The Arteries of the Encephalon Base in Caracal (Caracal caracal; Felidae; Carnivora)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Methods
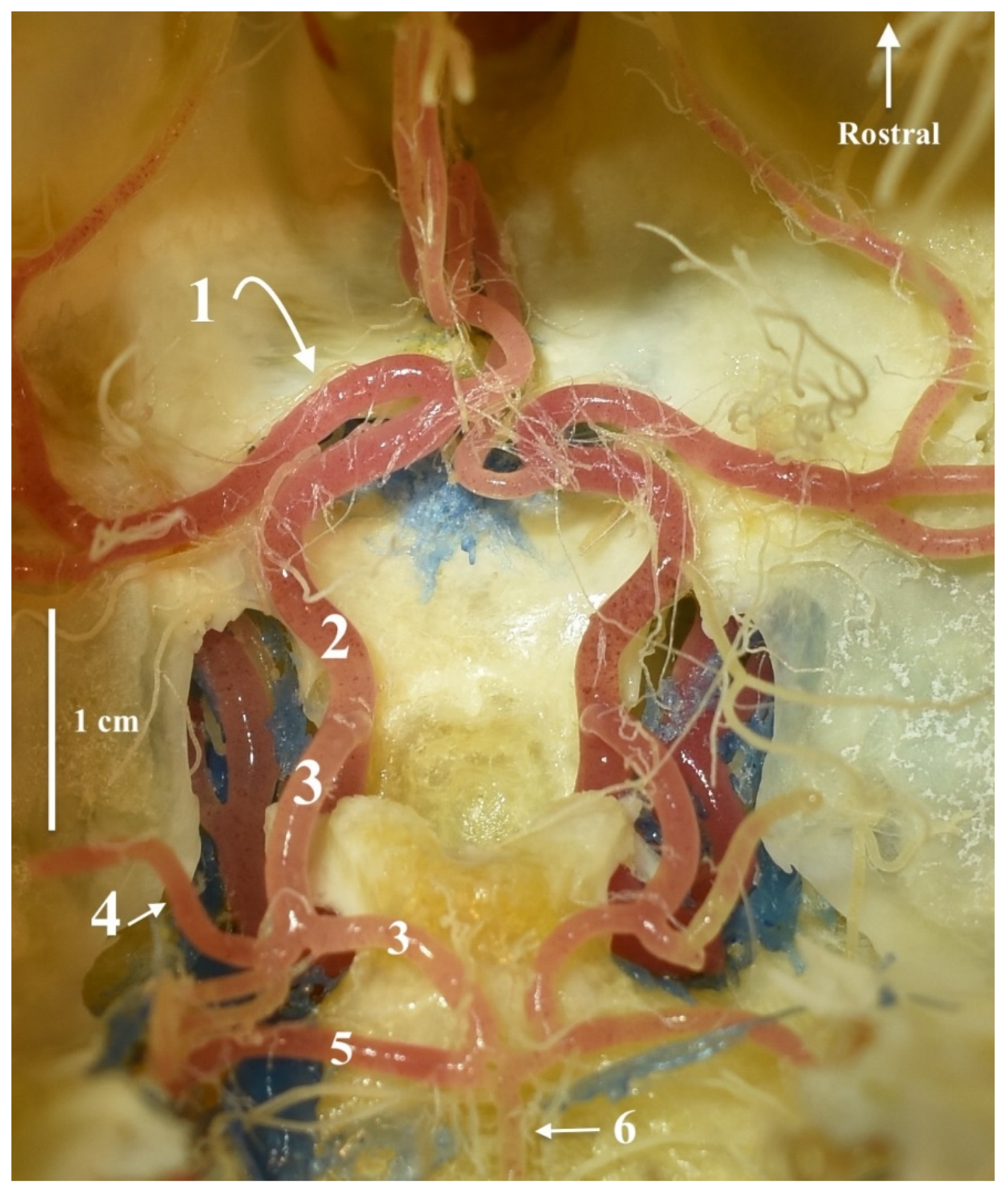
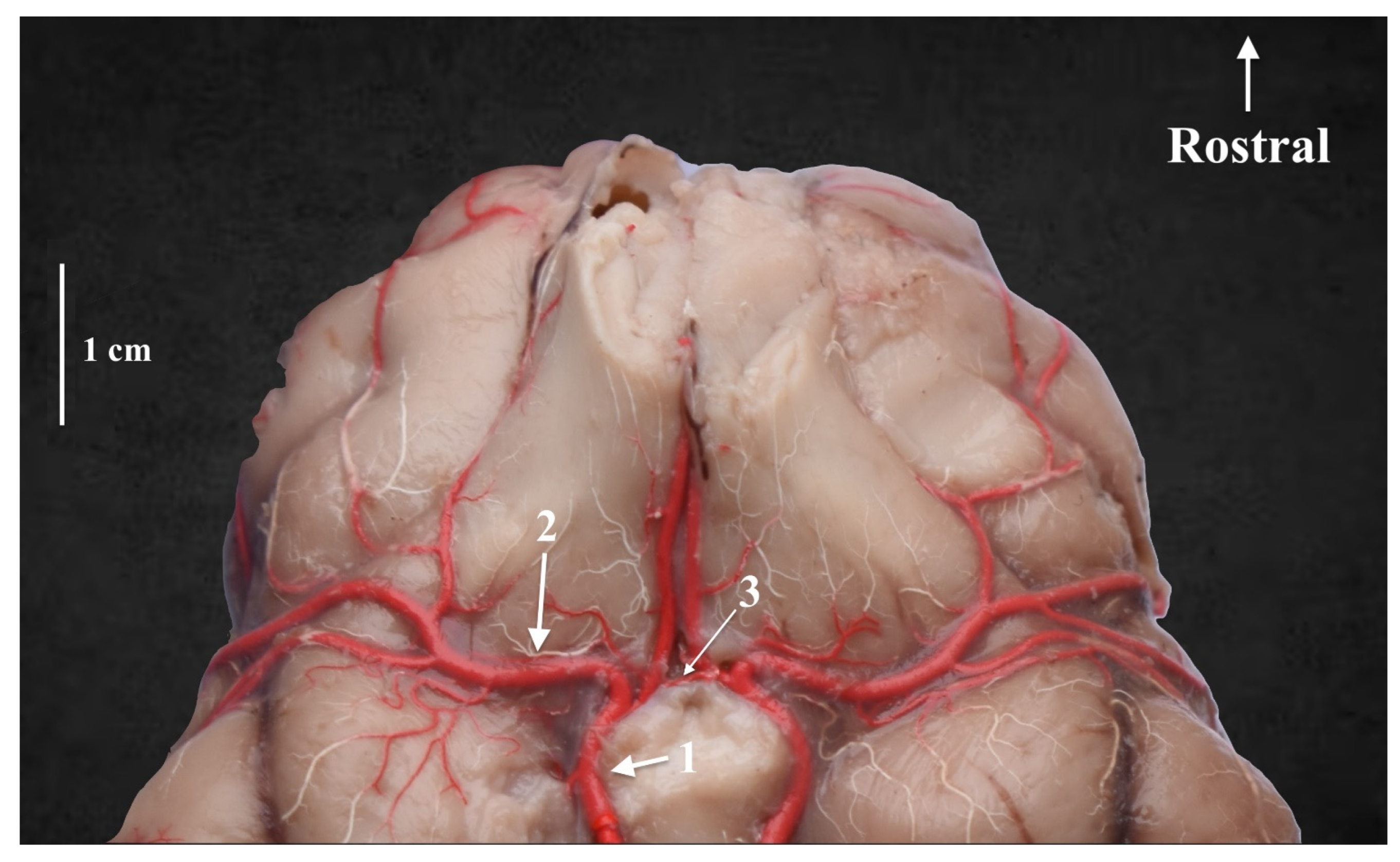
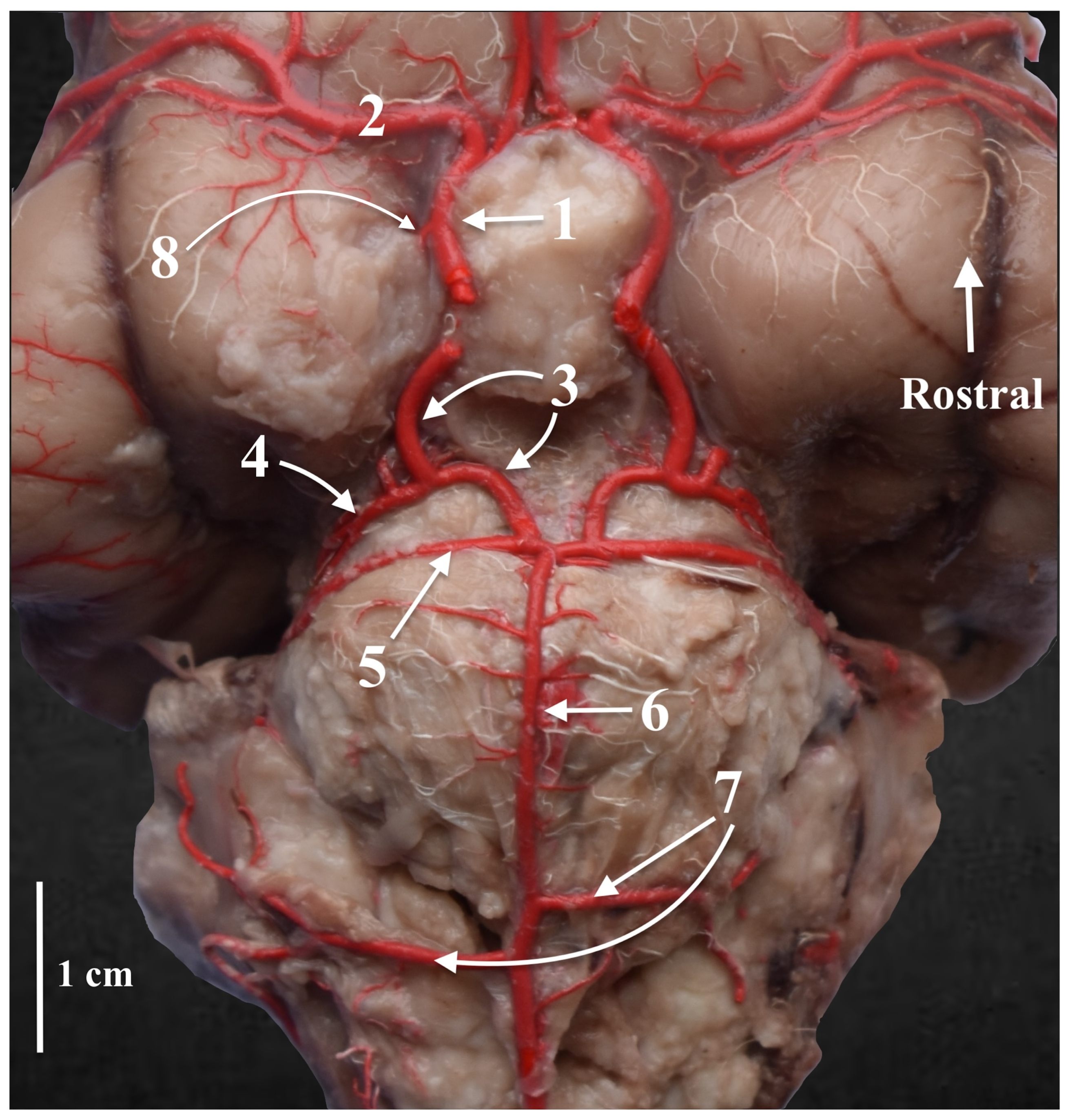
