Ulva prolifera Stress in the Yellow Sea of China: Suppressed Antioxidant Capacity and Induced Inflammatory Response of the Japanese Flounder (Paralichthys olivaceus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Fish Acclimation and Exposure Experiment
2.2. Sample Collection
2.3. Enzyme Activity Assays in Serum and Tissue
2.4. Total RNA Extraction, cDNA Synthesis, and Real-Time Quantitative Polymerase Chain Reaction PCR (RT-qPCR)
2.5. Western Blot Analysis
2.6. Calculations and Statistical Analysis
3. Results
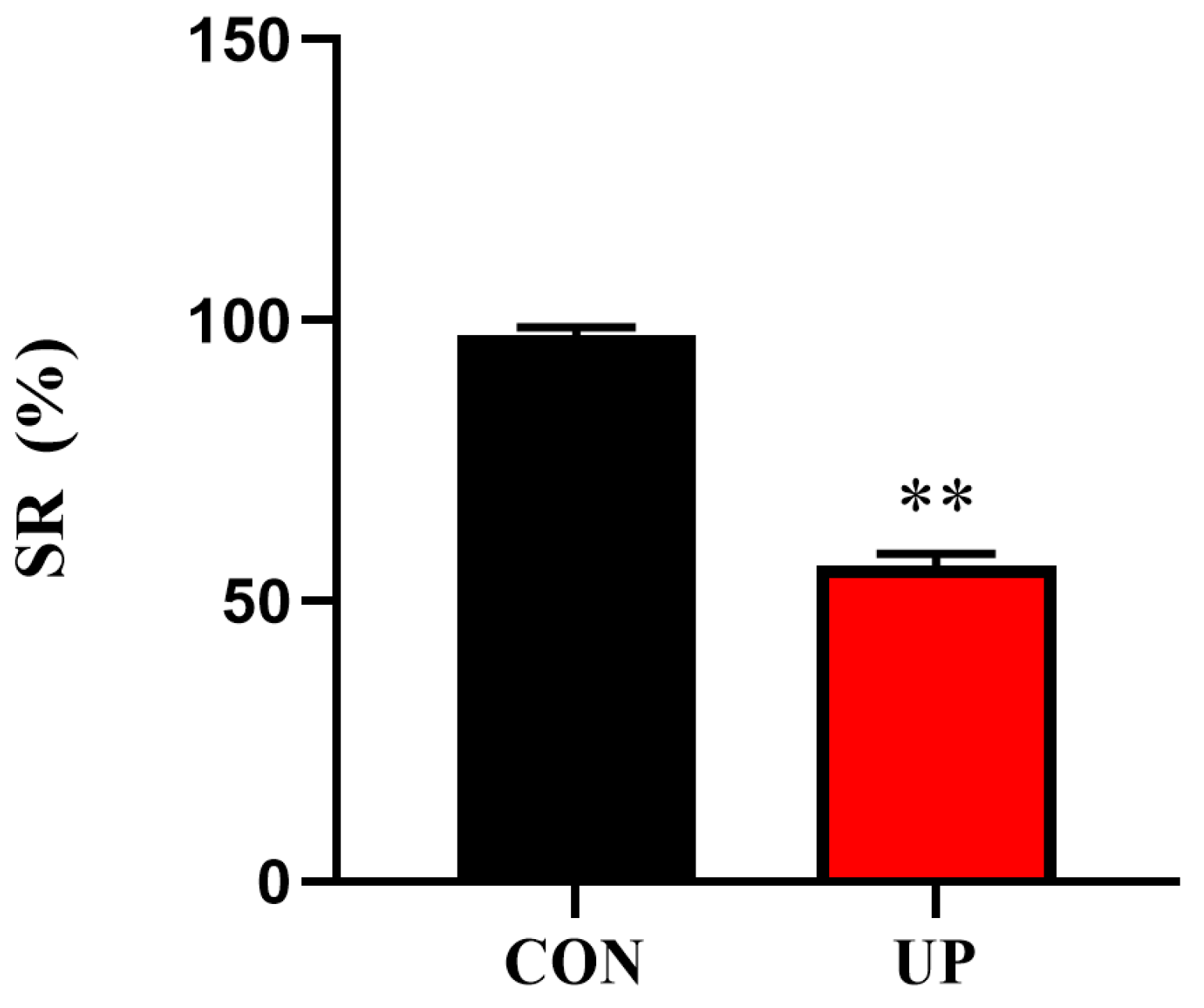
3.1. Survival Rate
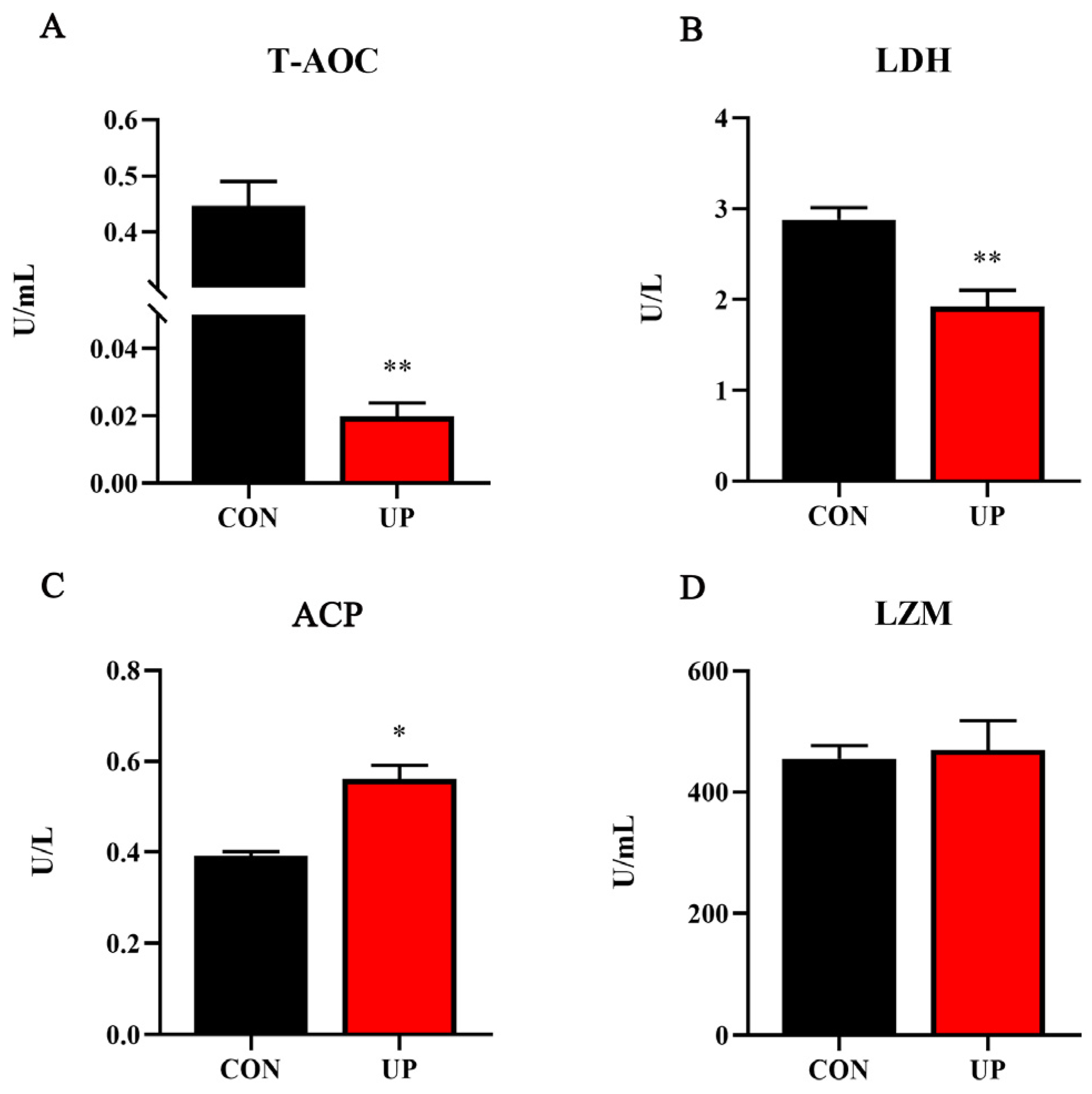
3.2. Serum T-AOC Capacity and Non-Specific Immune-Related Enzyme Activities
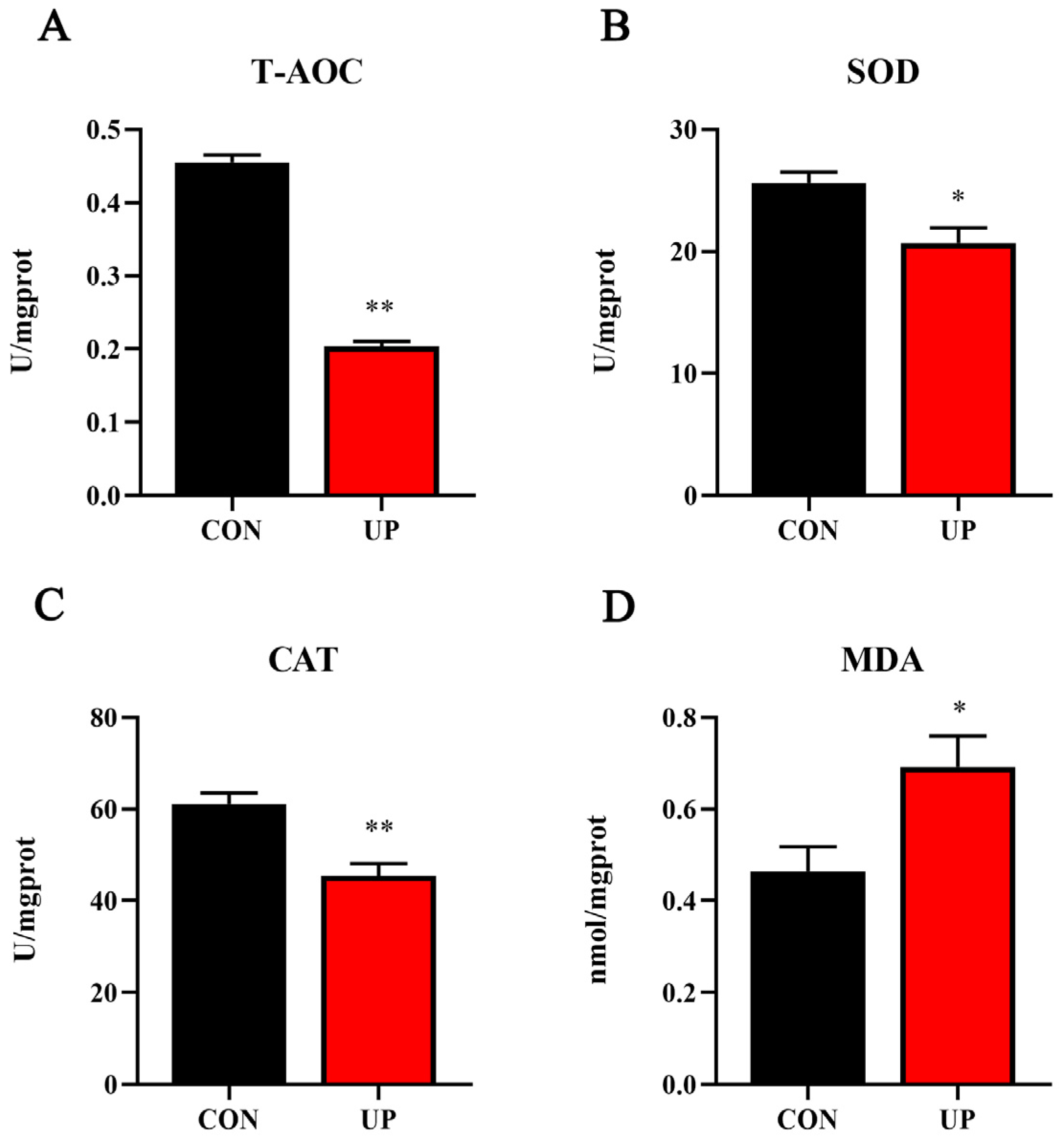
3.3. Liver Antioxidant Capacity and Related Genes Expression
3.4. Head Kidney Inflammatory Genes and MAPK Signaling Pathway Expression
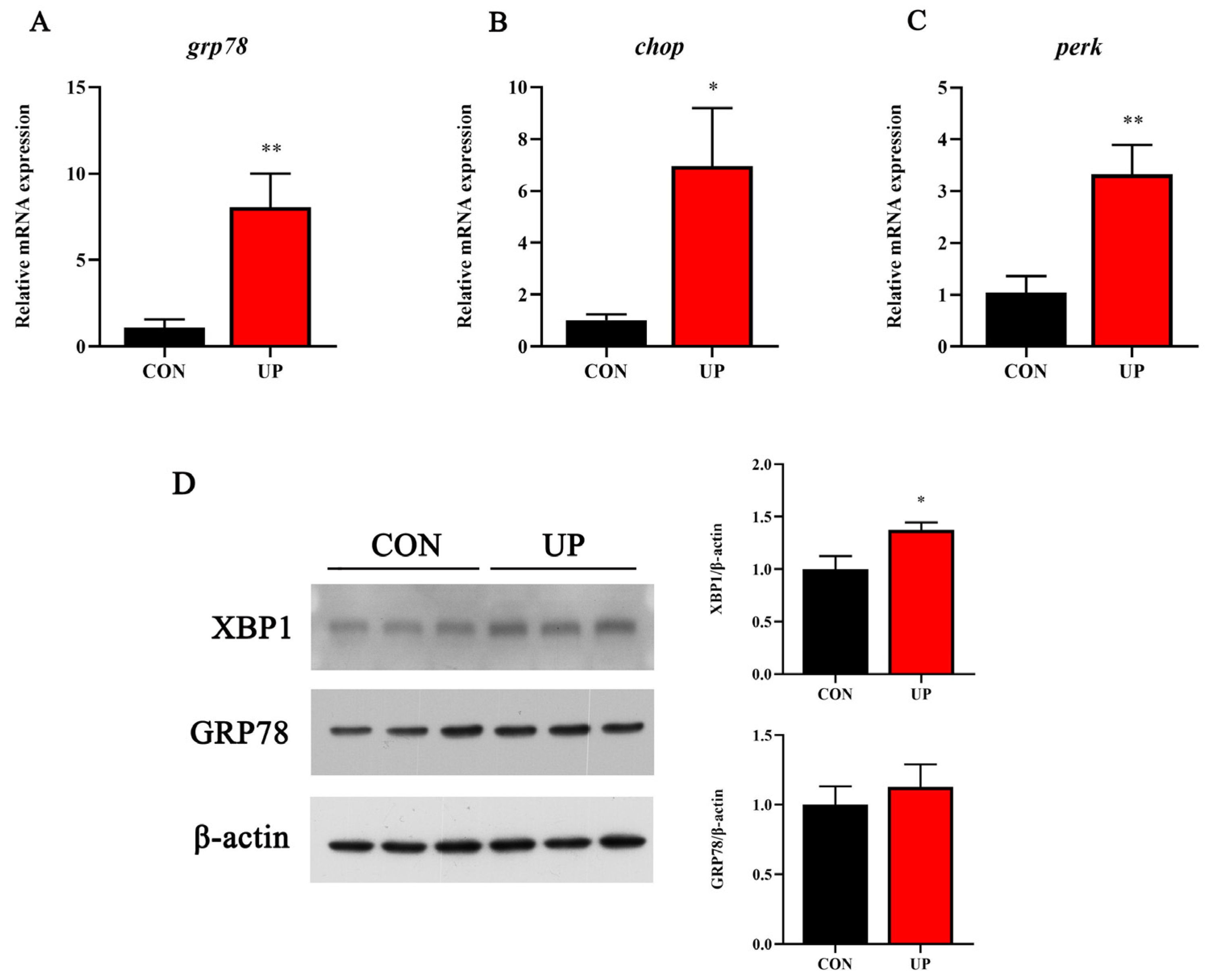
3.5. Head Kidney Endoplasmic Reticulum Stress-Related Genes and Proteins Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Wang, Z.; Zhang, X. A review of the green tides in the Yellow Sea, China. Mar. Environ. Res. 2016, 119, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, F.; Li, C.; Qin, S.; Zhou, M.; Ding, L.; Pang, S.; Duan, D.; Wang, G.; Yin, B. Emerging challenges: Massive green algae blooms in the Yellow Sea. Nat. Preced. 2008, 1. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Z.; Liu, D.; Fu, M.; Yuan, C.; Yan, T. Harmful macroalgal blooms (HMBs) in China’s coastal water: Green and golden tides. Harmful Algae 2021, 107, 102061. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhou, M. Green Tides of the Yellow Sea: Massive Free-Floating Blooms of Ulva prolifera. In Global Ecology and Oceanography of Harmful Algal Blooms; Springer: Berlin/Heidelberg, Germany, 2018; pp. 317–326. [Google Scholar]
