A Comprehensive Biochemical Characterization of Hybrid Grouper Larvae (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂) during Yolk-Sac Larval Development
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Raising Conditions
2.2. Sampling and Design
2.3. Proximate Composition Analysis
2.4. Fatty Acid Analysis
2.5. Amino Acids Analysis
2.6. Statistical Analysis
3. Results
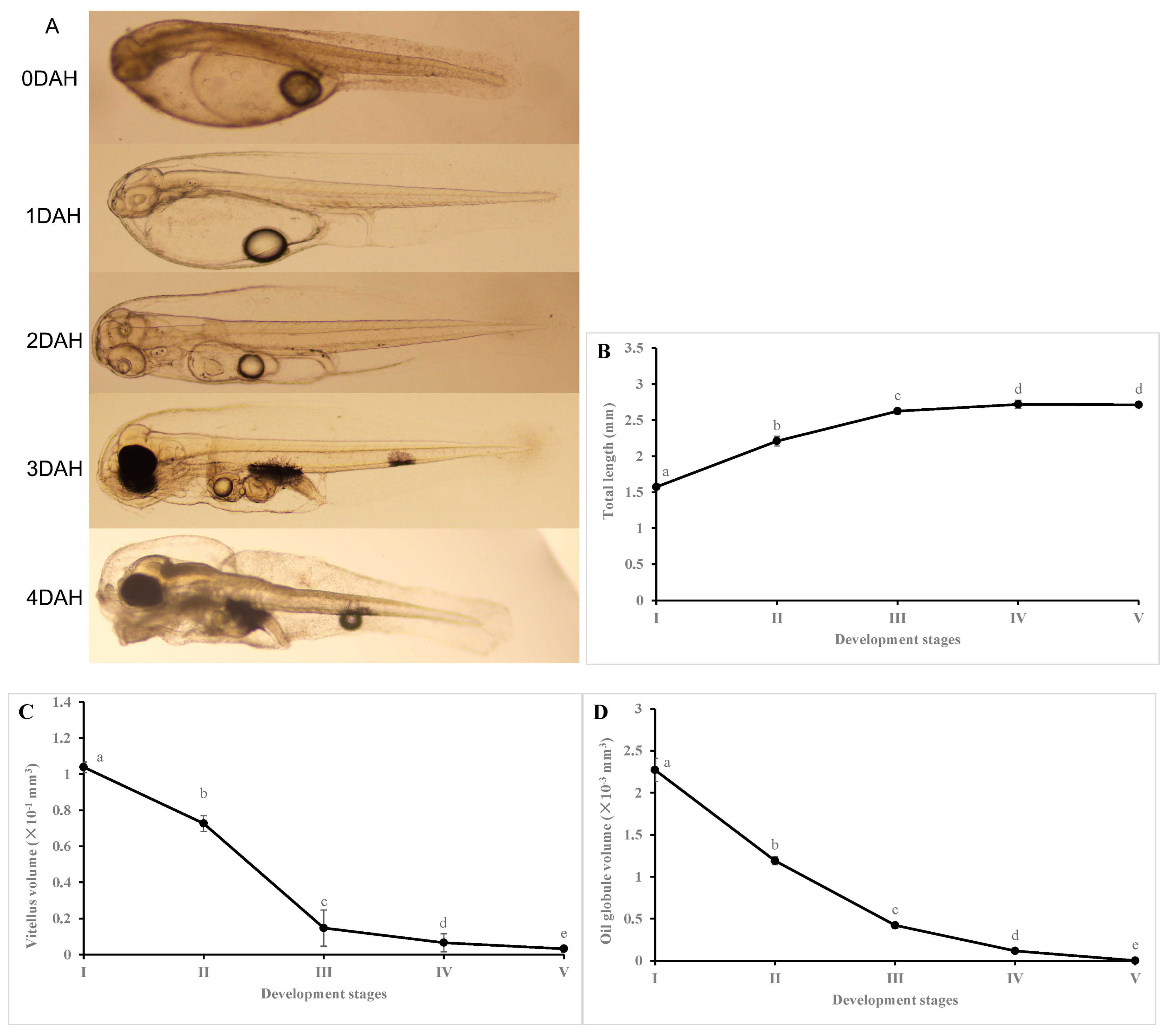
3.1. Changes in Larval Growth, Yolk Volume, and Oil Volume during Early Development
3.2. Changes in Proximate Composition during Early Development of Hybrid Grouper
3.3. Changes in Lipid Classes during Early Development of Hybrid Grouper
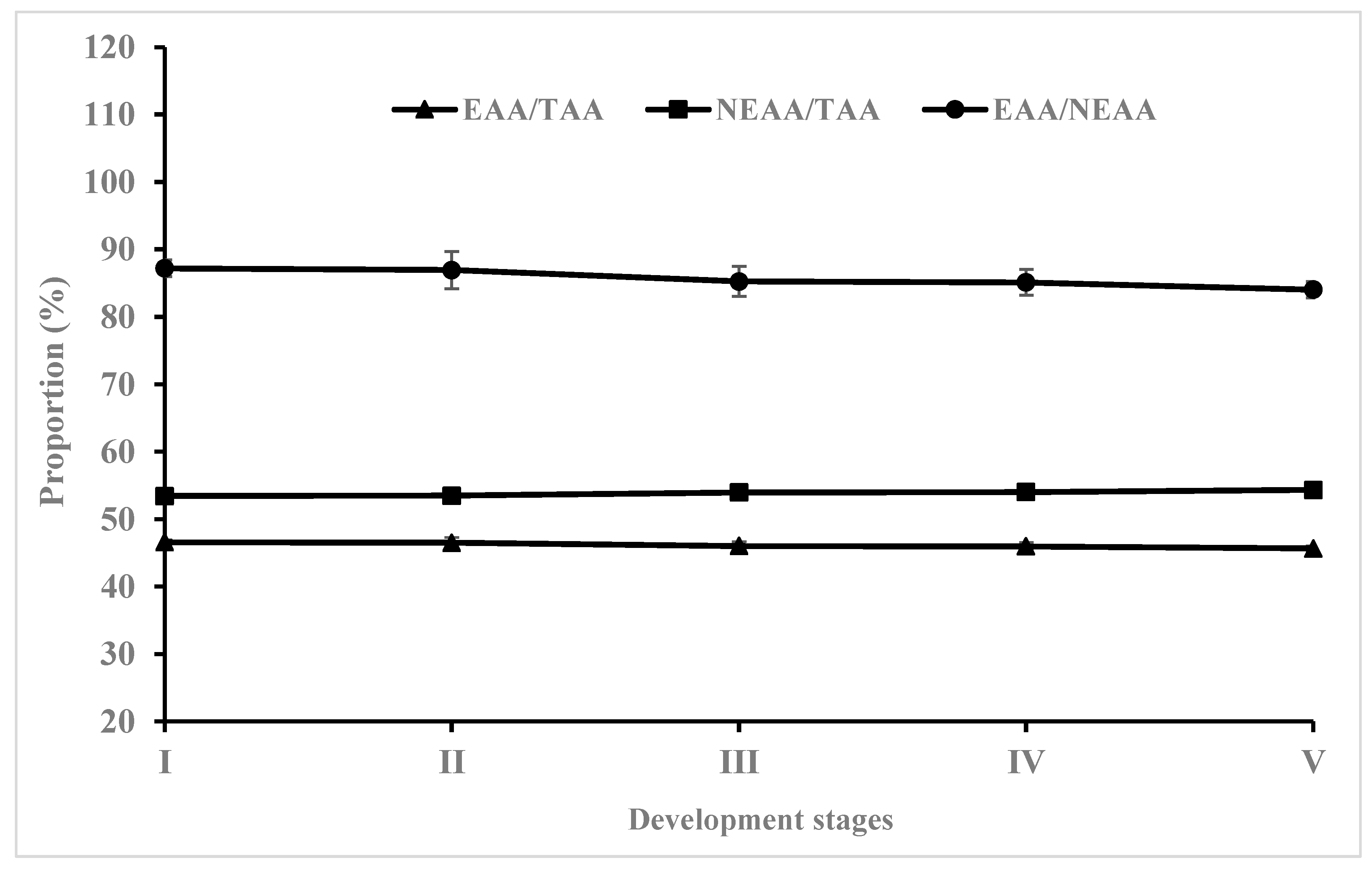
3.4. Changes in Amino Acid Composition during Early Development of Hybrid Grouper
3.5. Changes in Fatty Acid Composition during Early Development of Hybrid Grouper
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, B.; Liu, X.F.; Zhang, Y.F.; Liu, H.; Huang, B.; Gao, Y.Y. Study on auditory thresholds of Epinephelus fuscogutatus♀ × E. lanceolatus♂. Fish. Mod. 2019, 1, 6–12. [Google Scholar]
- Qiu, D.G.; Ping, W.; Qi, L.; Zhu, Z.H.; Wu, S.Q.; Liu, Y.H.; Chen, X.M.; Qiu, F.Y. The effect of feeding frequency on the growth and plasma anti-stress enzyme activities of hybrid grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂) in industrialized recirculating aquaculture systems. J. Fish. Res. 2018, 40, 441–448. [Google Scholar]
- Kjørsvik, E.; Hoehne-Reitan, K.; Reitan, K.I. Egg and larval quality criteria as predictive measures for juvenile production in turbot (Scophthalmus maximus L.). Aquaculture 2003, 227, 9–20. [Google Scholar] [CrossRef]
- Lazo, J.P.; Darias, M.J.; Gisbert, E. Ontogeny of the digestive tract. In Larval Fish Nutrition; Holt, G.J., Ed.; Wiley: West Sussex, UK, 2001; pp. 1–47. [Google Scholar]
- Lubzens, E.; Young, G.; Bobe, J.; Cerdà, J. Oogenesis in teleosts: How fish eggs are formed. Gen. Comp. Endocrinol. 2010, 165, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Lin, S.; Gong, J.; Qiu, C.; Zhao, C.; Xu, S. Composition and variation of amino acid and fatty acid in fertilized eggs and larvae of Sebastiscus marmoratus in different developmental stages. Oceanol. Limnol. Sin. 2016, 47, 173–181. [Google Scholar]
