Structural Features of Connective Tissue Formed around Resin Implants Subcutaneously Embedded in Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Implants
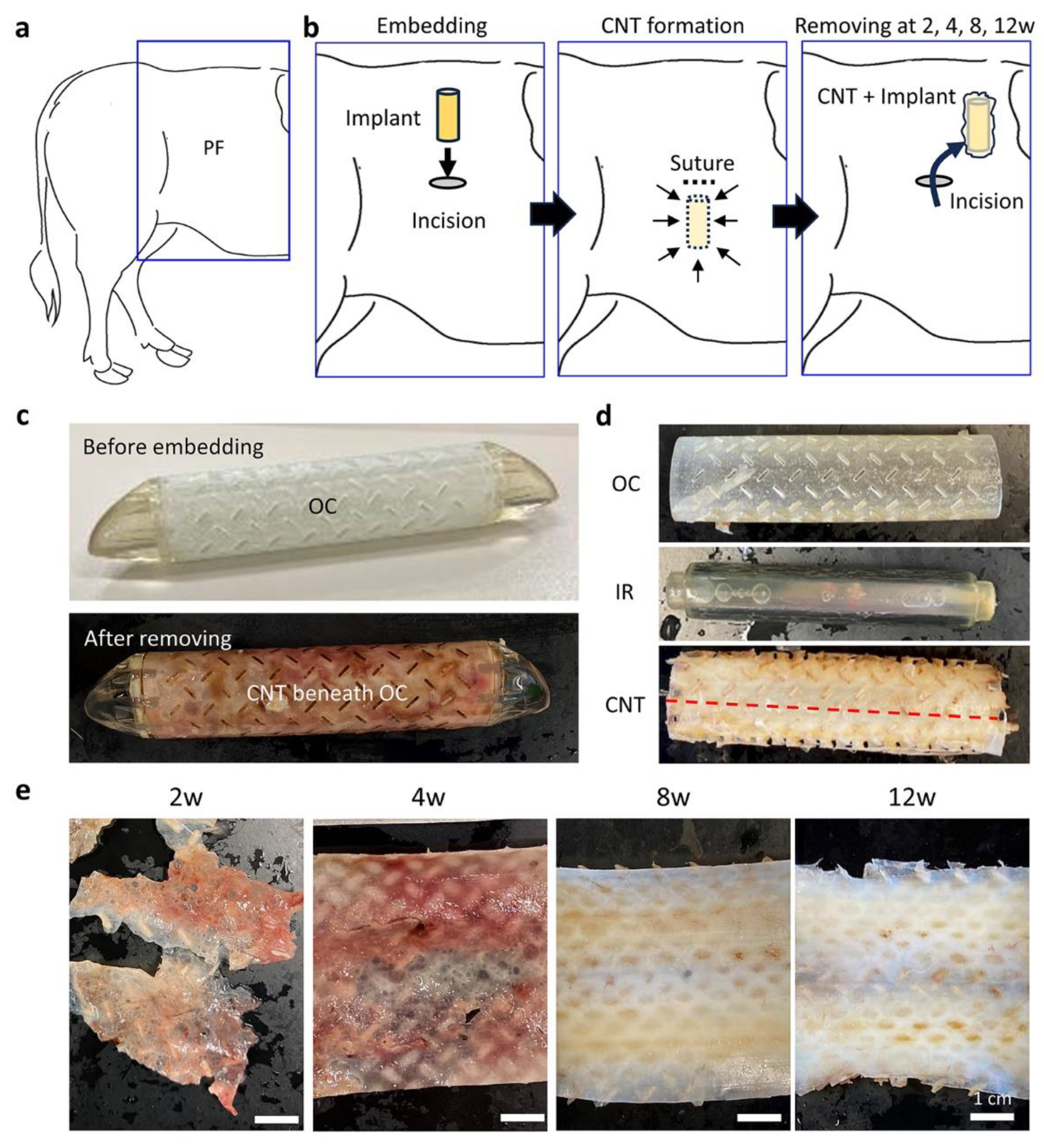
2.3. Surgical Embedding of the Resin Implants
2.4. Surgical Removal of the Resin Implant and Sample Preparations
2.5. Biochemical Analysis
2.6. Histological Analysis
2.7. Histoplanimetry
2.7.1. Thickness of the CNTs
2.7.2. Elastic Fiber Area and Positive Cell Area in the IHC
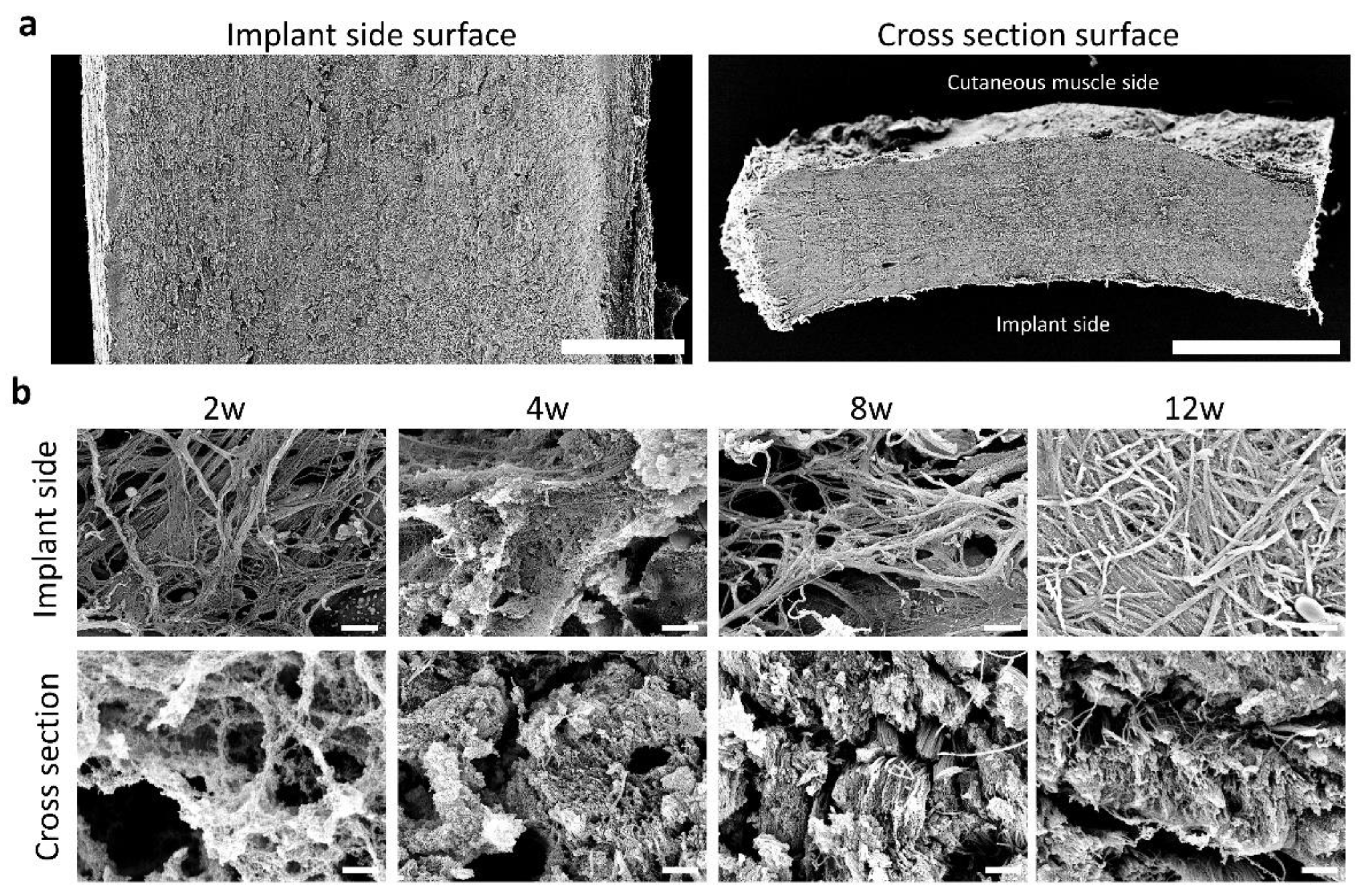
2.8. Scanning Electron Microscopy
2.9. Statistical Analysis
3. Results
3.1. CNTs Formed around the Embedded Resin Implant
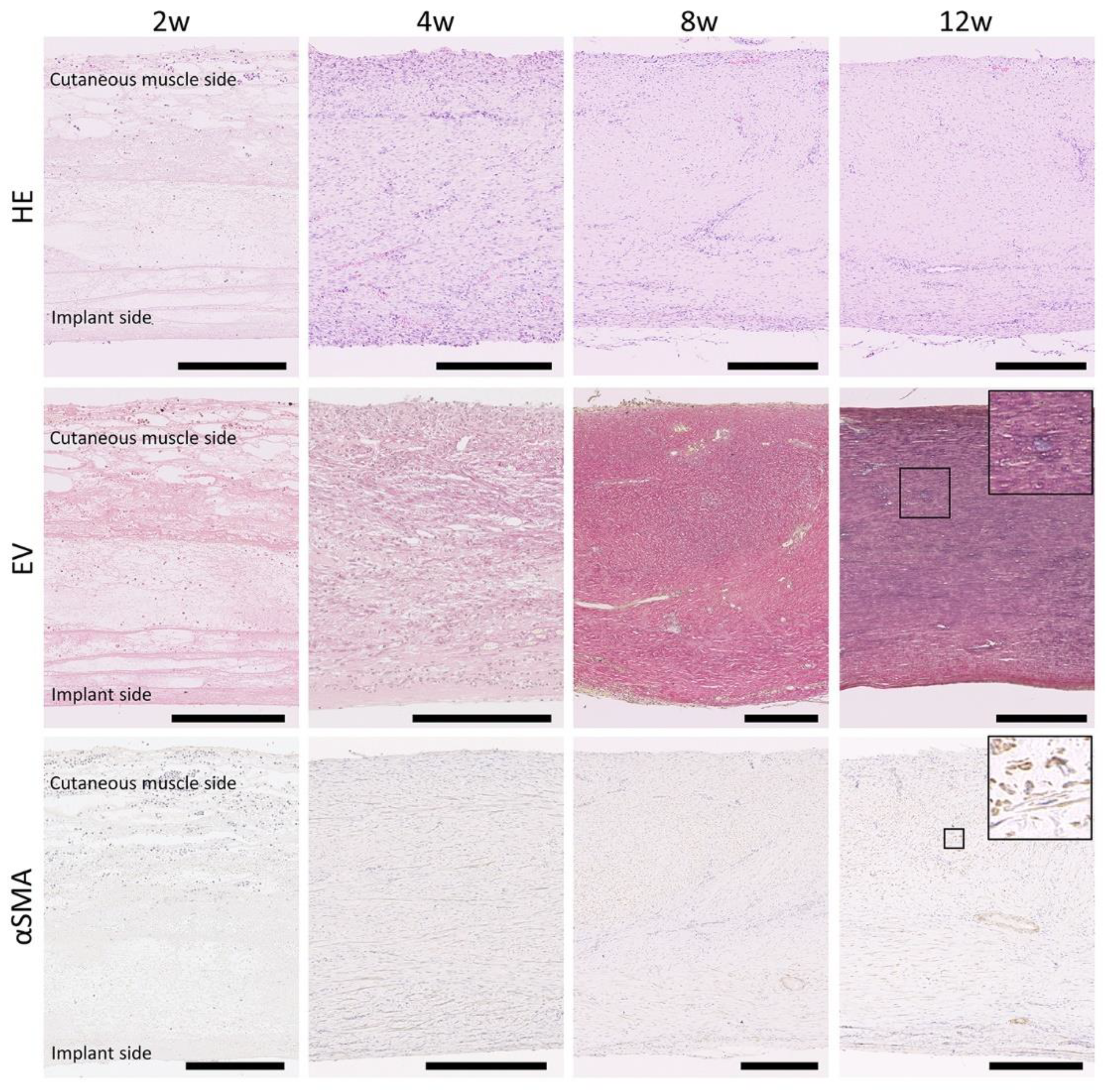
3.2. Histology of CNTs Formed around the Embedded Resin Implant
3.3. Fiber Arrangement in the CNTs Formed around the Embedded Resin Implant
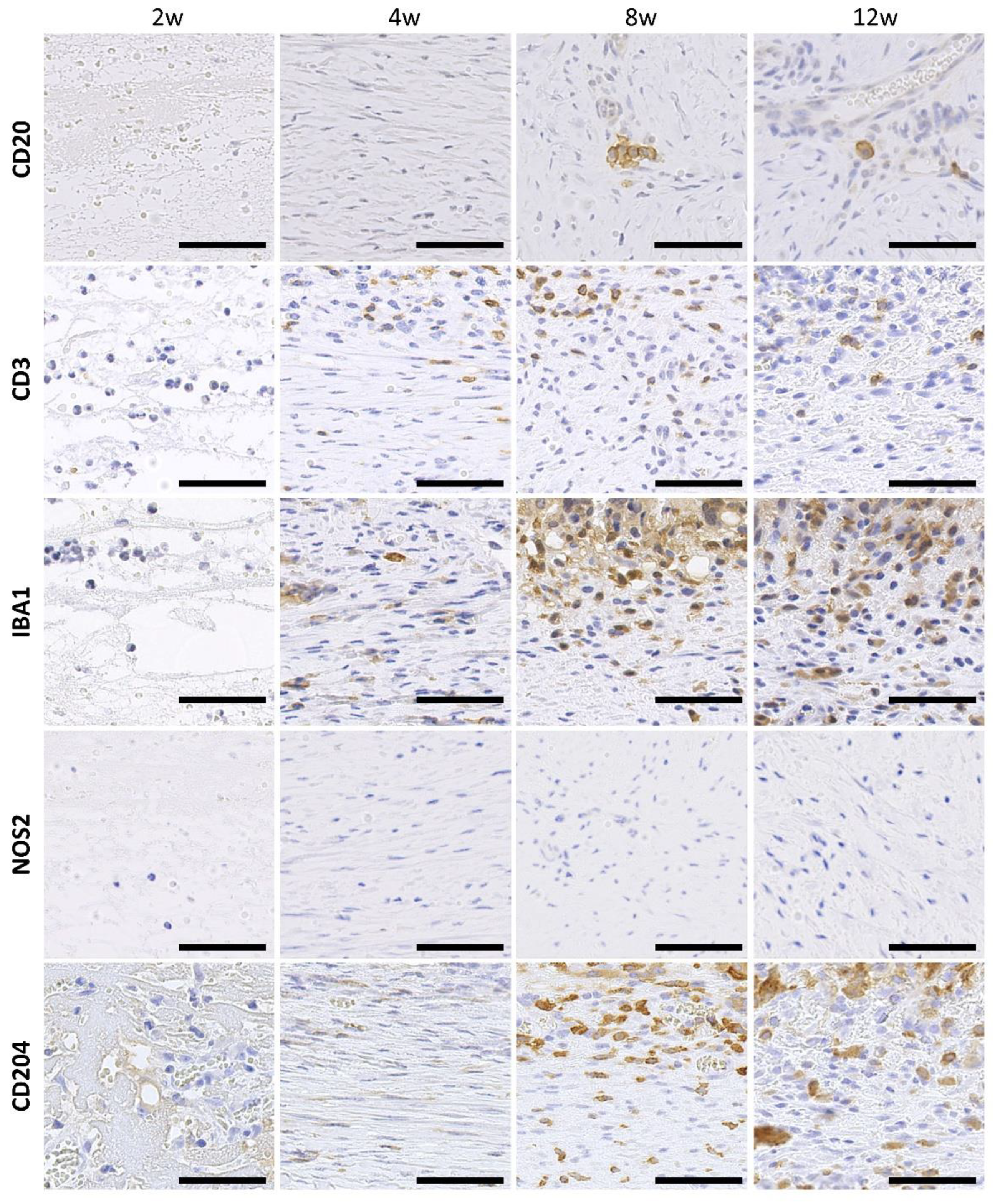
3.4. Infiltration of Immune Cells to the CNTs Formed around the Embedded Resin Implant
3.5. Proliferation Activity and Angiogenesis in the CNTs Formed around the Embedded Resin Implant
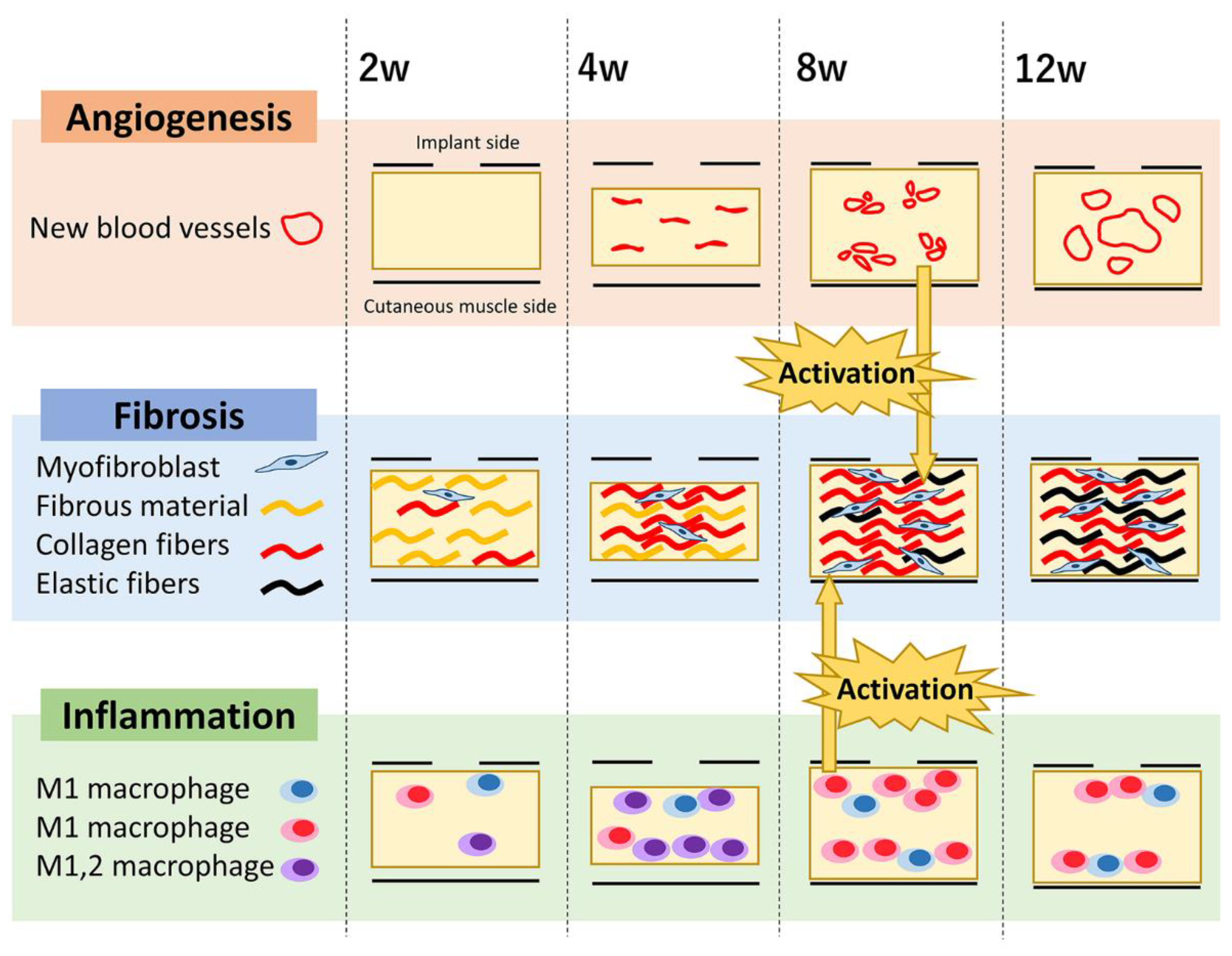
3.6. Summary of Quantitative Changes in the CNT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Greaves, N.S.; Ashcroft, K.J.; Baguneid, M.; Bayat, A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J. Dermatol. Sci. 2013, 72, 206–217. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.A.; Zakuan, N.; Billet, F.; Desmoulière, A. The myofibroblast, a key cell in normal and pathological tissue repair. Cell Mol. Life Sci. 2016, 73, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Delavary, B.M.; van der Veer, W.M.; van