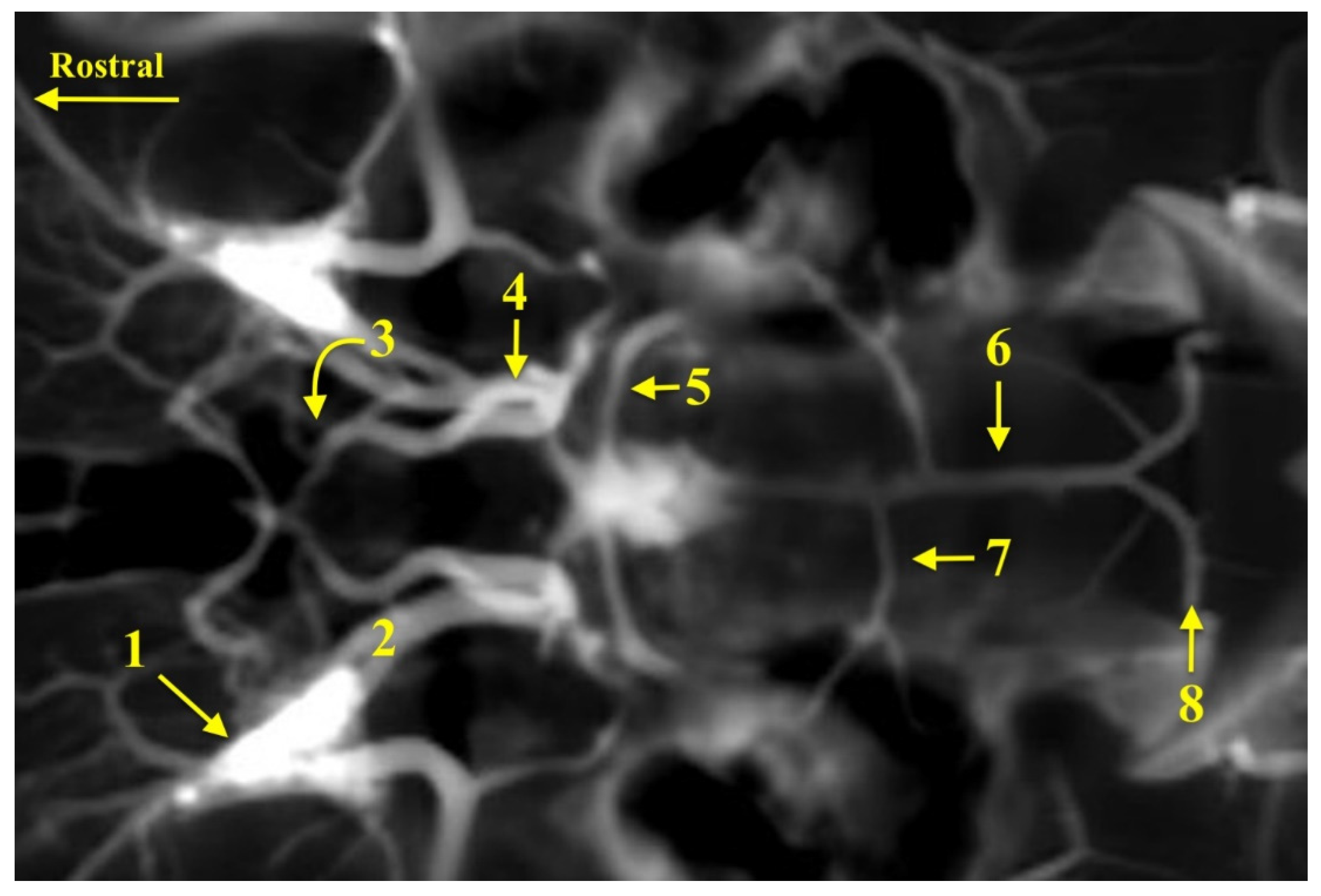
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassanin, A.; Veron, G.; Ropiquet, A.; Jansen van Vuuren, B.; Lécu, A.; Goodman, S.M.; Haider, J.; Nguyen, T.T. Evolutionary history of Carnivora (Mammalia, Laurasiatheria) inferred from mitochondrial genomes. PLoS ONE 2021, 16, e0240770. [Google Scholar]
- Wilson, D.E.; Reeder, D.M. Mammal Species of the World. In A Taxonomic and Geographic Reference, 3rd ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2005; pp. 1–728. [Google Scholar]
- Avgan, B.; Henschel, P.; Ghoddousi, A. Caracal Caracal. The IUCN Red List of Threatened Species; 2016, e. T3847A102424310. Available online: https://www.iucnredlist.org/species/3847/102424310 (accessed on 30 November 2023).
