Predation Risk, and Not Shelter or Food Availability, as the Main Determinant of Reproduction Investment in Island Lizards
Abstract
Simple Summary
Abstract
1. Introduction
- The amount of food available to the lizards (Food Limitation Hypothesis);
- The amount of shelter available to the lizards (Gravid Female Protection Hypothesis);
- The species richness of the local predator community (Predation Risk Hypothesis).
2. Materials and Methods
2.1. Study System
2.2. Study Organism
2.3. Reproductive Traits
2.4. Predation Pressure
2.5. Measurement of Food Availability
2.6. Measurement of Vegetation
2.7. Statistical Analyses
3. Results
3.1. Linear Models of Island Size and Independent Variables
3.2. Correlations
3.3. Effect of Predator Richness, Biomass of Arthropods, and NDVI on Reproductive Traits
3.4. Hypothesis Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
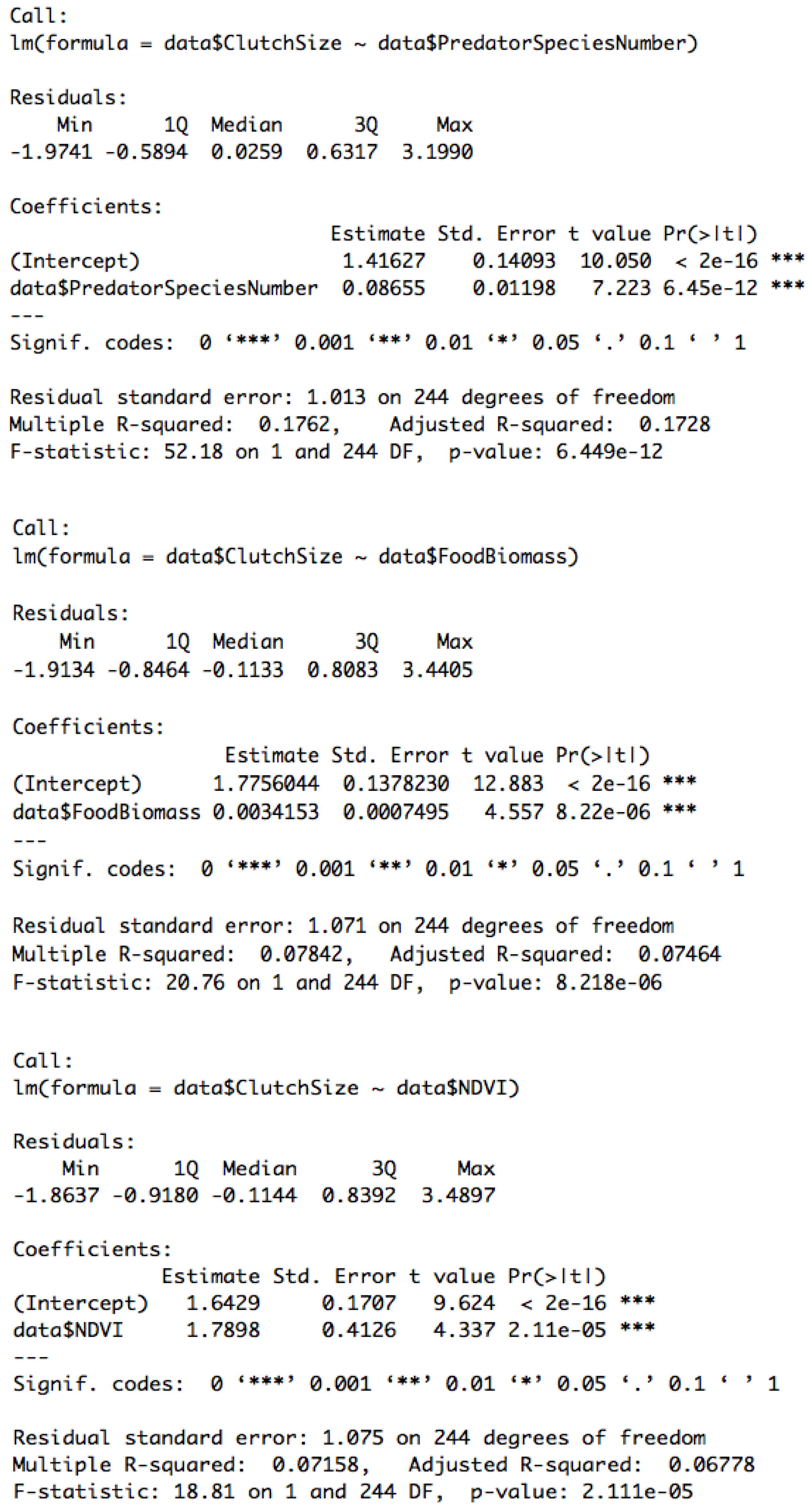
Appendix C

Appendix D

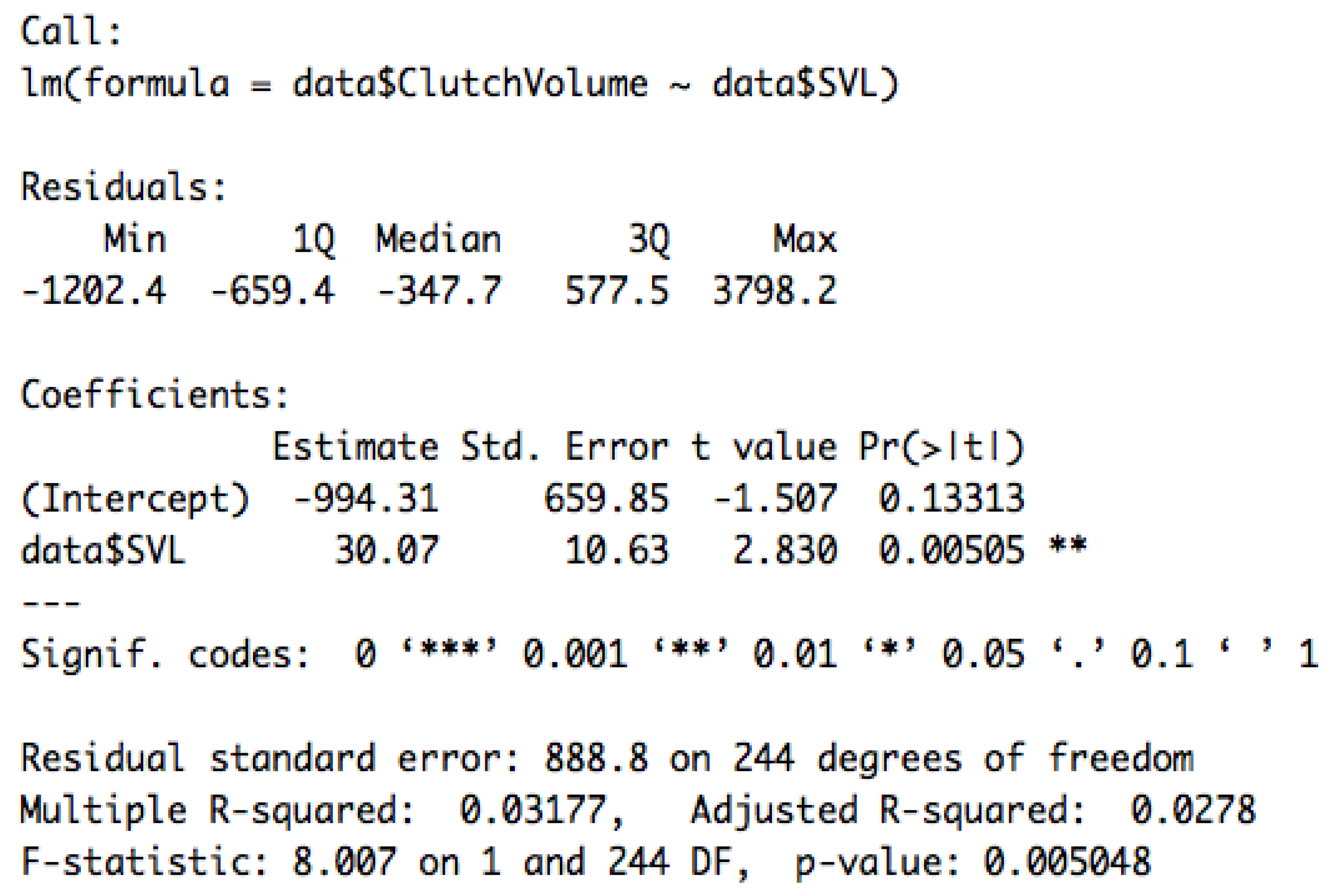
Appendix E


Appendix F
| Model | AICc | ∆ AICc | Akaike Weight |
|---|---|---|---|
| CS ~ P + SVL | 695.691 | 0 | 0.998 |
| CS ~ P | 708.225 | 12.533 | 1.895 × 10−3 |
| CS ~ B + SVL | 712.715 | 17.024 | 2.007 × 10−4 |
| CS ~ NDVI +SVL | 719.651 | 23.959 | 6.257 × 10−6 |
| CS ~ SVL | 733.504 | 37.812 | 6.141 × 10−9 |
| CS ~ B | 735.805 | 40.114 | 1.943 × 10−9 |
| CS ~ NDVI | 737.624 | 41.933 | 7.826 × 10−10 |
| Model | AIC | ∆ AIC | Akaike Weight |
|---|---|---|---|
| CV ~ P + SVL | 4030.073 | 0 | 0.753 |
| CV ~ P | 4032.505 | 2.432 | 0.223 |
| CV ~ B + SVL | 4037.205 | 7.132 | 2.128 × 10−2 |
| CV ~ SVL | 4042.707 | 12.634 | 1.359 × 10−2 |
| CV ~ B | 4043.606 | 13.533 | 8.668 × −10−4 |
| CV ~ NDVI + SVL | 4043.926 | 13.853 | 7.386 × 10−4 |
| CV ~ NDVI | 4049.325 | 19.252 | 4.967 × 10−5 |
Appendix G
| Island Name | Area (km2) | Predators |
|---|---|---|
| Kokkinonisi | 0.005 | 2 Snakes: — 0 Birds: Corvus, F. eleon. (H&D) Mammals: — |
| Mikropsathoura (Myiga) | 0.014 | 2 Snakes: — 0 Birds: Corvus (Wt), F. eleon. (H&D) Mammals: — |
| Ag. Ioannis | 0.033 | 2 Snakes: — 0 Birds: Corvus, F. eleon. (H&D) Mammals: — 0 |
| Kopria | 0.138 | 2 Snakes: — 0 Birds: Corvus Field observ. (JF) F. eleon (JF) Mammals: |
| Glaronisi | 0.188 | 2 Rats: F. eleon (JF) Birds: R. rattus (JF) |
| Ano Koufonisi | 5.770 | 5 Snakes: Eryx (JF), Vipera (JF). Birds: F. tinnunculus (JF), Corvus (JF) Mammals: Rattus sp. |
| Gioura | 11.052 | 6 Snakes: Colubridae sp. (Legakis) Birds: Corvus (JF), B. buteo, F. tinnunc (H&D), F. eleon (H&D), Mammals: Rattus sp. |
| Santorini | 76.197 | 9 Snakes: E. sit (Dimit), E. quat (Clarck 90), T. fall (Chon) Birds: B. buteo (H&D), F. tinnunc (H&D), F. eleon (H&D), A. noct (H&A), L. sen (H&A), Corvus (Wt), Mammals: Rattus sp. |
| Skopelos | 96.229 | 11 Snakes: E. sit (Dimit), M. monsp (Dimit), E. quat (Cattan 98), V. ammod (Cattan) Birds: Corvus (Wt), L. senat (Wt), B. buteo (H&D), F. tinnunc (H&D) F. eleon (H&D), A. noct (H&A), L. sen (H&A) Mammals: Rattus sp., M. foi (JF) |
| Tinos | 194.500 | 12 Snakes: E. sit (Dimit), E. quat (Dimit), D. caspius (Dimit), N. natr (Chon), V. amm (Chon), T. fall (Chon) Birds: B. buteo (H&D), F. tinnunc (H&D), F. eleon (H&D), A. noct (H&A), L. sen (H&A), Corvus (Wt), Mammals: |
| Andros | 380.000 | 13 Snakes: Z. sit, E. quat, D. casp, N. natr, T. fall, V. amm Birds: B. buteo, C. gall, L. sen, T. fall |
| Naxos | 429.785 | Mammals: R. rat, M. foi, V. vul 11 Snakes: E. quat (JF), N. natr (Chon), V. amm (Chon) Birds: Corvus (Wt), B. buteo (H&D), B. ruf (H&A), C. gallic (H&A), F. tinnunc (H&D), F. eleon (H&D), A. noct (H&A), L. sen (H&A) Mammals: R. rat, M. foi (JF) |
| Olympiada | 1000 | 16 Snakes: Z. sit, E. quat, Z. long, D. casp, P. naj, N. natr, V. amm Birds: B. buteo, C. gall, C. aer, F. tinnunc, A. noct, L. coll, L. sen, Corvus Mammals: M. foi |