Egmond, M.; Niessen, F.B.; Beelen, R.H. Macrophages in skin injury and repair. Immunobiology 2011, 216, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Daley, J.M.; Brancato, S.K.; Thomay, A.A.; Reichner, J.S.; Albina, J.E. The phenotype of murine wound macrophages. J. Leukoc. Biol. 2010, 87, 59–67. [Google Scholar] [CrossRef]
- Wu, P.; Wang, L.; Li, W.; Zhang, Y.; Wu, Y.; Zhi, D.; Wang, H.; Wang, L.; Kong, D.; Zhu, M. Construction of vascular graft with circumferentially oriented microchannels for improving artery regeneration. Biomaterials 2020, 242, 119922. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. A 2017, 105, 927–940. [Google Scholar] [CrossRef]
- Luttikhuizen, D.T.; Harmsen, M.C.; Van Luyn, M.J. Cellular and molecular dynamics in the foreign body reaction. Tissue Eng. 2006, 12, 1955–1970. [Google Scholar] [CrossRef]
- Lechleitner, S.; Kunstfeld, R.; Messeritsch-Fanta, C.; Wolff, K.; Petzelbauer, P. Peripheral lymph node addressins are expressed on skin endothelial cells. J. Investig. Dermatol. 1999, 113, 410–414. [Google Scholar] [CrossRef]
- Sussman, E.M.; Halpin, M.C.; Muster, J.; Moon, R.T.; Ratner, B.D. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann. Biomed. Eng. 2014, 42, 1508–1516. [Google Scholar] [CrossRef]
- Yoko, S.; Kobayashi, Y.; Iiri, T.; Kitazawa, H.; Okabe, M.; Kobayashi, H.; Okazaki, E.; Aizawa, Y. Pacing lead-induced granuloma in the atrium: A foreign body reaction to polyurethane. Case Rep. Cardiol. 2013, 2013, 396595. [Google Scholar] [CrossRef]