- Qi, L.; Hu, C.; Xing, Q.; Shang, S. Long-term trend of Ulva prolifera blooms in the western Yellow Sea. Harmful Algae 2016, 58, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, P.; Li, H.; Li, G.; Liu, J.; Jiao, F.; Zhang, J.; Huo, Y.; Shi, X.; Su, R.; et al. Ulva prolifera green-tide outbreaks and their environmental impact in the Yellow Sea, China. Natl. Sci. Rev. 2019, 6, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Van Alstyne, K.L.; Nelson, T.A.; Ridgway, R.L. Environmental Chemistry and Chemical Ecology of “Green Tide” Seaweed Blooms. Integr. Comp. Biol. 2015, 55, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, C.; Chen, H.; Zheng, Z. Metagenomic Analysis of the Effect of Enteromorpha prolifera Bloom on Microbial Community and Function in Aquaculture Environment. Curr. Microbiol. 2020, 77, 816–825. [Google Scholar] [CrossRef]
- Shumway, S.E. A Review of the Effects of Algal Blooms on Shellfish and Aquaculture. J. World Aquac. Soc. 1990, 21, 65–104. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Liu, D.; Keesing, J.K.; Gong, J. Macroalgal blooms favor heterotrophic diazotrophic bacteria in nitrogen-rich and phosphorus-limited coastal surface waters in the Yellow Sea. Estuar. Coast. Shelf Sci. 2015, 163, 75–81. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X. Release and microbial degradation of dissolved organic matter (DOM) from the macroalgae Ulva prolifera. Mar. Pollut. Bull. 2017, 125, 192–198. [Google Scholar] [CrossRef]
- Nelson, H.R.; Altieri, A.H. Oxygen: The universal currency on coral reefs. Coral Reefs 2019, 38, 177–198. [Google Scholar] [CrossRef]