- Sheikh, K.M.; Molur, S. (Eds.) Status and Red List of Pakistan’s Mammals. In Based on the Conservation Assessment and Management Plan; IUCN Pakistan: Islamabad, Pakistan, 2004; pp. 1–311. [Google Scholar]
- Cuzin, F. Les Grands Mammifères du Maroc Méridional (Haut Atlas, Anti Atlas et Sahara): Distribution, Écologie et Conservation. Master’s Thesis, Montpellier University, Montpellier, France, 2003. [Google Scholar]
- Thorn, M.; Green, M.; Keith, M.; Marnewick, K.; Bateman, P.W.; Cameron, E.Z.; Scott, D.M. Large-scale distribution patterns of carnivores in northern South Africa: Implications for conservation and monitoring. Oryx 2011, 45, 579–586. [Google Scholar] [CrossRef]
- Veals, A.M.; Burnett, A.D.; Morandini, M.; Drouilly, M.; Koprowski, J.L. Caracal caracal (Carnivora: Felidae). Mamm. Species 2020, 52, 71–85. [Google Scholar] [CrossRef]
- Kingdon, J. (Ed.) Carnivores, Pangolins, Equids, and Rhinoceroses; A&C Black: London, UK, 2014; pp. 173–179. [Google Scholar]
- Livingston, S.E. The nutrition and natural history of the serval (Felis serval) and caracal (Caracal caracal). Vet. Clin. N. Am. Exot. Anim. Pract. 2009, 12, 327–334. [Google Scholar] [CrossRef]
- Avenant, N.L.; Nel, J.A.J. Among habitat variation in prey availability and use by caracal Felis caracal. Mamm. Biol. 2002, 67, 18–33. [Google Scholar] [CrossRef]
- Braczkowski, A.; Watson, L.; Coulson, D.; Lucas, J.; Peiser, B.; Rossi, M. The diet of caracal, Caracal caracal, in two areas of the southern Cape, South Africa as determined by scat analysis. Afr. J. Wildl. Res. 2012, 42, 111–116. [Google Scholar] [CrossRef]
- Bothma, J.D.P.; Walker, C. Larger Carnivores of the African Savannas; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1999; pp. 1–262. [Google Scholar]
- Melville, H.I.A.S.; Bothma, J.D.P.; Mills, M.G.L. Prey selection by caracal in the Kgalagadi Transfrontier Park. Afr. J. Wildl. Res. 2004, 34, 67–75. [Google Scholar]
- Thorn, M.; Green, M.; Dalerum, F.; Bateman, P.W.; Scott, D.M. What drives human–carnivore conflict in the north West Province of South Africa? Biol. Conserv. 2012, 150, 23–32. [Google Scholar] [CrossRef]
- Crookes, D.J. Ecology, opportunity or threat? Drivers of the caracal (Caracal caracal) population decline in South Africa. Afr. J. Ecol. 2023, 61, 768–780. [Google Scholar] [CrossRef]
- Ijiri, A.; Yoshiki, K.; Tsuboi, S.; Shimazaki, H.; Akiyoshi, H.; Nakade, T. Surgical resection of twenty-three cases of brain meningioma. J. Vet. Med. Sci. 2014, 76, 331–338. [Google Scholar] [CrossRef]
- Caine, A.; Brash, R.; De Risio, L.; Van Dijk, J.; Cherubini, G.B.; Dennis, R. MRI in 30 cats with traumatic brain injury. J. Feline Med. Surg. 2019, 21, 1111–1119. [Google Scholar] [CrossRef]
- International Committee on Veterinary Gross Anatomical Nomenclature. Nomina Anatomica Veterinaria, 6th ed.; Editorial Committee: Hannover, Germany, 2017. [Google Scholar]
- Frąckowiak, H.; Godynicki, S. Brain basal arteries in various species of Felidae. Pol. J. Vet. Sci. 2003, 6, 195–200. [Google Scholar]
- Frąckowiak, H.; Jakubowski, H. Arterial vascularization in the giraffe brain. In Annales Zoologici Fennici; Finnish Zoological and Botanical Publishing Board: Helsinki, Finland, 2008; Volume 4, pp. 353–359. [Google Scholar]
- Zdun, M.; Frąckowiak, H.; Kiełtyka-Kurc, A.; Kowalczyk, K.; Nabzdyk, M.; Timm, A. The arteries of brain base in species of Bovini tribe. Anat. Rec. 2013, 296, 1677–1682. [Google Scholar] [CrossRef]