- Ward, W.K.; Wood, M.D.; Troupe, J.E. Rise in background current over time in a subcutaneous glucose sensor in the rabbit: Relevance to calibration and accuracy. Biosens. Bioelectron. 2000, 15, 53–61. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign bodies or tuners for the immune response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [PubMed]
- Frazer, R.Q.; Byron, R.T.; Osborne, P.B.; West, K.P. PMMA: An essential material in medicine and dentistry. J. Long. Term. Eff. Med. Implant. 2005, 15, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Ishibashi-Ueda, H.; Takamizawa, K. In vivo tissue-engineered small-caliber arterial graft prosthesis consisting of autologous tissue (biotube). Cell Transplant. 2004, 13, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Oshima, N.; Tatsumi, E.; Ichii, O.; Nishimura, T. iBTA-induced bovine Biosheet for repair of abdominal wall defects in a beagle model: Proof of concept. Hernia 2018, 22, 1033–1039. [Google Scholar] [CrossRef]
- Oe, S.; Masum, M.A.; Ichii, O.; Nishimura, T.; Nakamura, T.; Namba, T.; Otani, Y.; Nakayama, Y.; Elewa, Y.H.A.; Kon, Y. Spatiotemporal histological changes observed in mouse subcutaneous tissues during the foreign body reaction to silicone. J. Biomed. Mater. Res. 2021, 109, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Einaga, N.; Yoshida, A.; Noda, H.; Suemitsu, M.; Nakayama, Y.; Sakurada, A.; Kawaji, Y.; Yamaguchi, H.; Sasaki, Y.; Tokino, T.; et al. Assessment of the quality of DNA from various formalin-fixed paraffin-embedded (FFPE) tissues and the use of this DNA for next-generation sequencing (NGS) with no artifactual mutation. PLoS ONE 2017, 12, e0176280. [Google Scholar] [CrossRef]