- Migaud, H.; Bell, G.; Cabrita, E.; McAndrew, B.; Davie, A.; Bobe, J.; Herráez, M.P.; Carillo, M. Gamete quality and broodstock management in temperate fish. In Success Factors for Fish Larval Production; Conceição, L.E.C., Tandler, A., Eds.; Wiley: Blackwell, UK, 2018; pp. 3–39. [Google Scholar]
- Mourente, G.; Vázquez, R. Changes in the content of total lipid, lipid classes and their fatty acids of developing eggs and unfed larvae of the Senegal sole, Solea senegalensis Kaup. Fish Physiol. Biochem. 1996, 15, 221–235. [Google Scholar] [CrossRef]
- Chang, Q.; Liang, M.Q.; Chen, S.Q.; Zhang, H.H.; Wang, J.L.; Zhang, X.M. Changes in amino acid and fatty acid composition during development in tongue sole (Cynoglossus semilaevis) eggs and larvae. Acta Hydrobiol. Sin. 2007, 31, 767–773. [Google Scholar]
- Fraser, A.J. Changes in lipid content, lipid class composition and fatty acid composition of developing eggs and unfed larvae of cod (Gadus morhua). Mar. Biol. 1988, 3, 99. [Google Scholar] [CrossRef]
- Han, H.; Wang, T.; Zhang, M.; Wang, F.; Liu, Y.; Sun, N.; Jiang, H. Analysis on the composition of amino acid and fatty acid and their changes during early growth stage of Sebastes schlegelii. Acta Hydrobiol. Sin. 2019, 43, 526–536. [Google Scholar]
- Cruzado, I.H.; Rodríguez, E.; Herrera, M.; Lorenzo, A.; Almansa, E. Changes in lipid classes, fatty acids, protein and amino acids during egg development and yolk-sac larvae stage in brill (Scophthalmus rhombus L.). Aquac. Res. 2013, 44, 1568–1577. [Google Scholar] [CrossRef]
- Gimenez, G.; Estévez, A.; Henderson, R.J.; Bell, J.G. Changes in lipid content, fatty acid composition and lipid class composition of eggs and developing larvae (0–40 days old) of cultured common dentex (Dentex dentex Linnaeus 1758). Aquac. Nutr. 2018, 14, 300–308. [Google Scholar] [CrossRef]
- Harlioğlu, A.G.; Gölbaşi, S. Changes in fatty acid composition, cholesterol and fat-soluble vitamins during development of eggs and larvae in shabbout (Barbus grypus, Heckel 1843). J. Appl. Ichthyol. 2013, 29, 1357–1360. [Google Scholar] [CrossRef]
- Finn, R.N.; Rønnestad, I.; Fyhn, H.J. Respiration, nitrogen and energy metabolism of developing yolk-sac larvae of Atlantic halibut (Hippoglossus hippoglossus L.). Comp. Biochem. Physiol. Part A Physiol. 1996, 111, 647–671. [Google Scholar] [CrossRef]