- Birnie-Gauvin, K.; Costantini, D.; Cooke, S.J.; Willmore, W.G. A comparative and evolutionary approach to oxidative stress in fish: A review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative Stress and Antioxidant Defense in Fish: The implications of probiotic, prebiotic, and synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Mahboob, S. Environmental pollution of heavy metals as a cause of oxidative stress in fish: A review. Life Sci. J. 2013, 10, 336–347. [Google Scholar]
- Cui, W.; Cao, L.; Liu, J.; Ren, Z.; Zhao, B.; Dou, S. Effects of seawater acidification and cadmium on the antioxidant defense of flounder Paralichthys olivaceus larvae. Sci. Total Environ. 2020, 718, 137234. [Google Scholar] [CrossRef] [PubMed]
- Zapata, A.; Diez, B.; Cejalvo, T.; Gutierrez-de Frias, C.; Cortes, A. Ontogeny of the immune system of fish. Fish Shellfish Immunol. 2006, 20, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Magnadottir, B. Immunological control of fish diseases. Mar. Biotechnol. 2010, 12, 361–379. [Google Scholar] [CrossRef]
- Rauta, P.R.; Nayak, B.; Das, S. Immune system and immune responses in fish and their role in comparative immunity study: A model for higher organisms. Immunol. Lett. 2012, 148, 23–33. [Google Scholar] [CrossRef]
- Geven, E.J.W.; Klaren, P.H.M. The teleost head kidney: Integrating thyroid and immune signalling. Dev. Comp. Immunol. 2017, 66, 73–83. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Fan, Q.; Shi, K.; Zhan, M.; Xu, Q.; Liu, X.; Li, Z.; Liu, H.; Xia, Y.; Chen, Y.; Shi, X.; et al. Acute damage from the degradation of Ulva prolifera on the environmental microbiota, intestinal microbiota and transcriptome of Japanese flounder Paralichthys olivaceus. Environ. Pollut. 