- Govender, S.; Naidoo, R. Do antigen retrieval techniques improve DNA yield from formalin fixed paraffin embedded tissue? Adv. Tech. Biol. Med. 2015, 04, 1000176. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M.; Al-Harbi, F.A.; Näpänkangas, R.; Raustia, A. PMMA denture base material enhancement: A review of fiber, filler, and nanofiller addition. Int. J. Nanomed. 2017, 12, 3801–3812. [Google Scholar] [CrossRef]
- Silva, R.; Raposo-Amaral, C.A.; Guidi, M.C.; Raposo-Amaral, C.E.; Buzzo, C.L. Customized acrylic implants for reconstruction of extensive skull defects: An exception approach for selected patients. Rev. Col. Bras. Cir. 2017, 44, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Chen, C.; Xu, X.; Wang, J.; Hou, X.; Li, K.; Lu, X.; Shi, H.; Lee, E.S.; Jiang, H.B. A review of 3D printing in dentistry: Technologies, affecting factors, and applications. Scanning 2021, 2021, 9950131. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, T.; Umeno, T.; Terazawa, T.; Wada, T.; Shuto, T.; Nishida, H.; Anai, H.; Nakayama, Y.; Miyamoto, S. Aortic valve neocuspidization with in-body tissue-engineered autologous membranes: Preliminary results in a long-term goat model. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Komura, M.; Terawaki, K.; Kodaka, T.; Gohara, T.; Komura, H.; Nakayama, Y. Engineering and repair of diaphragm using biosheet (a collagenous connective tissue membrane) in rabbits. J. Pediatr. Surg. 2018, 53, 330–334. [Google Scholar] [CrossRef]
- Terazawa, T.; Nishimura, T.; Mitani, T.; Ichii, O.; Ikeda, T.; Kosenda, K.; Tatsumi, E.; Nakayama, Y. Wall thickness control in biotubes prepared using type-C mold. J. Artif. Organs 2018, 21, 387–391. [Google Scholar] [CrossRef]