| Vevi | 1000 | 18 Snakes: Z. sit, E. quat, D. casp, H. gem, N. natr, P. naj, M. monsp, V. ammo Birds: B. buteo, C. gall, C. aer, F. tinnunc, A. noct, L. coll, L. sen, Corvus Mammals: Rattus, M. foi (JF) |
References
- Spatz, D.R.; Zilliacus, K.M.; Holmes, N.D.; Butchart, S.H.M.; Genovesi, P.; Ceballos, G.; Tershy, B.R.; Croll, D.A. Globally threatened vertebrates on islands with invasive species. Sci. Adv. 2017, 3, e1603080. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.J.; Fernández-Palacios, J.M.; Matthews, T.J.; Borregaard, M.K.; Triantis, K.A. Island biogeography: Taking the long view of nature’s laboratories. Science 2017, 357, eaam8326. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef] [PubMed]
- Wikelski, M.; Foufopoulos, J.; Vargas, H.; Snell, H. Galápagos birds and diseases: Invasive pathogens as threats for island species. Ecol. Soc. 2004, 9, 5. [Google Scholar] [CrossRef]
- Foufopoulos, J.; Kilpatrick, A.M.; Ives, A.R. Climate change and elevated extinction rates of reptiles from Mediterranean islands. Am. Nat. 2011, 177, 119–129. [Google Scholar] [CrossRef]
- Adler, G.H.; Levins, R. The island syndrome in rodent populations. Q. Rev. Biol. 1994, 69, 473–490. [Google Scholar] [CrossRef]
- Goltsman, M.; Kruchenkova, E.P.; Sergeev, S.; Volodin, I.; Macdonald, D.W. ‘Island syndrome’ in a population of Arctic foxes (Alopex lagopus) from Mednyi Island. J. Zool. 2005, 267, 405–418. [Google Scholar] [CrossRef]
- Clegg, S.M.; Owens, P.F. The ‘island rule’ in birds: Medium body size and its ecological explanation. Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Novosolov, M.; Meiri, S. The effect of island type on lizard reproductive traits. J. Biogeogr. 2013, 40, 2385–2395. [Google Scholar] [CrossRef]
- Case, T.J. A general explanation for insular body size trends in terrestrial vertebrates. Ecology 1978, 59, 1–18. [Google Scholar] [CrossRef]
- Meiri, S. Size evolution in island lizards. Glob. Ecol. Biogeogr. 2007, 16, 702–708. [Google Scholar] [CrossRef]
- Pafilis, P.; Meiri, S.; Foufopoulos, J.; Valakos, E. Intraspecific competition and high food availability are associated with insular gigantism in a lizard. Naturwissenschaften 2009, 96, 1107–1113. [Google Scholar] [CrossRef]
- Herrel, A.; Huyghe, K.; Vanhooydonck, B.; Backeljau, T.; Breugelmans, K.; Grbac, I.; Damme, R.V.; Irschick, D.J. Rapid large-scale evolutionary divergence in morphology and performance associated with exploitation of a different dietary resource. Proc. Natl. Acad. Sci. USA 2008, 105, 4792–4795. [Google Scholar] [CrossRef] [PubMed]
- Runemark, A.; Hansson, B.; Pafilis, P.; Valakos, E.D.; Svensson, E.I. Island biology and morphological divergence of the Skyros wall lizard Podarcis gaigeae: A combined role for local selection and genetic drift on color morph frequency divergence? BMC Evol. Biol. 2010, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.M. Growth rate in island and mainland anoline lizards. Copeia 1976, 1976, 477–482. [Google Scholar] [CrossRef]
- Case, T.J. Species numbers, density compensation, and colonizing ability of lizards on islands in the Gulf of California. Ecology 1975, 56, 3–18. [Google Scholar] [CrossRef]
- Novosolov, M.; Rodda, G.H.; Feldman, A.; Kadison, A.E.; Dor, R.; Meiri, S. Power in numbers. Drivers of high population density in insular lizards. Glob. Ecol. Biogeogr. 2016, 25, 87–95. [Google Scholar] [CrossRef]
- BeVier, G.T.; Brock, K.M.; Foufopoulos, J. Ecology and home range of the Aegean Wall Lizard (Podarcis erhardii). Herpetol. Conserv. Biol. 2021, 16, 394–404. [Google Scholar]
