Modified Ultrasound-Guided Dorsal Quadratus Lumborum Block in Cat Cadavers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
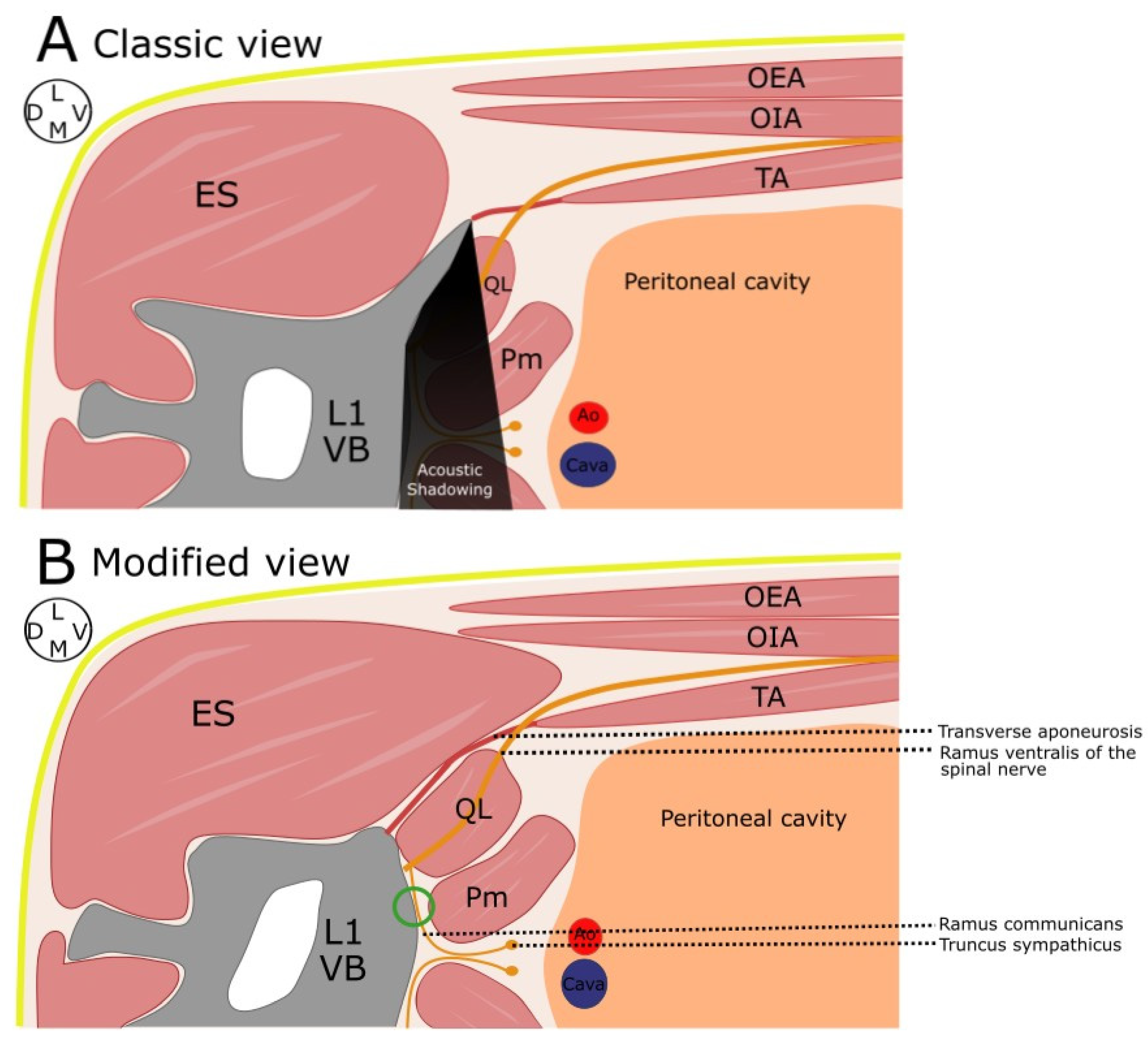
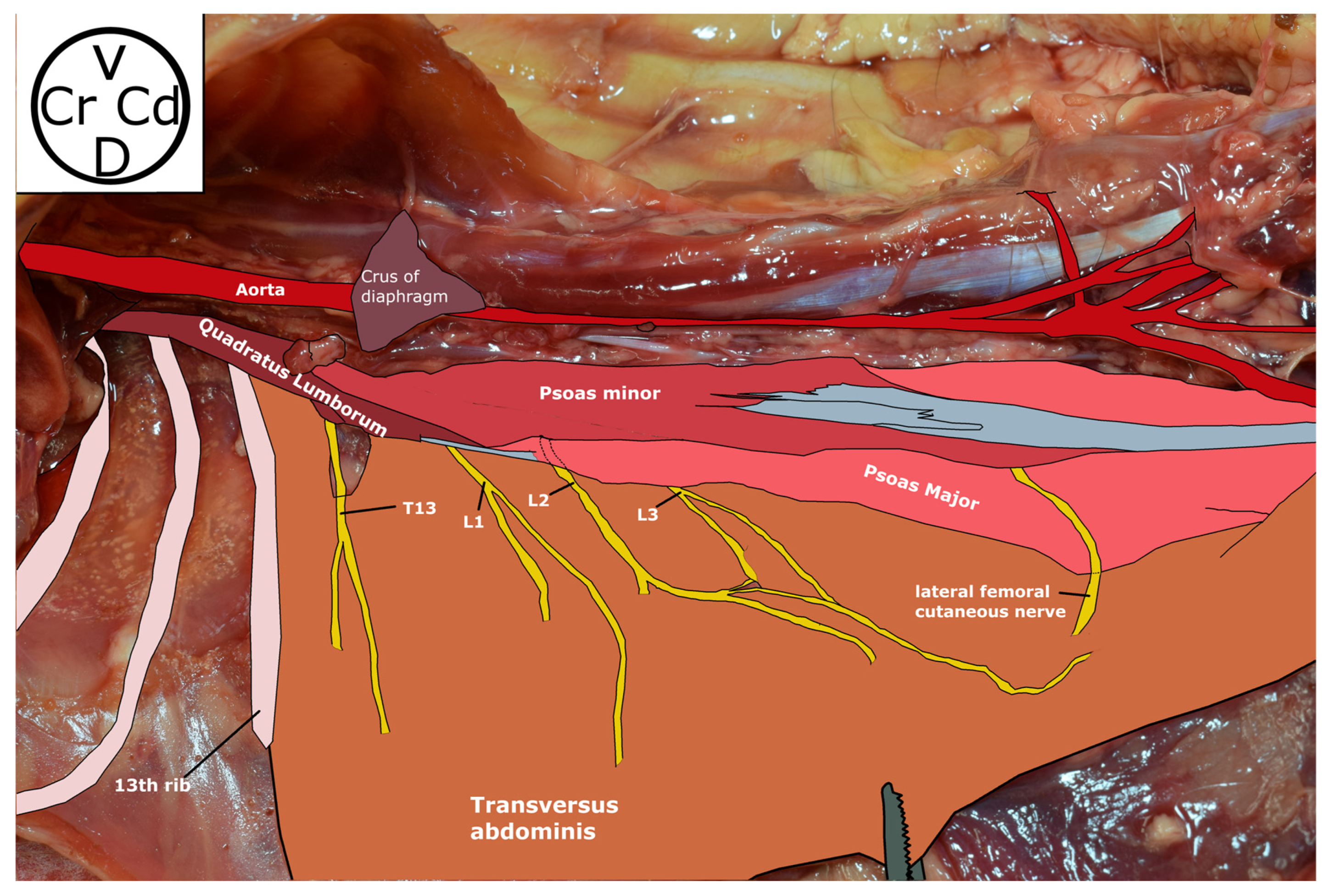
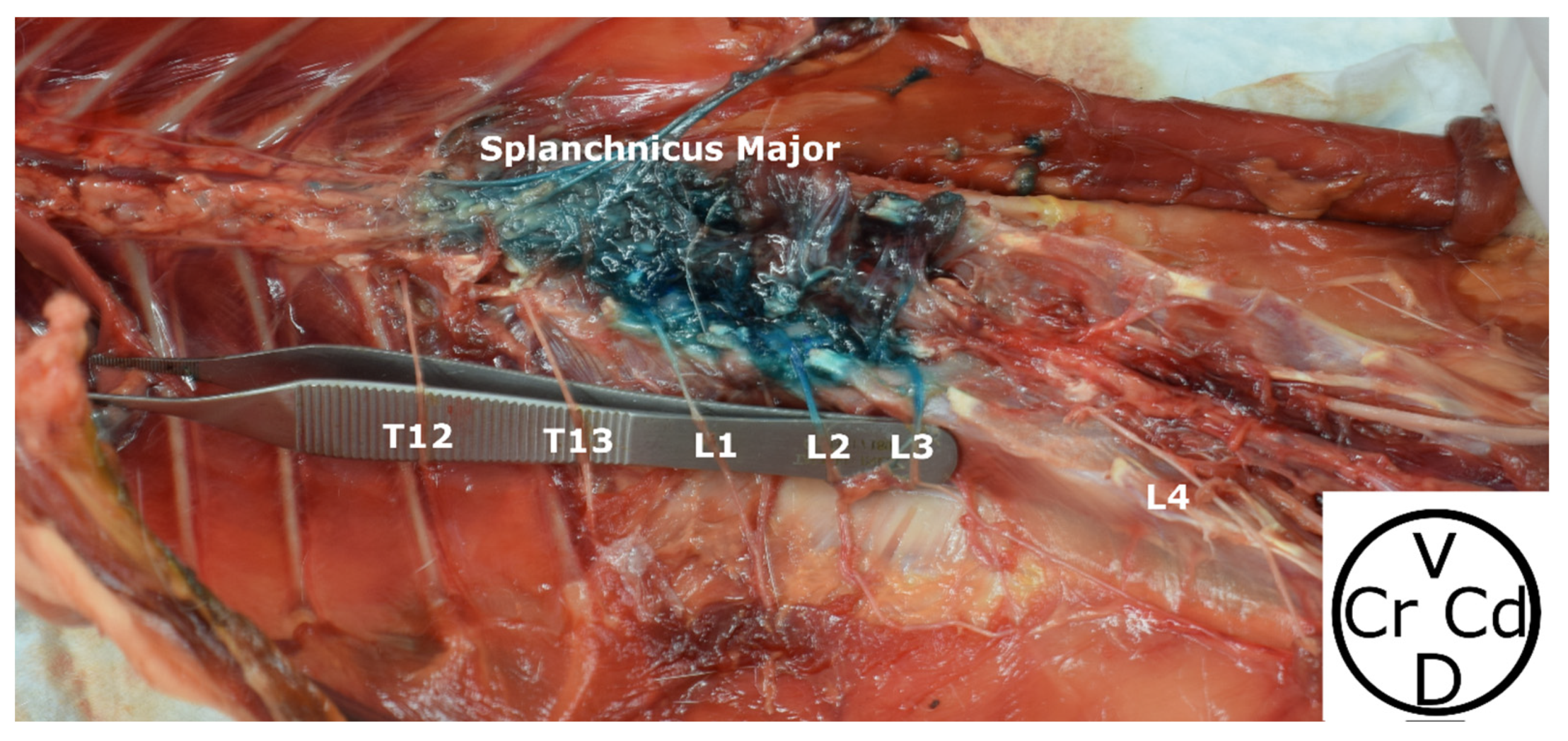
2.1. Phase 1: Anatomical Study
2.2. Phase 2
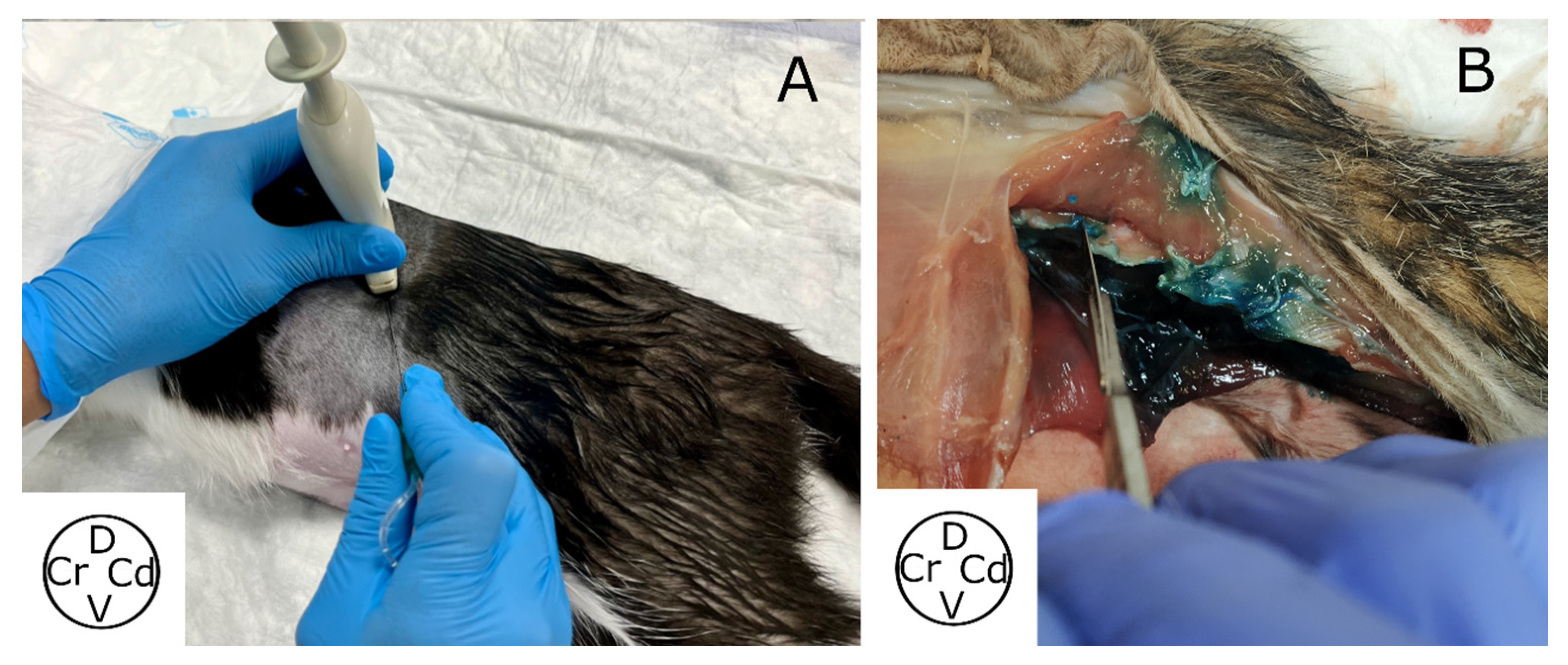
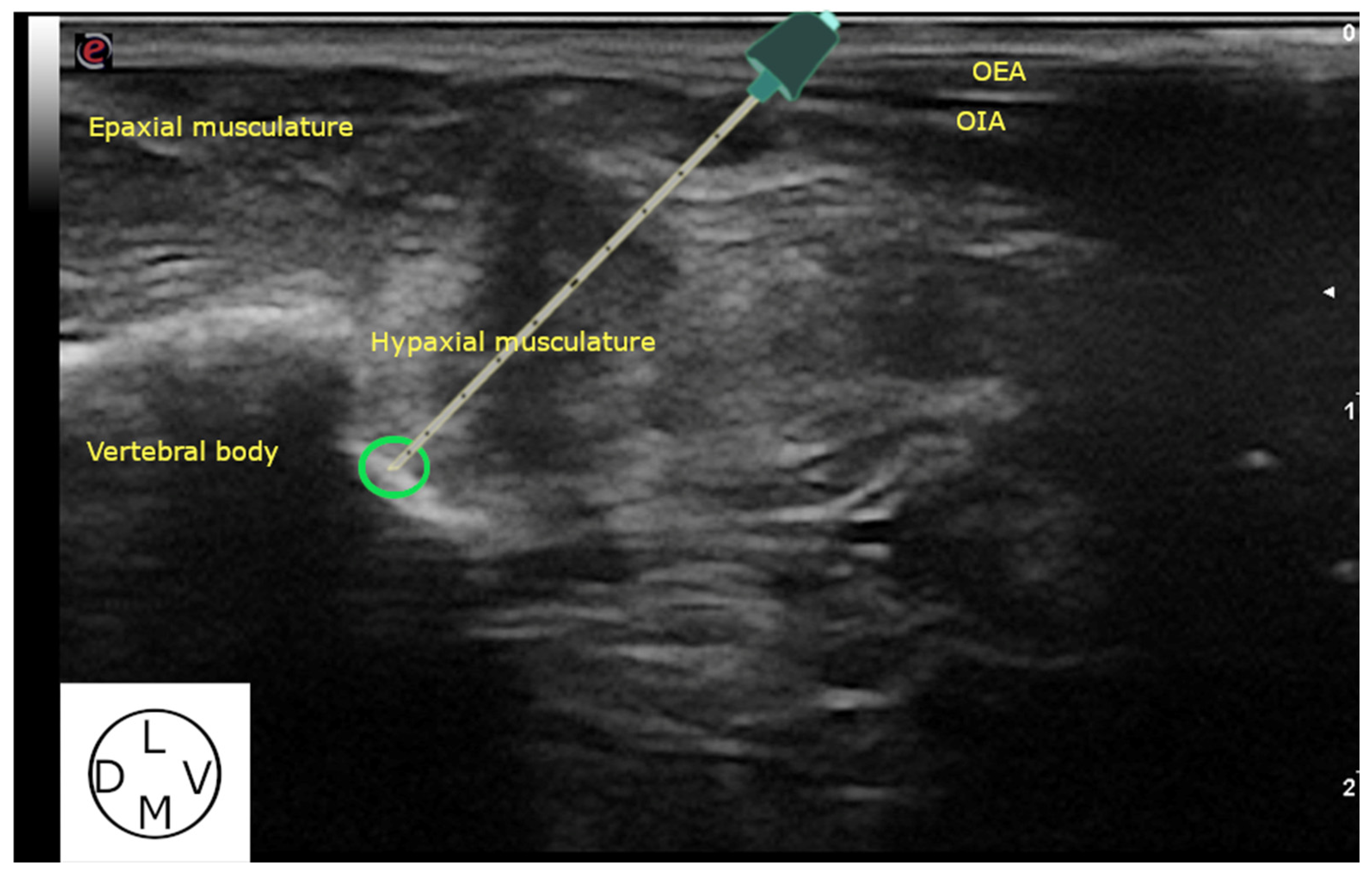
2.2.1. Ultrasound-Guided Technique
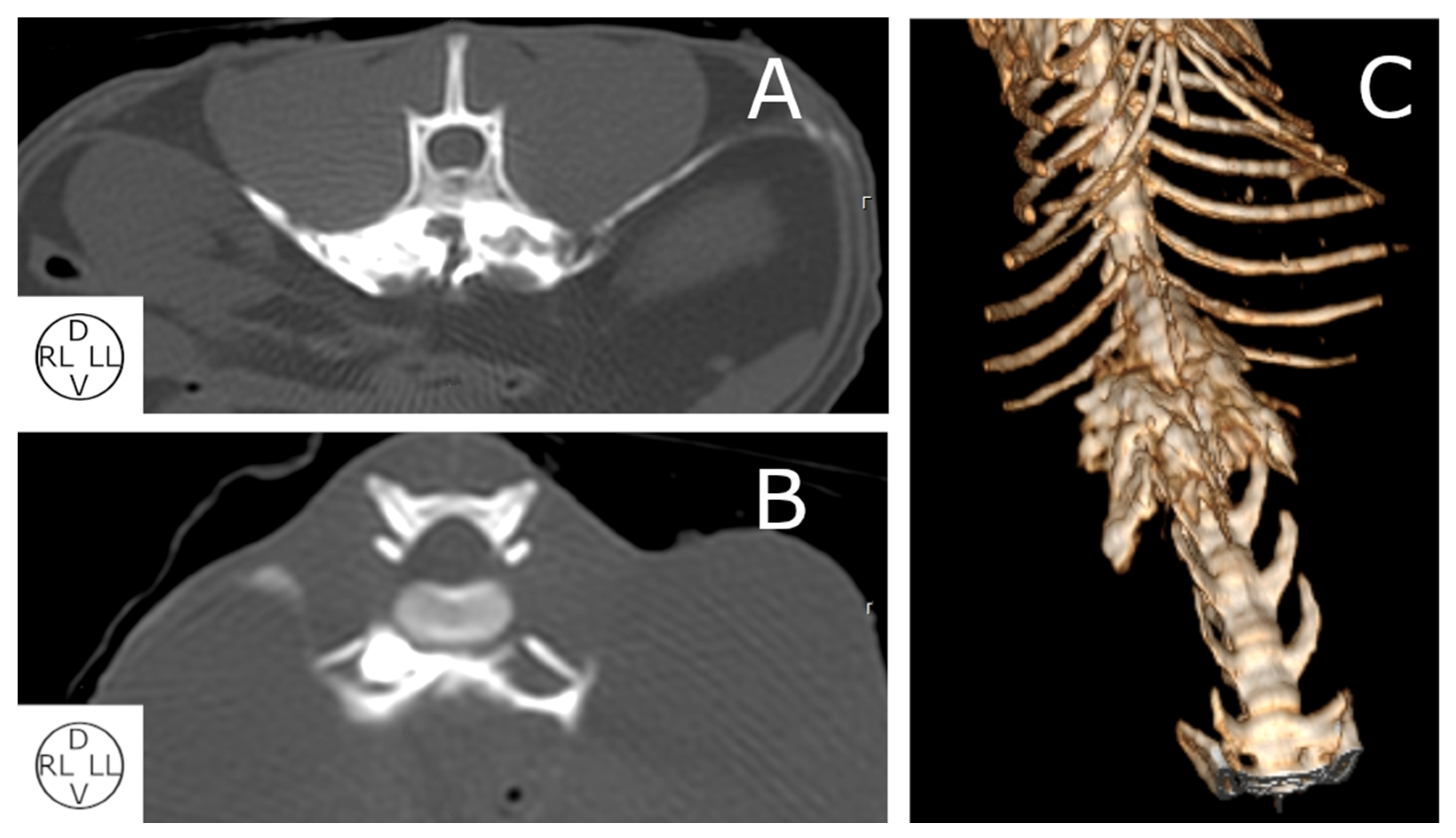
2.2.2. Computed Tomography (CT) Study
2.2.3. Spread Study
3. Results
3.1. Phase 1: Anatomical Study
3.2. Phase 2
3.2.1. Demographic Distribution
3.2.2. Ultrasound-Guided Technique
3.2.3. Computed Tomography Study
3.2.4. Spread Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Portela, D.A.; Verdier, N.; Otero, P.E. Regional Anesthetic Techniques for the Pelvic Limb and Abdominal Wall in Small Animals: A Review of the Literature and Technique Description. Vet. J. 2018, 238, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Bugada, D.; Ghisi, D.; Mariano, E.R. Continuous Regional Anesthesia: A Review of Perioperative Outcome Benefits. Minerva Anestesiol. 