- Watanabe, T.; Kanda, K.; Ishibashi-Ueda, H.; Yaku, H.; Nakayama, Y. Autologous small-caliber “biotube” vascular grafts with argatroban loading: A histomorphological examination after implantation to rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 236–242. [Google Scholar] [CrossRef]
- Umeda, S.; Nakayama, Y.; Terazawa, T.; Iwai, R.; Hiwatashi, S.; Nakahata, K.; Takama, Y.; Okuyama, H. Long-term outcomes of patch tracheoplasty using collagenous tissue membranes (biosheets) produced by in-body tissue architecture in a beagle model. Surg. Today 2019, 49, 958–964. [Google Scholar] [CrossRef]
- Okamoto, K.; Umeno, T.; Shuto, T.; Wada, T.; Anai, H.; Nishida, H.; Nakayama, Y.; Miyamoto, S. Three-month outcomes of aortic valve reconstruction using collagenous membranes (biosheets) produced by in-body tissue architecture in a goat model: A preliminary study. BMC Cardiovasc. Disord. 2021, 21, 184. [Google Scholar] [CrossRef]
- Sato, Y.; Iwai, R.; Fukushima, M.; Nakayama, Y. Involvement of somatic stem cells in encapsulation of foreign-body reaction in canine subcutaneous Biotube tissue formation. J. Biosci. Bioeng. 2021, 132, 524–530. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Hu, W.J.; Eaton, J.W.; Ugarova, T.P.; Tang, L. Molecular basis of biomaterial-mediated foreign body reactions. Blood 2001, 98, 1231–1238. [Google Scholar] [CrossRef]
- Balabiyev, A.; Podolnikova, N.P.; Kilbourne, J.A.; Baluch, D.P.; Lowry, D.; Zare, A.; Ros, R.; Flick, M.J.; Ugarova, T.P. Fibrin polymer on the surface of biomaterial implants drives the foreign body reaction. Biomaterials 2021, 277, 121087. [Google Scholar] [CrossRef] [PubMed]