- Cooper, W.E., Jr.; Dimopoulos, I.; Pafilis, P. Sex, age, and population density affect aggressive behaviors in island lizards promoting cannibalism. Ethology 2015, 121, 260–269. [Google Scholar] [CrossRef]
- Donihue, C.M.; Brock, K.M.; Foufopoulos, J.; Herrel, A. Feed or fight: Testing the impact of food availability and intraspecific aggression on the functional ecology of an island lizard. Funct. Ecol. 2016, 30, 566–575. [Google Scholar] [CrossRef]
- Brock, K.M.; Bednekoff, P.A.; Pafilis, P.; Foufopoulos, J. Evolution of antipredator behavior in an island lizard species, Podarcis erhardii (Reptilia: Lacertidae): The sum of all fears? Evolution 2015, 69, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, F.; Guo, Z.; Liu, X.; Jin, C.; Wang, Y.; Wang, S. Reduced predator species richness drives the body gigantism of a frog species on the Zhoushan Archipelago in China. J. Anim. Ecol. 2011, 80, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Pafilis, P.; Foufopoulos, J.; Poulakakis, N.; Lymberakis, P.; Valakos, E.D. Tail shedding in island lizards [Lacertidae, Reptilia]: Decline of antipredator defenses in relaxed predation environments. Evolution 2009, 63, 1262–1278. [Google Scholar] [CrossRef]
- Huang, W.S. Ecology and reproductive patterns of the agamid lizard Japalura swinhonis on an east Asian island, with comments on the small clutch sizes of island lizards. Zool. Sci. 2007, 24, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.C.; Fretwell, S.D. The optimal balance between size and number of offspring. Am. Nat. 1974, 108, 499–506. [Google Scholar] [CrossRef]
- Blondel, J. Evolution and ecology of birds on islands: Trends and prospects. Vie Milieu 2000, 50, 205–220. [Google Scholar]
- Galán, P. Reproductive characteristics of an insular population of the lizard Podarcis hispanica from Northwest Spain (Cies Islands, Galicia). Copeia 2003, 2003, 657–665. [Google Scholar] [CrossRef]
- Pafilis, P.; Foufopoulos, J.; Sagonas, K.; Runemark, A.; Svensson, E.; Valakos, E.D. Reproductive biology of insular reptiles: Marine subsidies modulate expression of the “island syndrome”. Copeia 2011, 2011, 545–552. [Google Scholar] [CrossRef]
- Du, W.G.; Ji, X.; Zhang, Y.P.; Xu, X.F.; Shine, R. Identifying sources of variation in reproductive and life-history traits among five populations of a Chinese lizard (Takydromus septentrionalis, Lacertidae). Biol. J. Linn. Soc. 2005, 85, 443–453. [Google Scholar] [CrossRef]
- Mesquita, D.O.; Costa, G.C.; Colli, G.R.; Costa, T.B.; Shepard, D.B.; Vitt, L.J.; Pianka, E.R. Life-history patterns of lizards of the world. Am. Nat. 2016, 187, 689–705. [Google Scholar] [CrossRef] [PubMed]
- Lundblad, C.G.; Conway, C.J. Ashmole’s hypothesis and the latitudinal gradient in clutch size. Ecol. Evol. 2021, 94, 1349–1366. [Google Scholar] [CrossRef] [PubMed]
- Angilletta, M.J., Jr.; Niewiarowski, P.H.; Dunham, A.E.; Leaché, A.D.; Porter, W.P. Bergmann’s clines in ectotherms: Illustrating a life-history perspective with sceloporine lizards. Am. Nat. 2004, 164, E168–E183. [Google Scholar] [CrossRef]
- Roitberg, E.S.; Kuranova, V.N.; Bulakhova, N.A.; Orlova, V.F.; Eplanova, G.V.; Zinenko, O.I.; Shamgunova, R.R.; Hofmann, F.; Yakovlev, V.A. Variation of reproductive traits and female body size in the most widely-ranging terrestrial reptile: Testing the effects of reproductive mode, lineage, and climate. Evol. Biol. 2013, 40, 420–438. [Google Scholar] [CrossRef] [PubMed]
- Sinervo, B. The evolution of maternal investment in lizards: An experimental and comparative analysis of egg size and its effects on offspring performance. Evolution 1990, 44, 279–294. [Google Scholar] [CrossRef]
- Meiri, S.; Avila, L.; Bauer, A.M.; Chapple, D.G.; Das, I.; Doan, T.M.; Doughty, P.; Ellis, R.; Grismer, L.; Kraus, F.; et al. The global diversity and distribution of lizard clutch sizes. Glob. Ecol. Biogeogr. 2020, 29, 1515–1530. [Google Scholar] [CrossRef]