- Rønnestad, I.; Thorsen, A.; Finn, R.N. Fish larval nutrition: A review of recent advances in the roles of amino acids. Aquaculture 1999, 177, 201–216. [Google Scholar] [CrossRef]
- Zhu, P.; Parrish, C.C.; Brown, J.A. Lipid and amino acid metabolism during early development of Atlantic halibut (Hippoglossus hippoglossus). Aquac. Int. 2003, 11, 43–52. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, J.; Liu, Y.; Zhang, H.; Deng, P.; Lu, G.; Zhang, Z. Changes in fatty acid composition during early developmental stage of tawny puffer (Takifugu flavidus). J. Fish. China 2017, 4, 1203–1212. [Google Scholar]
- Wang, D.L.; Xu, S.L.; Yan, X.J.; Wang, Y. Fatty acid composition and their changes in larvae and juveniles of Pseudosciaena crocea. J. Fish. China 2006, 30, 241–245. [Google Scholar]
- Xu, S.L.; Wang, Y.J.; Wang, D.L.; Yan, X.J. The study of fatty acid components in early developmental stage of Oplegnathus fasciatus. Oceanol. Limnol. Sin. 2013, 44, 438–444. [Google Scholar]
- Santos, J.A.D.; Martins, S.C.; Bialetzki, A. Early ontogeny of yellowtail tetra fish Astyanax lacustris (Characiformes: Characidae). Aquac. Res. 2020, 2, 1–13. [Google Scholar] [CrossRef]
- Khemis, I.B.; Hamza, N.; Messaoud, N.B.; Rached, S.B.; M’Hetli, M. Comparative study of pikeperch sander lucioperca (Percidae; linnaeus, 1758) eggs and larvae from wild females or from captive females fed chopped marine fish. Fish Physiol. Biochem. 2014, 40, 375–384. [Google Scholar] [CrossRef]
- Parrish, C.C. Determination of total lipid, lipid classes, and fatty acids in aquatic samples. Lipids Freshw. Ecosyst. 1999, 5, 4–20. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.F.; Gao, X.Q.; Yu, J.X.; Qian, X.M.; Xue, G.P.; Zhang, Q.Y. Effects of different salinities on growth performance, survival, digestive enzyme activity, immune response, and muscle fatty acid composition in juvenile American shad (Alosa sapidissima). Fish Physiol. Biochem. 2016, 11, 1–13. [Google Scholar] [CrossRef]
- Gao, X.Q.; Fei, F.; Liu, Z.F.; Guan, C.T.; Huang, B.; Liu, B.L.; Hong, L. Lipid content and fatty acid composition in female American shad, Alosa sapidissima, at different stages of ovarian development under reared conditions. Aquac. Res. 2019, 50, 439–448. [Google Scholar] [CrossRef]
- Hastey, R.P.; Phelps, R.P.; Davis, D.A.; Cummins, K.A. Changes in free amino acid profile of red snapper Lutjanus campechanus, eggs, and developing larvae. Fish Physiol. Biochem. 2010, 36, 473–481. [Google Scholar] [CrossRef]