- Barron, L.; Wynn, T.A. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G723–G728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Alanazi, Y.F.; Jowitt, T.A.; Roseman, A.M.; Baldock, C. Elastic fibre proteins in elastogenesis and wound healing. Int. J. Mol. Sci. 2022, 23, 4087. [Google Scholar] [CrossRef] [PubMed]
- Godwin, A.R.F.; Singh, M.; Lockhart-Cairns, M.P.; Alanazi, Y.F.; Cain, S.A.; Baldock, C. The role of fibrillin and microfibril binding proteins in elastin and elastic fibre assembly. Matrix Biol. 2019, 84, 17–30. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Davis, E.C.; Starcher, B.C.; Ouchi, T.; Yanagisawa, M.; Richardson, J.A.; Olson, E.N. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature 2002, 415, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef]
- Miyazaki, T.; Haraguchi, S.; Kim-Kaneyama, J.R.; Miyazaki, A. Endothelial calpain systems orchestrate myofibroblast differentiation during wound healing. FASEB J. 2019, 33, 2037–2046. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Leach, K.J.; Hoffman, A.S.; Ratner, B.D.; Bornstein, P. Mice that lack the angiogenesis inhibitor, thrombospondin 2, mount an altered foreign body reaction characterized by increased vascularity. Proc. Natl. Acad. Sci. USA 1999, 96, 4449–4454. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Zhu, Y.H.; Yang, Z.; Huynh, G.; Bornstein, P. Altered extracellular matrix remodeling and angiogenesis in sponge granulomas of thrombospondin 2-null mice. Am. J. Pathol. 2001, 159, 1255–1262. [Google Scholar] [CrossRef]