- Ballinger, R.E. Intraspecific variation in demography and life history of the lizard, Sceloporus jarrovi, along an altitudinal gradient in southeastern Arizona. Ecology 1979, 60, 901–909. [Google Scholar] [CrossRef]
- Cruz-Elizalde, R.; Ramírez-Bautista, A. Reproductive cycles and reproductive strategies among populations of the Rose-bellied Lizard Sceloporus variabilis (Squamata: Phrynosomatidae) from central Mexico. Ecol. Evol. 2016, 6, 1753–1768. [Google Scholar] [CrossRef] [PubMed]
- Vitt, L.J.; Congdon, J.D. Body shape, reproductive effort, and relative clutch mass in lizards: Resolution of a paradox. Am. Nat. 1978, 112, 595–608. [Google Scholar] [CrossRef]
- Mesquita, D.O.; Colli, G.R. Life history patterns in South American tropical lizards. Reprod. Reptiles Morfol. Ecol. Evol. 2010, 45–71. [Google Scholar]
- Werneck FD, P.; Giugliano, L.G.; Collevatti, R.G.; Colli, G.R. Phylogeny, biogeography and evolution of clutch size in South American lizards of the genus Kentropyx (Squamata: Teiidae). Mol. Ecol. 2009, 18, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Vitt, L.J. Lizard reproduction: Habitat specificity and constraints on relative clutch mass. Am. Nat. 1981, 117, 506–514. [Google Scholar] [CrossRef]
- Goodman, B.A.; Hudson, S.C.; Isaac, J.L.; Schwarzkopf, L. The evolution of body shape in response to habitat: Is reproductive output reduced in flat lizards? Evolution 2009, 63, 1279–1291. [Google Scholar] [CrossRef]
- Ballinger, R.E. Reproductive strategies: Food availability as a source of proximal variation in a lizard. Ecology 1977, 58, 628–635. [Google Scholar] [CrossRef]
- Hermansson, I.; von Numers, M.; Jaatinen, K.; Öst, M. Predation risk and landscape properties shape reproductive output of an endangered sea duck from two subpopulations with contrasting predation risk. J. Ornithol. 2023, 164, 311–326. [Google Scholar] [CrossRef]
- Foufopoulos, J. Host-Parasite Interactions in the Mountain Spiny Lizard Sceloporus jarrovi. Ph.D. Thesis, University of Wisconsin, Madison, WI, USA, 1999; 210p. [Google Scholar]
- Jordan, M.A.; Snell, H.L. Life history trade-offs and phenotypic plasticity in the reproduction of Galapagos lava lizards (Microlophus delanonis). Oecologia 2002, 130, 44–52. [Google Scholar] [CrossRef]
- Hoy, S.R.; Millon, A.; Petty, S.J.; Whitfield, D.P.; Lambin, X. Food availability and predation risk, rather than intrinsic attributes, are the main factors shaping the reproductive decisions of a long-lived predator. J. Anim. Ecol. 2016, 85, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Warne, R.W.; Gilman, C.A.; Garcia, D.A.; Wolf, B.O. Capital breeding and allocation to life-history demands are highly plastic in lizards. Am. Nat. 2012, 180, 130–141. [Google Scholar] [CrossRef]
- Du, W.G. Phenotypic plasticity in reproductive traits induced by food availability in a lacertid lizard, Takydromus septentrionalis. Oikos 2006, 112, 363–369. [Google Scholar] [CrossRef]
- Schaffer, W.M. Optimal reproductive effort in fluctuating environments. Am. Nat. 1974, 108, 783–790. [Google Scholar] [CrossRef]
- Stearns, S.C. The evolution of life history traits: A critique of the theory and a review of the data. Annu. Rev. Ecol. Syst. 1977, 8, 145–171. [Google Scholar] [CrossRef]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, NY, USA, 1992. [Google Scholar]
- Magnhagen, C. Predation risk as a cost of reproduction. Trends Ecol. Evol. 1991, 6, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Schwarzkopf, L. Measuring trade-offs: A review of studies of costs of reproduction in lizards. In Lizard Ecology: Historical and Experimental Perspectives; Vitt, L.J., Pianka, E.R., Eds.; Princeton University Press: Princeton, NJ, USA, 1994; pp. 7–30. [Google Scholar]