2017, 83, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Hutton, M.; Brull, R.; Macfarlane, A.J.R. Regional Anaesthesia and Outcomes. BJA Educ. 2018, 18, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Campoy, L. Development of Enhanced Recovery After Surgery (ERAS) Protocols in Veterinary Medicine through a One-Health Approach: The Role of Anesthesia and Locoregional Techniques. Javma 2022, 260, 1751–1759. [Google Scholar] [CrossRef]
- Munirama, S.; McLeod, G. A Systematic Review and Meta-Analysis of Ultrasound versus Electrical Stimulation for Peripheral Nerve Location and Blockade. Anaesthesia 2015, 70, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, H.; Pawa, A.; Mariano, E.R. Interfascial Plane Blocks: Back to Basics. Reg. Anesth. Pain Med. 2018, 43, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.J.; McDonnell, J.G.; Carvalho, B.; Sharkey, A.; Pawa, A.; Gadsden, J. Essentials of Our Current Understanding: Abdominal Wall Blocks. Reg. Anesth. Pain Med. 2017, 42, 133–183. [Google Scholar] [CrossRef]
- Garbin, M.; Ruel, H.L.; Watanabe, R.; Malo, A.; Monteiro, B.P.; Steagall, P.V. Analgesic Efficacy of an Ultrasound-Guided Transversus Abdominis Plane Block with Bupivacaine in Cats: A Randomised, Prospective, Masked, Placebo-Controlled Clinical Trial. J. Feline Med. Surg. 2023, 25, 1098612X2311544. [Google Scholar] [CrossRef]