- Tong, X.; Yang, X.; Bao, C.; Wang, J.; Tang, X.; Jiang, D.; Yang, L. Changes of biochemical compositions during development of eggs and yolk-sac larvae of turbot Scophthalmus maximus. Aquaculture 2017, 473, 317–326. [Google Scholar] [CrossRef]
- Li, M.; Mai, K.; Ai, Q.H.; He, G.; Xu, W.; Zhang, W.B.; Zhang, Y.J.; Zhou, H.H.; Liufu, Z.G. Effect of dietary lipid on the growth, fatty acid composition and Δ5 Fads expression of abalone (Haliotis discus hannai Ino) hepatopancreas. J. Ocean Univ. China 2015, 14, 317–324. [Google Scholar] [CrossRef]
- Liu, W.; Li, Q.; Kong, L. Reproductive cycle and seasonal variations in lipid content and fatty acid composition in gonad of the cockle Fulvia mutica in relation to temperature and food. J. Ocean Univ. China 2013, 12, 427–433. [Google Scholar] [CrossRef]
- Ohkubo, N.; Sawaguchi, S.; Nomura, K.; Tanaka, H.; Matsubara, T. Utilization of free amino acids, yolk protein and lipids in developing eggs and yolk-sac larvae of Japanese eel Anguilla japonica. Aquaculture 2008, 282, 130–137. [Google Scholar] [CrossRef]
- Huang, J.S.; Amenyogbe, E.; Chen, G.; Wang, W.Z. Biochemical composition and activities of digestive and antioxidant enzymes during the egg and yolk-sac larval development of the cobia (Rachycentron canadum). Aquac. Res. 2021, 52, 1643–1656. [Google Scholar] [CrossRef]
- Cejas, J.R.; Almansa, E.; Jérez, S.; Bolaños, A.; Felipe, B.; Lorenzo, A. Changes in lipid class and fatty acid composition during development in white seabream (Diplodus sargus) eggs and larvae. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2004, 139, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, X.; Yu, J.; Wang, Y.; Guo, Z.; Huang, B.; Hong, L. Changes of protein and lipid contents, amino acid and fatty acid compositions in eggs and yolk-sac larvae of American shad (Alosa sapidissima). J. Ocean Univ. China 2018, 17, 413–419. [Google Scholar] [CrossRef]
- Ohkubo, N.; Matsubara, T. Sequential utilization of free amino acids, yolk proteins and lipids in developing eggs and yolk-sac larvae of barfin flounder Verasper moseri. Mar. Biol. 2002, 140, 187–196. [Google Scholar]