- Schwarz, R.; Itescu, Y.; Antonopoulos, A.; Gavriilidi, I.-A.; Tamar, K.; Pafilis, P.; Meiri, S. Isolation and predation drive gecko life-history evolution on islands. Biol. J. Linn. Soc. 2020, 129, 618–629. [Google Scholar] [CrossRef]
- Van Damme, R.; Bauwens, D.; Verheyen, R.F. Effect of relative clutch mass on sprint speed in the lizard Lacerta vivipara. J. Herpetol. 1989, 23, 459–461. [Google Scholar] [CrossRef]
- Miles, D.B.; Sinervo, B.; Frankino, W.A. Reproductive burden, locomotor performance, and the cost of reproduction in free ranging lizards. Evolution 2000, 54, 1386–1395. [Google Scholar]
- Reznick, D. Costs of reproduction: An evaluation of the empirical evidence. Oikos 1985, 44, 257–267. [Google Scholar] [CrossRef]
- Reznick, D. Measuring the costs of reproduction. Trends Ecol. Evol. 1992, 7, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.E.; Vitt, L.J.; Hedges, R.; Huey, R.B. Locomotor impairment and defense in gravid lizards (Eumeces laticeps): Behavioral shift in activity may offset costs of reproduction in an active forager. Behav. Ecol. Sociobiol. 1990, 27, 153–157. [Google Scholar] [CrossRef]
- Braña, F. Shifts in body temperature and escape behaviour of female Podarcis muralis during pregnancy. Oikos 1993, 66, 216–222. [Google Scholar] [CrossRef]
- Bauwens, D.; Thoen, C. Escape tactics and vulnerability to predation associated with reproduction in the lizard Lacerta vivipara. J. Anim. Ecol. 1981, 50, 733–743. [Google Scholar] [CrossRef]
- Brown, G.P.; Shine, R. Effects of reproduction on the antipredator tactics of snakes (Tropidonophis mairii, Colubridae). Behav. Ecol. Sociobiol. 2004, 56, 257–262. [Google Scholar] [CrossRef]
- Lima, S.L.; Dill, L.M. Behavioral decisions made under the risk of predation: A review and prospectus. Can. J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Cruz-Elizalde, R.; Ramírez-Bautista, A.; Stephenson, B.P.; Luja, V.H.; Hernández-Salinas, U. Variation in female reproduction between populations of the arboreal lizard Urosaurus bicarinatus (Squamata: Phrynosomatidae) from two different environments in Mexico. Salamandra 2017, 53, 359–367. [Google Scholar]
- Haenel, G. Effects of habitat on clutch size of ornate tree lizards, Urosaurus ornatus. West. N. Am. Nat. 2011, 71, 247–256. [Google Scholar] [CrossRef][Green Version]
- Dunham, A.E.; Miles, D.B. Patterns of covariation in life history traits of squamate reptiles: The effects of size and phylogeny reconsidered. Am. Nat. 1985, 126, 231–257. [Google Scholar] [CrossRef]
- Niewiarowski, P.H.; Angilletta, M.J.; Leaché, A.D. Phylogenetic comparative analysis of life-history variation among populations of the lizard Sceloporus undulatus: An example and prognosis. Evolution 2004, 58, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.; Pérez-Tris, J.; Tellería, J.; Carbonell, R.; Santos, T. Reproductive investment of a Lacertid lizard in fragmented habitat. Conserv. Biol. 2005, 19, 1578–1585. [Google Scholar] [CrossRef]
- Iverson, J.B.; Higgins, H.; Sirulnik, A.; Griffiths, C. Local and geographic variation in the reproductive biology of the snapping turtle (Chelydra serpentina). Herpetologica 1997, 53, 96–117. [Google Scholar]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Change 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Fielding, J.; Turland, N. Flowers of Crete, 2nd ed.; Royal Botanic Gardens, Kew: London, UK, 2008. [Google Scholar]
- Grove, A.T.; Rackham, O. The Nature of Mediterranean Europe: An Ecological History; Yale University Press: New Haven, CT, USA, 2023. [Google Scholar]