- Touzot-Jourde, G. Ultrasound-Guided Rectus Abdominis Sheath Block in Cats Undergoing Ovariectomy: A Prospective, Randomized, Investigator-Blinded, Placebo-Controlled Clinical Trial. OAJVSR 2022, 7, 1–8. [Google Scholar] [CrossRef]
- Otero, P.E.; Campoy, L. Epidural and Spinal Anesthesia. In Small Animal Regional Anesthesia and Analgesia; Campoy, L., Read, M.R., Eds.; Wiley: Ames, IA, USA, 2013; pp. 227–259. ISBN 978-0-8138-1994-5. [Google Scholar]
- Børglum, J.; Moriggl, B.; Jensen, K.; Lønnqvist, P.-A.; Christensen, A.F.; Sauter, A.; Bendtsen, T.F. Ultrasound-Guided Transmuscular Quadratus Lumborum Blockade. BJA Br. J. Anaesth. 2013, 111. [Google Scholar] [CrossRef]
- Garbin, M.; Portela, D.A.; Bertolizio, G.; Garcia-Pereira, F.; Gallastegui, A.; Otero, P.E. Description of Ultrasound-Guided Quadratus Lumborum Block Technique and Evaluation of Injectate Spread in Canine Cadavers. Vet. Anaesth. Analg. 2020, 47, 249–258. [Google Scholar] [CrossRef]
- Viscasillas, J.; Terrado, J.; Marti-Scharfhausen, R.; Castiñeiras, D.; Esteve, V.; Clancy, N.; Redondo, J.I. A Modified Approach for the Ultrasound-Guided Quadratus Lumborum Block in Dogs: A Cadaveric Study. Animals 2021, 11, 2945. [Google Scholar] [CrossRef] [PubMed]