2022, 302, 119022. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Cui, K.; Li, Q.; Zhu, S.; Zhang, J.; Gao, S.; Hao, T.; Mai, K.; Ai, Q. Docosahexaenoic acid alleviates palmitic acid-induced inflammation of macrophages via TLR22-MAPK-PPARgamma/Nrf2 pathway in large yellow croaker (Larimichthys crocea). Antioxidants 2022, 11, 682. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q.; Pang, Y.; Xu, X.; Cui, K.; Zhang, Y.; Mai, K.; Ai, Q. Molecular cloning and the involvement of IRE1alpha-XBP1s signaling pathway in palmitic acid induced—Inflammation in primary hepatocytes from large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2020, 98, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Zhang, J.; Ding, Y. Energy utilization of algae biomass waste enteromorpha resulting in green tide in China: Pyrolysis kinetic parameters estimation based on shuffled complex evolution. Sustainability 2020, 12, 2086. [Google Scholar] [CrossRef]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.R.; Dietrich, T.; Chapple, I.L. Prediction of serum total antioxidant activity from the concentration of individual serum antioxidants. Clin. Chim. Acta 2006, 372, 188–194. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Welker, A.F.; Moreira, D.C.; Campos, E.G.; Hermes-Lima, M. Role of redox metabolism for adaptation of aquatic animals to drastic changes in oxygen availability. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 165, 384–404. [Google Scholar] [CrossRef]