- Valakos, E.; Pafilis, P.; Sotiropoulos, K.; Lymberakis, P.; Maragou, P.; Foufopoulos, J. The Amphibians and Reptiles of Greece; Chimaira: Frankfurt, Germany, 2008. [Google Scholar]
- Brock, K.M.; Madden, I.E. Morph-specific differences in escape behavior in a color polymorphic lizard. Behav. Ecol. Sociobiol. 2022, 76, 104. [Google Scholar] [CrossRef]
- Arnold, E.N. Resource partition among lacertid lizards in southern Europe. J. Zool. 1987, 1, 739–782. [Google Scholar] [CrossRef]
- Gruber, U. Podarcis erhardii (Bedriaga, 1876)–Ägäische Mauereidechse. In Handbuch der Reptilien und Amphibien Europas; AULA Wiesbaden: Wiesbaden, Germany, 1986; Volume 2, pp. 25–49. [Google Scholar]
- Adamopoulou, C.; Valakos, E.D.; Pafilis, P. Summer diet of Podarcis milensis, P. gaigeae and P. erhardii (Sauria: Lacertidae). Bonn. Zool. Beiträge 1999, 48, 275–282. [Google Scholar]
- Madden, I.E.; Brock, K.M. An extreme record of cannibalism in Podarcis erhardii mykonensis (Reptilia: Lacertidae) from Siros island, Cyclades, Greece. Herpetol. Notes 2018, 11, 291–292. [Google Scholar]
- Mayhew, W.W. Reproduction in the granite spiny lizard, Sceloporus orcutti. Copeia 1963, 1963, 144–152. [Google Scholar] [CrossRef]
- Itescu, Y.; Schwarz, R.; Meiri, S.; Pafilis, P. Intraspecific competition, rather than predation, drives tail loss in insular geckos. J. Anim. Ecol. 2017, 86, 66–74. [Google Scholar] [CrossRef]
- Rogers, L.E.; Hinds, W.T.; Buschbom, R.L. A General Weight vs. Length Relationship for Insects. Ann. Entomol. Soc. Am. 1976, 69, 387–389. [Google Scholar] [CrossRef]
- Kohn, D.D.; Walsh, D.M. Plant species richness—The effect of island size and habitat diversity. J. Ecol. 1994, 82, 367–377. [Google Scholar] [CrossRef]
- Shure, D.J.; Phillips, D.L. Patch size of forest openings and arthropod populations. Oecologia 1991, 86, 325–334. [Google Scholar] [CrossRef]
- Polis, G.A.; Hurd, S.D. Extraordinarily high spider densities on islands: Flow of energy from the marine to terrestrial food webs and the absence of predation. Proc. Natl. Acad. Sci. USA 1995, 92, 4382–4386. [Google Scholar] [CrossRef]
- Abell, A.J.; Cole, B.J.; Reyes, R.; Wiernasz, D.C. Sexual selection on body size and shape in the western harvester ant, Pogonomyrmex occidentalis Cresson. Evolution 1999, 53, 535–545. [Google Scholar] [CrossRef]
- Turkheimer, E.; Haley, A.; Waldron, M.; d’Onofrio, B.; Gottesman, I.I. Socioeconomic status modifies heritability of IQ in young children. Psychol. Sci. 2003, 14, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Tinkle, D.W. The concept of reproductive effort and its relation to the evolution of life histories of lizards. Am. Nat. 1969, 103, 501–516. [Google Scholar] [CrossRef]
- Tinkle, D.W.; Wilbur, H.M.; Tilley, S.G. Evolutionary strategies in lizard reproduction. Evolution 1970, 24, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Siliceo, I.; Díaz, J.A. A comparative study of clutch size, range size, and the conservation status of island vs. mainland lacertid lizards. Biol. Conserv. 2010, 143, 2601–2608. [Google Scholar] [CrossRef]
- Shine, R. Life-history evolution in reptiles. Annu. Rev. Ecol. Syst. 2005, 36, 23–46. [Google Scholar] [CrossRef]
- Qualls, C.P.; Shine, R. Maternal body-volume as a constraint on reproductive output in lizards: Evidence from the evolution of viviparity. Oecologia 1995, 103, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Shine, R. Locomotor speeds of gravid lizards: Placing ‘costs of reproduction’within an ecological context. Funct. Ecol. 2003, 17, 526–533. [Google Scholar] [CrossRef]
- Shine, R.; Schwarzkopf, L. The evolution of reproductive effort in lizards and snakes. Evolution 1992, 46, 62–75. [Google Scholar] [CrossRef]