- Irani, A.; Noori, F. Comparative study on the biochemical factors and antioxidant enzymes of rainbow trout eggs and larvae in a recirculating and flow-through system. Aquaculture 2020, 523, 735–742. [Google Scholar] [CrossRef]
- Liu, C.L.; Zhao, F.Z.; Chen, S.Q.; Qin, B.; Teng, F.R.; Long, G.J. Changes of the main biochemical composition during embryonic development stage of Sepia esculenta. Period. Ocean Univ. China 2016, 46, 62–72. [Google Scholar]
- Laurel, B.J.; Copeman, L.A.; Hurst, T.P.; Parrish, C.C. The ecological significance of lipid/fatty acid synthesis in developing eggs and newly hatched larvae of Pacific cod (Gadus macrocephalus). Mar. Biol. 2010, 157, 1713–1724. [Google Scholar] [CrossRef]
- Rainuzzo, J.R.; Reitan, K.I.; Olsen, Y. The significance of lipids at early stages of marine fish: A review. Aquaculture 1997, 155, 103–115. [Google Scholar] [CrossRef]
- Mejri, S.; Adams, A.J.; Shenker, J.M.; Cianciotto, A.C.; Robinson, C.; Uribe, V.; Wills, P.S. Lipid composition and utilization in early stage leptocephalus larvae of bonefish (Albula vulpes). Lipids 2021, 56, 81–91. [Google Scholar] [CrossRef]
- Uribe, V.; Wills, P.S.; Shenker, J.M.; Adams, A.J.; Mejri, S. A comprehensive biochemical characterization of settlement stage leptocephalus larvae of bonefish (Albula vulpes). J. Fish Biol. 2021, 99, 99–109. [Google Scholar] [CrossRef]
- Hu, X.C.; Li, J.K.; Zhao, Y.L. Changes in the protein content and amino acid pool during embryonic and larval development of Odontobutis Potamophila. Chin. J. Zool. 2020, 55, 776–783. [Google Scholar]
- Liu, J.K.L. Research outline of amino acids nutrition physiology at early stages of marine fish larvae. Mar. Fish. Res. 2003, 24, 75–79. [Google Scholar]
- Huang, J.S.; Li, R.X.; Xie, R.T.; Chen, Y.M.; Zhang, J.D.; Amenyogbe, E.; Chen, G. Changes in amino acid and fatty acid composition during early development in cobia (Rachycentron canadum). Front. Mar. Sci. 2022, 9, 901–913. [Google Scholar] [CrossRef]
- Shi, Y.H.; Xu, J.B.; Yan, Y.L.; Zhang, Z.W.; Liu, Y.S.; Yu, A.Q.; Deng, P.P.; Jiang, F.; Yuan, X.C. Composition and utilization of fatty acids during the endogenous nutrient stage of Alosa sapidissima. Mar. Fish. 2022, 44, 303–314. [Google Scholar]
- Xu, H.G.; Dong, X.J.; Zuo, R.T.; Mai, K.S.; Ai, Q.H. Response of juvenile Japanese seabass (Lateolabrax japonicus) to different dietary fatty acid profiles: Growth performance, tissue lipid accumulation, liver histology and flesh texture. Aquaculture 2016, 461, 40–47. [Google Scholar] [CrossRef]