- Marchina-Gonçalves, A.; Gil, F.; Laredo, F.G.; Soler, M.; Agut, A.; Belda, E. Evaluation of High-Volume Injections Using a Modified Dorsal Quadratus Lumborum Block Approach in Canine Cadavers. Animals 2021, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Alaman, M.; Bonastre, C.; de Blas, I.; Gomez-Alvarez, C.M.; Laborda, A. Description of a Novel Ultrasound-Guided Approach for a Dorsal Quadratus Lumborum Block: A Canine Cadaver Study. Vet. Anaesth. Analg. 2022, 49, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Marchina-Gonçalves, A.; Laredo, F.G.; Gil, F.; Soler, M.; Agut, A.; Redondo, J.I.; Belda, E. An Ultrasound-Guided Latero-Ventral Approach to Perform the Quadratus Lumborum Block in Dog Cadavers. Animals 2023, 13, 2214. [Google Scholar] [CrossRef] [PubMed]
- Garbin, M.; Portela, D.A.; Bertolizio, G.; Gallastegui, A.; Otero, P.E. A Novel Ultrasound-Guided Lateral Quadratus Lumborum Block in Dogs: A Comparative Cadaveric Study of Two Approaches. Vet. Anaesth. Analg. 2020, 47, 810–818. [Google Scholar] [CrossRef] [PubMed]
- dos-Santos, J.D.; Ginja, M.; Alves-Pimenta, S.; Otero, P.E.; Ribeiro, L.; Colaço, B. A Description of an Ultrasound-Guided Technique for a Quadratus Lumborum Block in the Cat: A Cadaver Study. Vet. Anaesth. Analg. 2021, 48, 804–808. [Google Scholar] [CrossRef]