- Liu, W.; Qiao, Q.; Chen, Y.; Wu, K.; Zhang, X. Microcystin-LR exposure to adult zebrafish (Danio rerio) leads to growth inhibition and immune dysfunction in F1 offspring, a parental transmission effect of toxicity. Aquat. Toxicol. 2014, 155, 360–367. [Google Scholar] [CrossRef]
- Li, H.; Gu, X.; Chen, H.; Mao, Z.; Zeng, Q.; Yang, H.; Kan, K. Comparative toxicological effects of planktonic Microcystis and benthic Oscillatoria on zebrafish embryonic development: Implications for cyanobacteria risk assessment. Environ. Pollut. 2021, 274, 115852. [Google Scholar] [CrossRef]
- Kubrak, O.I.; Husak, V.V.; Rovenko, B.M.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Cobalt-induced oxidative stress in brain, liver and kidney of goldfish Carassius auratus. Chemosphere 2011, 85, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.F.; Shen, W.L.; Hou, C.C.; Liu, C.; Wu, X.F.; Zhu, J.Q. Physiological responses and changes in gene expression in the large yellow croaker Larimichthys crocea following exposure to hypoxia. Chemosphere 2017, 169, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Le Luherne, E.; Réveillac, E.; Ponsero, A.; Sturbois, A.; Ballu, S.; Perdriau, M.; Le Pape, O. Fish community responses to green tides in shallow estuarine and coastal areas. Estuar. Coast. Shelf Sci. 2016, 175, 79–92. [Google Scholar] [CrossRef]
- Sun, J.L.; Zhao, L.L.; Liao, L.; Tang, X.H.; Cui, C.; Liu, Q.; He, K.; Ma, J.D.; Jin, L.; Yan, T.; et al. Interactive effect of thermal and hypoxia on largemouth bass (Micropterus salmoides) gill and liver: Aggravation of oxidative stress, inhibition of immunity and promotion of cell apoptosis. Fish Shellfish Immunol. 2020, 98, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Qin, J.G.; Ma, Z.; Yu, G. Acute acidification stress weakens the head kidney immune function of juvenile Lates calcarifer. Ecotoxicol. Environ. Saf. 2021, 225, 112712. [Google Scholar] [CrossRef] [PubMed]