- Frąckowiak, H.; Zdun, M.; Kowalczyk, K.; Komosa, M.; Kiełtyka-Kurc, A. Comparison of cerebral base arteries in antelopes of Tragelaphus, Taurotragus and Boselaphus genera. Zoomorphology 2014, 133, 351–357. [Google Scholar] [CrossRef]
- Frąckowiak, H.; Dębiński, D.; Komosa, M.; Zdun, M. The arterial circle of the brain, its branches and connections in selected representatives of the Antilopinae. J. Morphol. 2015, 276, 766–771. [Google Scholar] [CrossRef]
- Zdun, M.; Jabłonski, R.; Dębinski, D.; Frąckowiak, H. The Eurasian elk’s (Alces alces) brain base arteries in view of vascular variation. Anat. Rec. 2019, 302, 339–345. [Google Scholar] [CrossRef]
- Al Aiyan, A.; Menon, P.; Al Darwich, A.; Almuhairi, F.; Alnuaimi, S.; Bulshawareb, A.; Qablan, M.; Shehab, S. Descriptive analysis of cerebral arterial vascular architecture in dromedary camel (Camelus dromedarius). Front. Neuroanat. 2019, 13, 67. [Google Scholar] [CrossRef]
- Jerbi, H.; Vazquez, N.; Perez, W. Morphological Configuration and Topography of the brain arterial supply of the One-humped Camel (Camelus dromedarius, Linnaeus 1758). Int. J. Morphol. 2019, 37, 1095–1100. [Google Scholar] [CrossRef]
- Al Aiyan, A.; Menon, P.; AlDarwich, A.; Qablan, M.; Hammoud, M.; Shawaf, T.; Richardson, K. Vertebrobasilar contribution to cerebral arterial system of dromedary camels (Camelus dromedarius). Front. Vet. Sci. 2021, 8, 696707. [Google Scholar] [CrossRef]
- Kanan, C.V. The cerebral arteries of Camelus dromedarius. Acta Anat. 1970, 7, 605–616. [Google Scholar] [CrossRef]
- Evans, H.E.; de Lahunta, A. Miller’s Anatomy of the Dog, 4th ed.; Elsevier Saunders: St. Louis, MO, USA, 2012; pp. 1–872. [Google Scholar]
- Ozudogru, Z.; Can, M.; Balkaya, H. Macro-anatomical investigation of the cerebral arterial circle (Circle of Willis) in red fox (Vulpes vulpes). J. Anim. Vet. Adv. 2012, 11, 2861–2864. [Google Scholar]
- Fenrich, M.; Habjanovic, K.; Kajan, J.; Heffer, M. The circle of Willis revisited: Forebrain dehydration sensing facilitated by the anterior communicating artery: How hemodynamic properties facilitate more efficient dehydration sensing in amniotes. BioEssays 2021, 43, 2000115. [Google Scholar] [CrossRef]
- Rahmat, S.; Gillan, E. Comparative anatomy of the carotid-basilar arterial trunk and hindbrain penetrating arteries in vertebrates. Open Anat. J. 2014, 6, 1–26. [Google Scholar] [CrossRef]
- Klein, T. Korrosionsanatomische Untersuchungen am Blutgefaßsystem des Encephalon und der Meninges bei Felis domestica [Corrosion-anatomical study of the blood vessels of the encephalon and meninges of Felis domestica]. Anat. Histol. Embryol. 1980, 9, 236–279. [Google Scholar] [CrossRef]
- Jerbi, H.; Khaldi, S.; Pérez, W. Morphometric Study of the rostral epidural rete mirabile in the dromedary (Camelus dromedarius, Linnaeus 1758). Int. J. Morphol. 2016, 34, 1429–1435. [Google Scholar] [CrossRef]
- Zdun, M.; Ruszkowski, J.J.; Butkiewicz, A.F.; Gogulski, M. Arterial Blood Supply to the Cerebral Arterial Circle in the Selected Species of Carnivora Order from Poland. Animals 2023, 13, 3144. [Google Scholar] [CrossRef]
- Baldwin, B.A.; Bell, F.R. The contribution of the carotid and cerebral arteries to the blood pressure in sheep. J. Physiol. 1960, 151, 9–10. [Google Scholar]
- Baldwin, B.A.; Bell, F.R. Effect of clamping cartotid and vertebral arteries on the blood pressure in sheep. J. Physiol. 1960, 151, 39–40. [Google Scholar]
