Odorskaya M, Nikiforov-Nikishin A, Ponomarev A, et al. The Effects of Acute Bisphenol A Toxicity on the Hematological Parameters, Hematopoiesis, and Kidney Histology of Zebrafish (Danio rerio). Animals. 2023; 13(23):3685. https://doi.org/10.3390/ani13233685
Chicago/Turabian StyleSmorodinskaya, Svetlana, Nikita Kochetkov, Kirill Gavrilin, Dmitry Nikiforov-Nikishin, Diana Reznikova, Aleksey Vatlin, Anastasia Klimuk, Maya Odorskaya, Alexei Nikiforov-Nikishin, Andrey Ponomarev, and et al. 2023. "The Effects of Acute Bisphenol A Toxicity on the Hematological Parameters, Hematopoiesis, and Kidney Histology of Zebrafish (Danio rerio)" Animals 13, no. 23: 3685. https://doi.org/10.3390/ani13233685
APA StyleSmorodinskaya, S., Kochetkov, N., Gavrilin, K., Nikiforov-Nikishin, D., Reznikova, D., Vatlin, A., Klimuk, A., Odorskaya, M., Nikiforov-Nikishin, A., Ponomarev, A., Marsova, M., & Danilenko, V. (2023). The Effects of Acute Bisphenol A Toxicity on the Hematological Parameters, Hematopoiesis, and Kidney Histology of Zebrafish (Danio rerio). Animals, 13(23), 3685. https://doi.org/10.3390/ani13233685