- Kvamme, B.O.; Gadan, K.; Finne-Fridell, F.; Niklasson, L.; Sundh, H.; Sundell, K.; Taranger, G.L.; Evensen, O. Modulation of innate immune responses in Atlantic salmon by chronic hypoxia-induced stress. Fish Shellfish Immunol. 2013, 34, 55–65. [Google Scholar] [CrossRef]
- Wang, J.; Lu, D.Q.; Jiang, B.; Luo, H.L.; Lu, G.L.; Li, A.X. The effect of intermittent hypoxia under different temperature on the immunomodulation in Streptococcus agalactiae vaccinated Nile tilapia (Oreochromis niloticus). Fish. Shellfish Immunol. 2018, 79, 181–192. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Zhang, D.; Hannink, M.; Arvisais, E.; Kaufman, R.J.; Diehl, J.A. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell Biol. 2003, 23, 7198–7209. [Google Scholar] [CrossRef]
- Urano, F.; Wang, X.; Bertolotti, A.; Zhang, Y.; Chung, P.; Harding, H.P.; Ron, D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000, 287, 664–666. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Zhang, Z.; He, C.; Shi, Q.; Jiao, N.; Zhang, Y. DOC dynamics and bacterial community succession during long-term degradation of Ulva prolifera and their implications for the legacy effect of green tides on refractory DOC pool in seawater. Water Res. 2020, 185, 116268. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Lim, W.A.; Lu, D.; Dai, X.; Orlova, T.; Iwataki, M. Harmful algal blooms and associated fisheries damage in East Asia: Current status and trends in China, Japan, Korea and Russia. Harmful Algae 2021, 102, 101787. [Google Scholar] [CrossRef]