- dos-Santos, J.D.; Ginja, M.; Alves-Pimenta, S.; E Otero, P.; Ribeiro, L.; Colaço, B. Comparison of Dorsoventral and Ventrodorsal Approaches for Ultrasound-Guided Quadratus Lumborum Block in Cats: A Cadaver Study. Vet. Anaesth. Analg. 2022, 49, 481–489. [Google Scholar] [CrossRef]
- Grubb, T.; Lobprise, H. Local and Regional Anaesthesia in Dogs and Cats: Overview of Concepts and Drugs (Part 1). Vet. Med. Sci. 2020, 6, 209–217. [Google Scholar] [CrossRef]
- WSAVA Nutritional Assessment Guidelines Task Force Members; Freeman, L.; Becvarova, I.; Cave, N.; MacKay, C.; Nguyen, P.; Rama, B.; Takashima, G.; Tiffin, R.; Tsjimoto, H.; et al. WSAVA Nutritional Assessment Guidelines. J. Small Anim. Pract. 2011, 52, 385–396. [Google Scholar] [CrossRef]
- Getty, R. Sisson and Grossman’s-The Anatomy of the Domestic Animals; 5. Aufl.: Philadelphia, PA, USA, 1975; Volume 2, ISBN 978-0-7216-4107-2. [Google Scholar]
- Argus, A.P.V.; Freitag, F.A.V.; Bassetto, J.E.; Vilani, R.G. Quadratus Lumbar Block for Intraoperative and Postoperative Analgesia in a Cat. Vet. Anaesth. Analg. 2020, 47, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Torres Cantó, L.; Felisberto, R.; Economou, A.; Flaherty, D.; Moreno Aguado, B.; Tayari, H. Ultrasound-Guided Dorsolateral Approach for Quadratus Lumborum Block in Rabbits (Oryctolagus Cuniculus): A Prospective, Randomized, Blinded, Cadaveric Study Comparing Four Different Injectate Volumes. Animals 2023, 13, 2559. [Google Scholar] [CrossRef] [PubMed]