- Baldwin, B.A.; Bell, F.R. The anatomy of the cerebral circulation in the sheep and ox. J. Anat. 1963, 97, 203–215. [Google Scholar]
- Kamijyo, Y.; Garcia, J.H. Carotid arterial supply of the feline brain. Applications to the study of regional cerebral ischemia. Stroke 1975, 6, 361–369. [Google Scholar] [CrossRef]
- Bugge, J. The cephalic arterial system in carnivores, with special reference to the systematic classification. Acta Anat. 1978, 101, 45–61. [Google Scholar] [CrossRef]
- Simoens, P.; Lauwers, H.; De Geest, J.; De Schaepdrijver, L. Functional morphology of the cranial retia mirabilia in the domestic mammals. Schw. Arch. Fur Tierheilkd. 1987, 129, 295–307. [Google Scholar]
- Kier, E.L.; Conlogue, G.J.; Zhuang, Z. High-resolution computed tomography imaging of the cranial arterial system and rete mirabile of the cat (Felis catus). Anat. Rec. 2019, 302, 1958–1967. [Google Scholar] [CrossRef]
- Ziemak, H.; Frackowiak, H.; Zdun, M. Domestic cat’s internal carotid artery in ontogenesis. Vet. Med. 2021, 66, 292–297. [Google Scholar] [CrossRef]
- Wiland, C. Zakres zmienności układu tętnic podstawy mózgowia u piesaków. Rocz. WSR. Pozn. 1967, 36, 215–231. [Google Scholar]
- Frąckowiak, H. Tętnice głowy u lisa pospolitego. Rocz. Akad. Rol. Pozn. 1978, 101, 59–66. [Google Scholar]
- Tanuma, K. A morphological study on the circle of Willis in the dog. Okajimas Folia Anat. Jap. 1981, 58, 155–175. [Google Scholar] [CrossRef]
- Wiland, C. Badania porównawcze gałęzi korowych tętnicy środkowej mózgu u niektórych gatunków drapieżnych (Carnivora). Zesz. Nauk. ATR Bydgoszcz. 1991, 44, 1–52. [Google Scholar]
- Skoczylas, B.; Brudnicki, W.; Kirkiłło-Stacewicz, K.; Nowicki, W.; Wach, J. Cortical branches of the middle cerebral artery in silver fox (Vulpes vulpes). Pesqui. Veterinária Bras. 2016, 36, 1053–1057. [Google Scholar] [CrossRef]
- Brudnicki, W. Basilar arteries of the brain in domestic goat (Capra hircus L.). Electron. J. Pol. Agric. Univ. Ser. Vet. Med. 2000, 3, 02. [Google Scholar]
- Brudnicki, W. Morphometric analysis of the brain base arteries in fallow deer (Dama dama). Vet. Med. 2011, 56, 462–468. [Google Scholar] [CrossRef]
- Godynicki, S. Tętnice głowy u sarny (Capreolus capreolus L.). Rocz. Akad. Rol. W Poznaniu. Wydział Zootech. 1971, 54, 35–45. [Google Scholar]
- König, H.E. Anatomie und Entwicklung der Blutgefässe in der Schädelhöhle der Hauswiederkäuer (Rind, Schaf und Ziege); Ferdinand Enke Verl: Stuttgart, Germany, 1979; pp. 1–215. [Google Scholar]
- Nickiel, R.; Schwarz, R. Vergleichende Betrachtung der Kopfarterien der Haussaugetiere (Katze, Hund, Schwein, Rind, Schaf, Ziege, Pferd). Zentralb. Veter. Reihe A 1963, 10, 89–120. (In German) [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdun, M.; Butkiewicz, A.F.; Zawadzki, M. The Arteries of the Encephalon Base in Caracal (Caracal caracal; Felidae; Carnivora). Animals 2023, 13, 3780. https://doi.org/10.3390/ani13243780
Zdun M, Butkiewicz AF, Zawadzki M. The Arteries of the Encephalon Base in Caracal (Caracal caracal; Felidae; Carnivora). Animals. 2023; 13(24):3780. https://doi.org/10.3390/ani13243780
Chicago/Turabian StyleZdun, Maciej, Aleksander F. Butkiewicz, and Marcin Zawadzki. 2023. "The Arteries of the Encephalon Base in Caracal (Caracal caracal; Felidae; Carnivora)" Animals 13, no. 24: 3780. https://doi.org/10.3390/ani13243780