- John, U.; Supraha, L.; Gran-Stadniczenko, S.; Bunse, C.; Cembella, A.; Eikrem, W.; Janouskovec, J.; Klemm, K.; Kuhne, N.; Naustvoll, L.; et al. Spatial and biological oceanographic insights into the massive fish-killing bloom of the haptophyte Chrysochromulina leadbeateri in northern Norway. Harmful Algae 2022, 118, 102287. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, K.; Tan, L.; Wang, J. Utilization and release of biogenic elements by macroalgae Ulva prolifera: A mesocosm experiment off the coast of Qingdao, China. Mar. Pollut. Bull. 2021, 170, 112612. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, R.-C.; Zhou, M.-J. Effects of the decomposing green macroalga Ulva (Enteromorpha) prolifera on the growth of four red-tide species. Harmful Algae 2012, 16, 12–19. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, H.; Wang, S.; Wen, S.; Qin, S. Hypolipidemic and antioxidant properties of a polysaccharide fraction from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 58, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Wan, X.; Wang, D.; Zhao, C.; Liu, D.; Gao, L.; Wang, M.; Wu, C.; Nabavid, S.M.; Daglia, M.; et al. Polysaccharides from Marine Enteromorpha: Structure and function. Trends Food Sci. Technol. 2020, 99, 11–20. [Google Scholar] [CrossRef]



| Gene | Forward (5′–3′) | Reverse (5′–3′) | Accession Number |
|---|---|---|---|
| β-actin | AGGTTCCGTTGTCCCG | TGGTTCCTCCAGATAGCAC | XM_020103099 |
| tnf-α | GTCCTGGCGTTTTCTTGGTA | CTTGGCTCTGCTGCTGATTT | XM_020104959 |
| il-1β | CTGTCGTTCTGGGCATCAAA | AACAGAAATCGCACCATCTCACT | XM_020105656 |
| ifn-γ | AGTGGTCTGTCTGTCCCTGTG | GCTTCCCGTTGAATCTGTCTT | AB435093 |
| p65 | GCTTCTCTGGGTAGCACACC | GGGTTCAGAAGGTCCACAAA | XM_020100108 |