- Martin-Flores, M. Clinical Pharmacology and Toxicology of Local Anesthetics and Adjuncts. In Small Animal Regional Anesthesia and Analgesia; Campoy, L., Read, M.R., Eds.; Wiley: Ames, IA, USA, 2013; pp. 25–40. ISBN 978-0-8138-1994-5. [Google Scholar]
- Zahra, J.O.L.; Segatto, C.Z.; Zanelli, G.R.; Bruno, T.D.S.; Nicácio, G.M.; Giuffrida, R.; Cassu, R.N. A Comparison of Intra and Postoperative Analgesic Effects of Sacrococcygeal and Lumbosacral Epidural Levobupivacaine in Cats Undergoing Ovariohysterectomy. J. Vet. Med. Sci. 2023, 85, 1172–1179. [Google Scholar] [CrossRef]
- Valverde, A. Epidural Analgesia and Anesthesia in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 1205–1230. [Google Scholar] [CrossRef]
- Garcia-Pereira, F. Epidural Anesthesia and Analgesia in Small Animal Practice: An Update. Vet. J. 2018, 242, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.I.; Hoang, K.; Gelbard, W. Treatment of Acute Flares of Chronic Pancreatitis Pain with Ultrasound Guided Transversus Abdominis Plane Block: A Novel Application of a Pain Management Technique in the Acute Care Setting. Case Rep. Emerg. Med. 2014, 2014, 759508. [Google Scholar] [CrossRef]