| Antibody | Ki67 | α-SMA | CD20 | IBA1 | CD3 | NOS2 | CD31 | CD204 |
|---|---|---|---|---|---|---|---|---|
| Host | Rabbit | Mouse | ||||||
| Dilution | 1:1500 | 1:100 | 1:500 | 1:3000 | 1:100 | 1:500 | 1:100 | 1:300 |
| Source | Abcam, Cambridge, UK | FUJIFILM Wako, Osaka, Japan | Nichirei, Tokyo, Japan | Santacruz, CA, USA | Abcam, Cambridge, UK | TransGenic, Fukuoka, Japan | ||
| Antigen retrieval | CB, 105 °C, 20 min | VT, 105 °C, 20 min | CB, 105 °C, 20 min | |||||
| Blocking serum | 10% normal goat serum (cat. #: 426042, Nichirei) | 10% normal rabbit serum (cat. #: 426052, Nichirei) | ||||||
| Indices | Parameters | Weeks after Embedding | ||||
|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 12 | |||
| Cellularity and collagen amount | Weight (mg/cm2) | 31.01 ± 8.06 a | 49.08 ± 18.52 a | 79.85 ± 14.22 b | 83.48 ± 23.41 b | |
| Total protein (mg/cm2) | 4.30 ± 0.53 a | 6.53 ± 4.55 a | 13.55 ± 3.57 b | 15.75 ± 5.33 b | ||
| Collagen (mg/cm2) | 0.02 ± 0.01 a | 0.64 ± 1.18 a | 3.83 ± 1.91 b | 5.28 ± 2.38 b | ||
| DNA (μg/cm2) | 36.01 ± 21.51 a | 77.51 ± 105.46 ab | 150.62 ± 54.07 bc | 186.20 ± 65.54 cd | ||
| Connective fiber development | Tissue thickness (μm) | 448.39 ± 140.66 a | 348.38 ± 168.56 a | 708.13 ± 180.64 b | 615.84 ± 157.96 b | |
| Elastic fiber area (%) | 0.10 ± 0.09 a | 0.47 ± 0.63 a | 2.22 ± 1.03 b | 4.48 ± 0.63 c | ||
| αSMA+ cell area (%) | 0.05 ± 0.02 a | 0.20 ± 0.21 a | 2.93 ± 0.88 b | 2.54 ± 1.83 b | ||
| Lymphocytes | B cells | CD20+ cell area (%) | 0.03 ± 0.01 a | 0.05 ± 0.04 a | 0.15 ± 0.08 b | 0.14 ± 0.11 b |