- Olsson, M.; Shine, R.; Wapstra, E. Costs of reproduction in a lizard species: A comparison of observational and experimental data. Oikos 2001, 93, 121–125. [Google Scholar] [CrossRef]
- Sinervo, B.; Licht, P. Proximate constraints on the evolution of egg size, number, and total clutch mass in lizards. Science 1991, 252, 1300–1302. [Google Scholar] [CrossRef]
- Slavenko, A.; Itescu, Y.; Foufopoulos, J.; Pafilis, P.; Meiri, S. Clutch size variability in an ostensibly fix-clutched lizard: Effects of insularity on a Mediterranean gecko. Evol. Biol. 2015, 42, 129–136. [Google Scholar] [CrossRef]
- Semegen, S.L. Predation Pressure as A Determinant of Locomotor Performance: Lizards Run Slower on Islands Without Predators. Master’s Thesis, University of Michigan, Ann Arbor, MI, USA, 2018. [Google Scholar]
- Vervust, B.; Grbac, I.; Van Damme, R. Differences in morphology, performance and behaviour between recently diverged populations of Podarcis sicula mirror differences in predation pressure. Oikos 2017, 116, 1343–1352. [Google Scholar] [CrossRef]
- Pérez-Mellado, V.; Corti, C.; LoCascio, P. Tail autotomy and extinction in Mediterranean lizards. A preliminary study of continental and insular populations. J. Zool. 1997, 243, 533–541. [Google Scholar] [CrossRef]
- Cooper, W.E. Tradeoffs between predation risk and feeding in a lizard, the broad-headed skink (Eumeces laticeps). Behaviour 2000, 137, 1175–1189. [Google Scholar] [CrossRef]
- Li, B.; Belasen, A.; Pafilis, P.; Bednekoff, P.; Foufopoulos, J. Effects of feral cats on the evolution of anti-predator behaviours in island reptiles: Insights from an ancient introduction. Proc. R. Soc. Lond. B 2014, 281, 20140339. [Google Scholar] [CrossRef] [PubMed]
- Mugabo, M.; Marquis, O.; Perret, S.; Le Galliard, J.F. Direct and socially-mediated effects of food availability late in life on life-history variation in a short-lived lizard. Oecologia 2011, 166, 949–960. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Tripathi, V. Physiological effects of food availability times in higher vertebrates. J. Exp. Biol. 2022, 225, jeb239004. [Google Scholar] [CrossRef] [PubMed]
- Adamopoulou, C.; Valakos, E.D. Small clutch size in a Mediterranean endemic lacertid (Podarcis milensis). Copeia 2000, 2000, 610–614. [Google Scholar] [CrossRef]
- Valakos, E. The feeding ecology of Podarcis erhardii (Reptilia-Lacertidae) in a main insular ecosystem. Herpetol. J. 1986, 1, 118–121. [Google Scholar]
- McLean, J.A.; Speakman, J.R. Morphological changes during postnatal growth and reproduction in the brown long-eared bat Plecotus auritus: Implications for wing loading and predicted flight performance. J. Nat. Hist. 2000, 34, 773–791. [Google Scholar] [CrossRef]
- Lee, S.J.; Witter, M.S.; Cuthill, I.C.; Goldsmith, A.R. Reduction in escape performance as a cost of reproduction in gravid starlings, Sturnus vulgaris. Proc. R. Soc. Lond. B 1996, 263, 619–623. [Google Scholar]
- Veasey, J.S.; Houston, D.C.; Metcalfe, N.B. A hidden cost of reproduction: The trade-off between clutch size and escape take-off speed in female zebra finches. J. Anim. Ecol. 2001, 70, 20–24. [Google Scholar]
- Seigel, R.A.; Huggins, M.M.; Ford, N.B. Reduction in locomotor ability as a cost of reproduction in gravid snakes. Oecologia 1987, 73, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Shine, R. “Costs” of reproduction in reptiles. Oecologia 1980, 46, 92–100. [Google Scholar] [CrossRef] [PubMed]

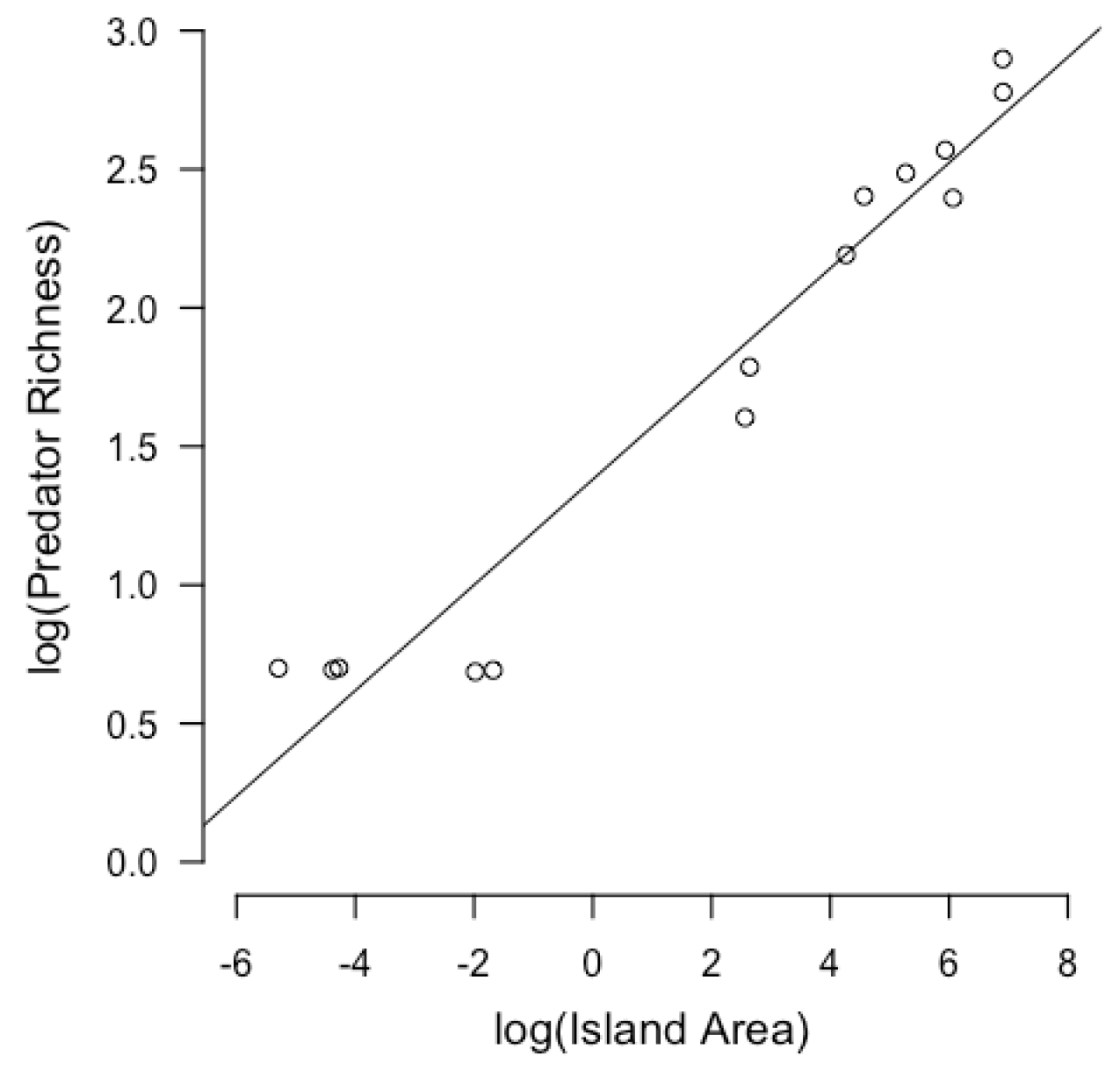
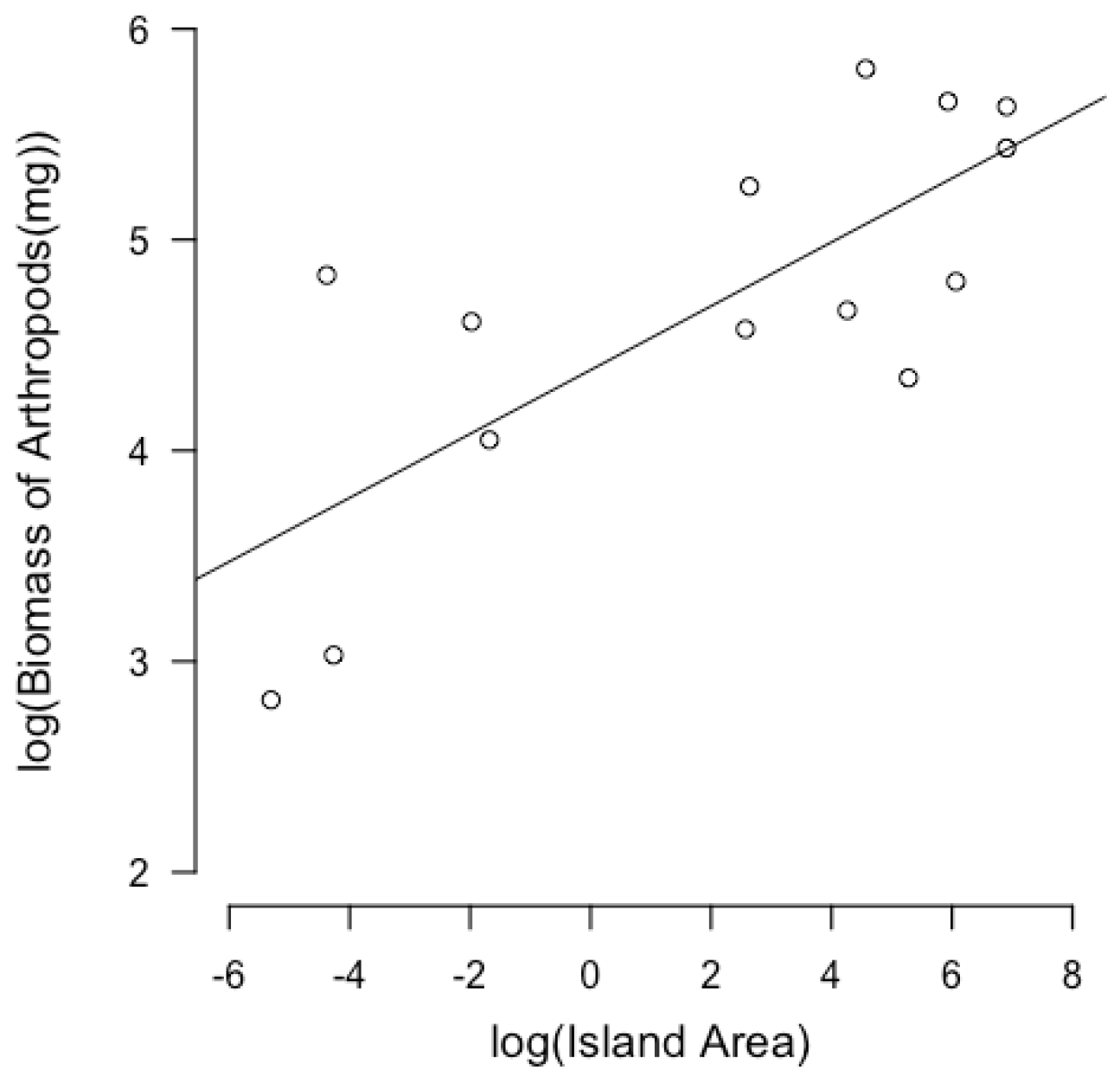
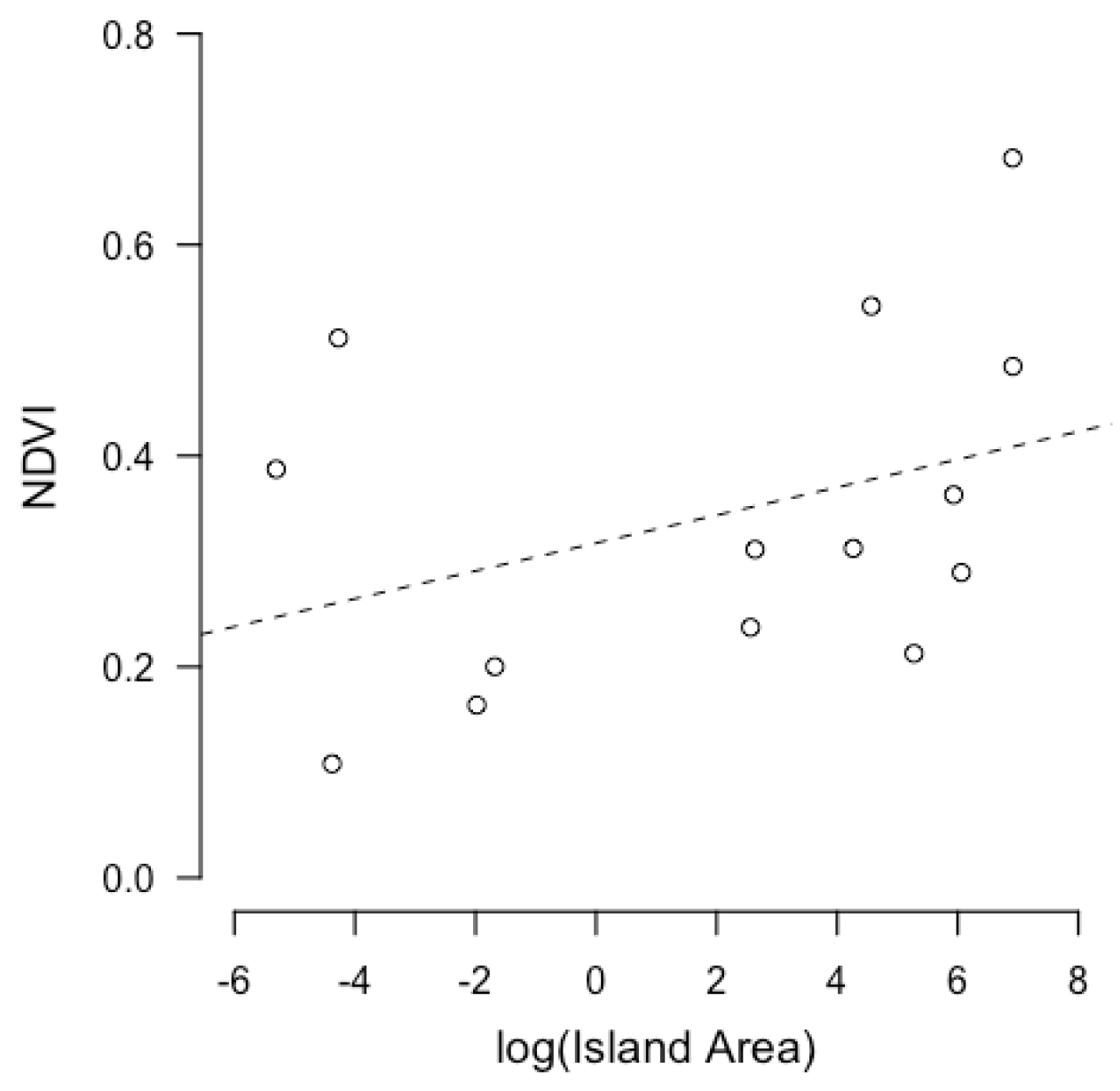
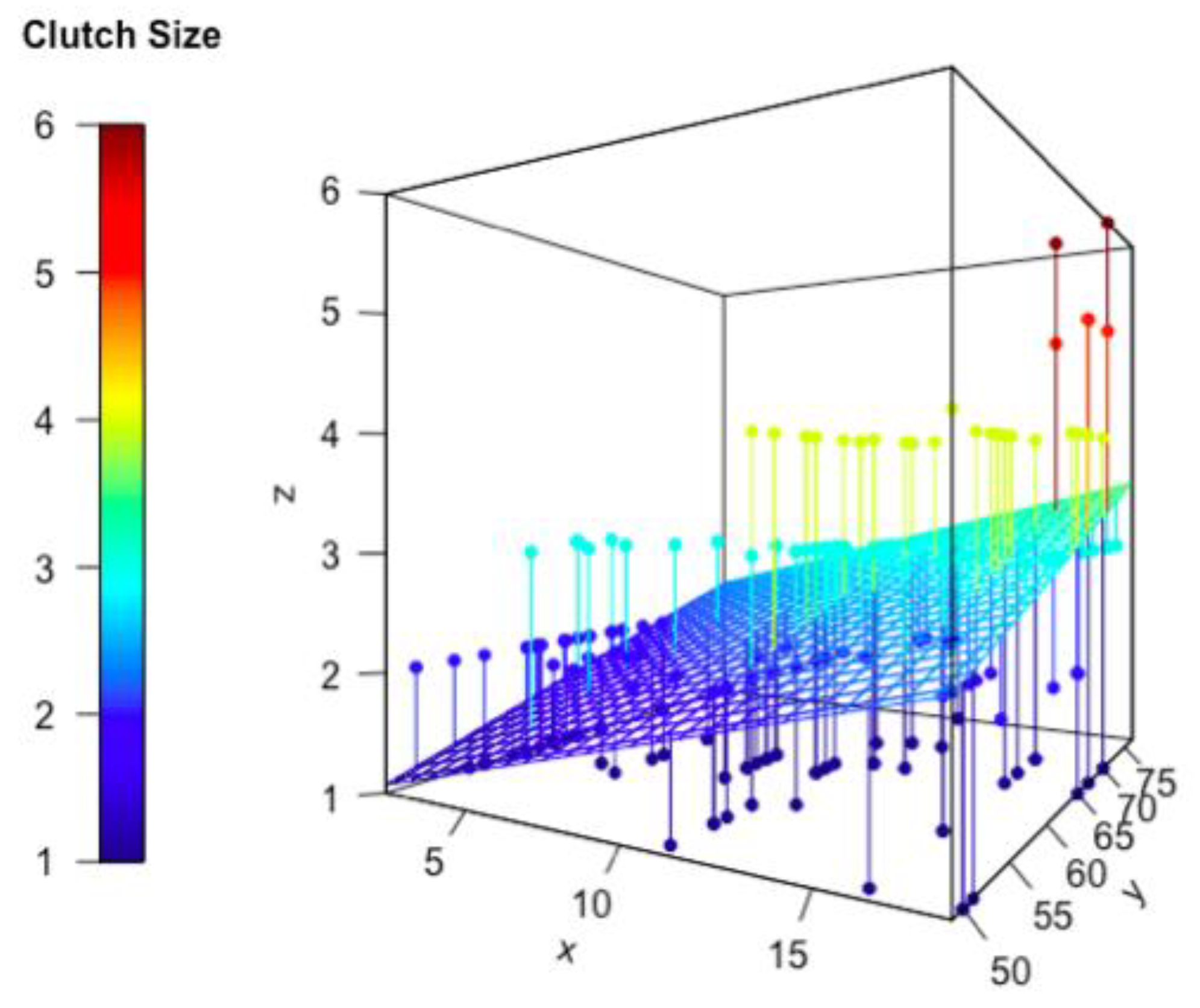

| Island Name | Coordinates | Island Size (km2) | Predator Richness | Biomass of Arthropods (mg) | NDVI | Clutch Size | Clutch Volume (mm3) |