- Bhatia, N.; Sen, I.M.; Mandal, B.; Batra, A. Postoperative Analgesic Efficacy of Ultrasound-Guided Ilioinguinal-Iliohypogastric Nerve Block Compared with Medial Transverse Abdominis Plane Block in Inguinal Hernia Repair: A Prospective, Randomised Trial. Anaesth. Crit. Care Pain Med. 2019, 38, 41–45. [Google Scholar] [CrossRef]
- Viscasillas, J.; Sanchis-Mora, S.; Burillo, P.; Esteve, V.; Del Romero, A.; Lafuente, P.; Redondo, J.I. Evaluation of Quadratus Lumborum Block as Part of an Opioid-Free Anaesthesia for Canine Ovariohysterectomy. Animals 2021, 11, 3424. [Google Scholar] [CrossRef]
- Klonner, M.E.; Verdier, N.; Otero, P.E. Bilateral Ultrasound-guided Lateral Quadratus Lumborum Block in a Minipig Undergoing Ovariectomy. Vet Rec. Case Rep. 2023, 11, e572. [Google Scholar] [CrossRef]
- Crouch, J.E. Text-Atlas of Cat Anatomy; Lea & Febiger: Philadelphia, PA, USA, 1969. [Google Scholar]
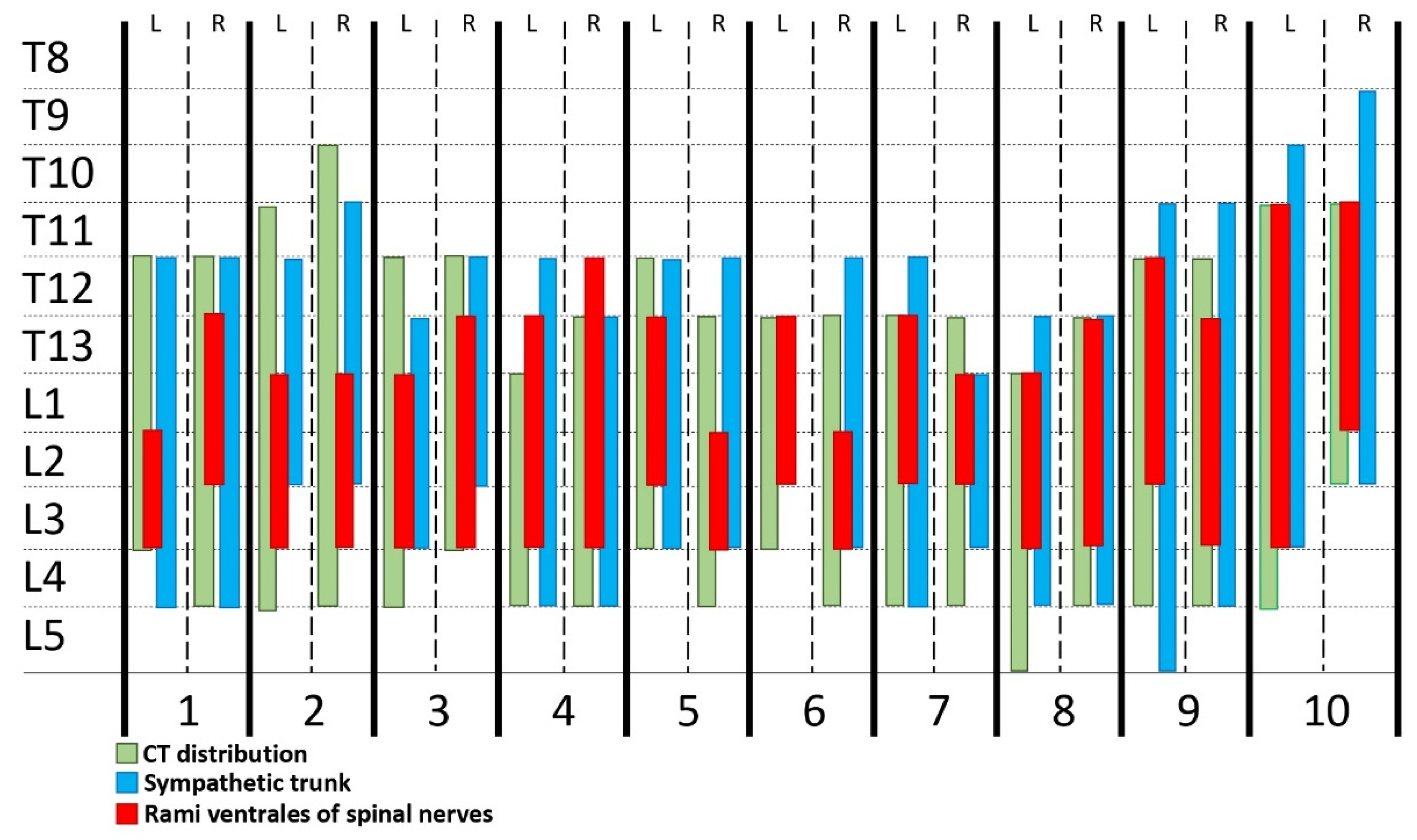
| Muscles | Dorsal to QL | Through QL | Between QL and Pm | Through PM | Between QL and PM |
|---|---|---|---|---|---|
| Rami Ventrales | |||||
| T13 | 3/5 | 2/5 | |||
| L1 | 5/5 | ||||
| L2 | 4/5 | 1/5 | |||
| L3 | 3/5 | 1/5 | 1/5 | ||
| L4 | 5/5 |
| Breed | Weight (kg) | BCS (1–9/9) |
|---|---|---|
| European Shorthair | 3.37 | 4/9 |