| pan T cells | CD3+ cell area (%) | 0.16 ± 0.05 | 0.17 ± 0.06 | 0.54 ± 0.34 | 0.77 ± 0.80 | |
| Mφ | M1/M2-type | IBA1+ cell area (%) | 0.54 ± 0.27 | 1.54 ± 1.75 | 1.41 ± 0.78 | 1.86 ± 1.36 |
| M1-type | NOS2+ cell area (%) | 0.03 ± 0.01 | 0.23 ± 0.20 | 0.12 ± 0.11 | 0.21 ± 0.14 | |
| M2-type | CD204+ cell area (%) | 0.18 ± 0.11 a | 0.22 ± 0.09 a | 1.73 ± 0.86 b | 1.13 ± 1.15 a | |
| Proliferation | Ki67+ cell area (%) | 0.51 ± 0.31 | 0.97 ± 0.97 | 0.82 ± 0.64 | 0.73 ± 0.26 | |
| Angiogenesis | CD31+ cell area (%) | 0.03 ± 0.01 a | 0.95 ± 1.38 a | 1.70 ± 1.29 a | 2.43 ± 0.94 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katayama, Y.; Ichii, O.; Nakamura, T.; Yanase, K.; Hiraishi, M.; Namba, T.; Otani, Y.; Ikeda, T.; Tsuji, E.; Tsuzuki, N.; et al. Structural Features of Connective Tissue Formed around Resin Implants Subcutaneously Embedded in Dairy Cows. Animals 2023, 13, 3700. https://doi.org/10.3390/ani13233700
Katayama Y, Ichii O, Nakamura T, Yanase K, Hiraishi M, Namba T, Otani Y, Ikeda T, Tsuji E, Tsuzuki N, et al. Structural Features of Connective Tissue Formed around Resin Implants Subcutaneously Embedded in Dairy Cows. Animals. 2023; 13(23):3700. https://doi.org/10.3390/ani13233700
Chicago/Turabian StyleKatayama, Yuka, Osamu Ichii, Teppei Nakamura, Keita Yanase, Masaya Hiraishi, Takashi Namba, Yuki Otani, Teppei Ikeda, Erika Tsuji, Natsuko Tsuzuki, and et al. 2023. "Structural Features of Connective Tissue Formed around Resin Implants Subcutaneously Embedded in Dairy Cows" Animals 13, no. 23: 3700. https://doi.org/10.3390/ani13233700