|---|---|---|---|---|---|---|---|
| Kokkinonisi | 39°9′38.757″ N, 23°54′7.129″ E | 0.005 | 2 | 16.734 | 0.387 | 1.7 ± 0.3 (10) | 598 ± 234 (10) |
| Mikropsathoura (Myga) | 39°28′56.262″ N, 24°10′51.691″ E | 0.014 | 2 | 20.724 | 0.511 | 1.6 ± 0.2 (11) | 219 ± 60 (11) |
| Agios Ioannis | 36°36′36.327″ N, 24°57′23.118″ E | 0.033 | 2 | 125.727 | 0.108 | 1.4 ± 0.2 (10) | 433 ± 143 (10) |
| Kopria | 36°59′27.899″ N, 25°38′14.122″ E | 0.138 | 2 | 100.870 | 0.164 | 1.6 ± 0.2 (10) | 805 ± 254 (10) |
| Glaronisi | 36°55′15.371″ N, 25°36′15.286″ E | 0.188 | 2 | 57.548 | 0.200 | 1.9 ± 0.1 (8) | 362 ± 100 (8) |
| Ano Koufonisi | 36°56′49.45″ N, 25°36′20.237″ E | 5.770 | 5 | 96.874 | 0.237 | 2.2 ± 0.2 (9) | 386 ± 120 (9) |
| Gioura | 39°23′46.899″ N, 24°10′20.407″ E | 11.052 | 6 | 191.591 | 0.311 | 1.8 ± 0.3 (9) | 549 ± 149 (9) |
| Santorini | 36°22′59.326″ N, 25°28′29.843″ E | 76.197 | 9 | 106.454 | 0.312 | 1.7 ± 0.1 (24) | 612 ± 121 (24) |
| Skopelos | 39°7′30.145″ N, 23°39′10.323″ E | 96.229 | 11 | 333.154 | 0.542 | 2.5 ± 0.4 (12) | 947 ± 235 (12) |
| Tinos | 37°33′31.293″ N, 25°7′40.568″ E | 194.500 | 12 | 77.232 | 0.213 | 2.7 ± 0.2 (23) | 1268 ± 269 (23) |
| Andros | 37°53′23.97″ N, 24°43′25.309″ E | 380.000 | 13 | 286.327 | 0.363 | 2.2 ± 0.4 (9) | 777 ± 289 (9) |
| Naxos | 37°4′54.364″ N, 25°29′16.147″ E | 429.785 | 11 | 121.837 | 0.289 | 2.4 ± 0.1 (42) | 1008 ± 139 (42) |
| Olympiada | 39°59′45.907″ N, 22°14′0.477″ E | 1000.000 | 16 | 278.560 | 0.485 | 2.9 ± 0.2 (35) | 1258 ± 175 (35) |
| Vevi | 40°46′27.065″ N, 21°36′53.896″ E | 1000.000 | 18 | 229.523 | 0.682 | 2.9 ± 0.2 (34) | 951 ± 165 (34) |
| Model | AICc | Δ AICc | Akaike Weight |
|---|---|---|---|
| CS ~ P + SVL + (1|Location) | 714.703 | 0 | 0.687 |
| CS ~ NDVI +SVL+ (1|Location) | 716.997 | 2.293 | 0.218 |
| CS ~ SVL + (1|Location) | 719.623 | 4.920 | 5.871 × 10−2 |
| CS ~ P + (1|Location) | 720.750 | 6.047 | 3.342 × 10−2 |
| CS ~ NDVI + (1|Location) | 727.220 | 12.517 | 1.315 × 10−3 |
| CS ~ B + SVL + (1|Location) | 727.674 | 12.971 | 1.049 × 10−3 |
| CS ~ B + (1|Location) | 739.570 | 24.867 | 2.737 × 10−6 |
| Model | AIC | Δ AIC | Akaike Weight |
|---|---|---|---|
| CV ~ P + SVL + (1|Location) | 4008.389 | 0 | 0.587 |
| CV ~ NDVI + SVL + (1|Location) | 4009.153 | 0.764 | 0.401 |
| CV ~ P + (1|Location) | 4017.407 | 9.018 | 6.461 × 10−3 |
| CV ~ B + SVL + (1|Location) | 4018.701 | 10.312 | 3.383 × 10−3 |
| CV ~ NDVI + (1|Location) | 4019.651 | 11.262 | 2.104 × 10−3 |
| CV ~ SVL + (1|Location) | 4022.031 | 13.642 | 6.401 × 10−4 |
| CV ~ B + (1|Location) | 4029.952 | 21.563 | 1.220 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foufopoulos, J.; Zhao, Y.; Brock, K.M.; Pafilis, P.; Valakos, E.D. Predation Risk, and Not Shelter or Food Availability, as the Main Determinant of Reproduction Investment in Island Lizards. Animals 2023, 13, 3689. https://doi.org/10.3390/ani13233689
Foufopoulos J, Zhao Y, Brock KM, Pafilis P, Valakos ED. Predation Risk, and Not Shelter or Food Availability, as the Main Determinant of Reproduction Investment in Island Lizards. Animals. 2023; 13(23):3689. https://doi.org/10.3390/ani13233689
Chicago/Turabian StyleFoufopoulos, Johannes, Yilun Zhao, Kinsey M. Brock, Panayiotis Pafilis, and Efstratios D. Valakos. 2023. "Predation Risk, and Not Shelter or Food Availability, as the Main Determinant of Reproduction Investment in Island Lizards" Animals 13, no. 23: 3689. https://doi.org/10.3390/ani13233689
APA StyleFoufopoulos, J., Zhao, Y., Brock, K. M., Pafilis, P., & Valakos, E. D. (2023). Predation Risk, and Not Shelter or Food Availability, as the Main Determinant of Reproduction Investment in Island Lizards. Animals, 13(23), 3689. https://doi.org/10.3390/ani13233689