| European Shorthair | 3.45 | 5/9 |
| Siamese | 2.20 | 3/9 |
| European Shorthair | 1.56 | 3/9 |
| European Shorthair | 2.17 | 4/9 |
| European Shorthair | 3.07 | 5/9 |
| European Shorthair | 5.38 | 5/9 |
| European Shorthair | 3.26 | 4/9 |
| Persian | 2.76 | 5/9 |
| European Shorthair | 2.70 | 4/9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polo-Paredes, G.; Laredo, F.G.; Gil, F.; Soler, M.; Agut, A.; Belda, E. Modified Ultrasound-Guided Dorsal Quadratus Lumborum Block in Cat Cadavers. Animals 2023, 13, 3798. https://doi.org/10.3390/ani13243798
Polo-Paredes G, Laredo FG, Gil F, Soler M, Agut A, Belda E. Modified Ultrasound-Guided Dorsal Quadratus Lumborum Block in Cat Cadavers. Animals. 2023; 13(24):3798. https://doi.org/10.3390/ani13243798
Chicago/Turabian StylePolo-Paredes, Gonzalo, Francisco G. Laredo, Francisco Gil, Marta Soler, Amalia Agut, and Eliseo Belda. 2023. "Modified Ultrasound-Guided Dorsal Quadratus Lumborum Block in Cat Cadavers" Animals 13, no. 24: 3798. https://doi.org/10.3390/ani13243798
APA StylePolo-Paredes, G., Laredo, F. G., Gil, F., Soler, M., Agut, A., & Belda, E. (2023). Modified Ultrasound-Guided Dorsal Quadratus Lumborum Block in Cat Cadavers. Animals, 13(24), 3798. https://doi.org/10.3390/ani13243798