| sod1 | CGTTGGAGACCTGGGGAATGTG | ATCGTCAGCCTTCTCGTGGATC | EF681883 |
| cat | CACGGACCAGATGAAGCAGTG | CCTTGGAGTAGCGGGTAATGTC | XM_020079314 |
| nrf2 | GAAGAACAAGGTGGCGGCTCAG | GAAGGTCAGGCTGTGCTGGAAC | XM_020096126 |
| keap1 | GGAGCCGTGCCAGAAAGAAGTG | GTGCCGCTGACTGTGGTGAAC | XM_020084284 |
| gpx | GGTGGATGTGAATGGGAAGGATGC | TTGTATCGTCGCTGGGAAATGGC | EU095498 |
| grp78 | GTCGTGAGGTTGAGAAGGCA | TCATGGTGGAACGGAACAGG | DQ662232 |
| chop | CGGCCAAAAAGAGTCGCAAA | TCTCCGCTTTCAATCGCTCA | XM_020096956 |
| perk | CTACCACCTACATCGTCCGC | ACCGGCTCAAAGTCAGTCAG | XM_020105998 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Tang, Y.; Li, W.; Yang, Y. Ulva prolifera Stress in the Yellow Sea of China: Suppressed Antioxidant Capacity and Induced Inflammatory Response of the Japanese Flounder (Paralichthys olivaceus). Animals 2023, 13, 3768. https://doi.org/10.3390/ani13243768
Xu D, Tang Y, Li W, Yang Y. Ulva prolifera Stress in the Yellow Sea of China: Suppressed Antioxidant Capacity and Induced Inflammatory Response of the Japanese Flounder (Paralichthys olivaceus). Animals. 2023; 13(24):3768. https://doi.org/10.3390/ani13243768
Chicago/Turabian StyleXu, Dan, Yongzheng Tang, Wenlong Li, and Yingming Yang. 2023. "Ulva prolifera Stress in the Yellow Sea of China: Suppressed Antioxidant Capacity and Induced Inflammatory Response of the Japanese Flounder (Paralichthys olivaceus)" Animals 13, no. 24: 3768. https://doi.org/10.3390/ani13243768
APA StyleXu, D., Tang, Y., Li, W., & Yang, Y. (2023). Ulva prolifera Stress in the Yellow Sea of China: Suppressed Antioxidant Capacity and Induced Inflammatory Response of the Japanese Flounder (Paralichthys olivaceus). Animals, 13(24), 3768. https://doi.org/10.3390/ani13243768