- Sorbera, L.A.; Zanuy, S.; Carrillo, M. A role for polyunsaturated fatty acids and prostaglandins in oocyte maturation in the sea bass (Dicentrarchus labrax). Ann. N. Y. Acad. Sci.-Pap. Ed. 1998, 839, 535–537. [Google Scholar] [CrossRef]
- Copeman, L.A.; Parrish, C.C.; Brown, J.A.; Harel, M. Effects of docosahexaenoic, eicosapentaenoic, and arachidonic acids on the early growth, survival, lipid composition and pigmentation of yellowtail flounder (Limanda ferruginea): A live food enrichment experiment. Aquaculture 2002, 210, 285–304. [Google Scholar] [CrossRef]
- Lauritzen, L.A.; Hansen, H.S.; Jørgensen, M.H.; Michaelsen, K.F. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res. 2001, 40, 1–94. [Google Scholar] [CrossRef]
- Jin, Y.H.; Xie, Z.G.; Lou, B.; Shi, H.L. The vary of amino acid, free amino acid and fatty acid of Nibea albiflora larvae during ontogeny. J. Zhejiang Ocean Univ. (Nat. Sci.) 2014, 34, 53–58. [Google Scholar]
- Huang, X.; Feng, L.; Wen, W.; Chen, Q.; Wei, L. The changes in lipid and fatty acid profiles of devil stinger Inimicus japonicas during the development of embryo and yolk-sac larvae. J. Fish. China 2013, 37, 526–535. [Google Scholar] [CrossRef]
- Gunasekera, R.M.; De Silva, S.S.; Ingram, B.A. The amino acid profiles in developing eggs and larvae of the freshwater Percichthyid fishes, trout cod, Maccullochella macquariensis and Murray cod, Maccullochella peelii peelii. Aquat. Living Resour. 1999, 12, 255–261. [Google Scholar] [CrossRef]
- Gunasekera, R.M.; De Silva, S.S.; Ingram, B.A. Chemical changes in fed and starved larval trout cod, Maccullochella macquarensis during early development. Fish Physiol. Biochem. 2001, 25, 255–268. [Google Scholar] [CrossRef]

| Parameter | I | II | II | IV | V |
|---|---|---|---|---|---|
| TAG | 8.38 ± 0.26 a | 6.15 ± 62 b | 5.37 ± 0.15 c | 4.23 ± 0.18 d | 3.36 ± 0.22 e |
| WE-SE | 6.70 ± 0.22 a | 6.33 ± 0.90 a | 4.14 ± 0.11 b | 3.87 ± 0.11 b | 2.17 ± 0.14 c |
| KET | 3.60 ± 0.19 | 3.36 ± 0.31 | 3.58 ± 0.14 | 3.44 ± 0.16 | 3.33 ± 0.24 |
| HC | 1.62 ± 0.26 | 1.62 ± 0.19 | 1.66 ± 0.21 | 1.63 ± 0.11 | 1.66 ± 0.12 |
| ST | 18.45 ± 1.49 | 18.15 ± 1.19 | 17.76 ± 1.96 | 17.23 ± 1.49 | 16.81 ± 1.70 |
| PL | 14.97 ± 0.75 a | 14.63 ± 0.35 a | 14.07 ± 1.25 a | 12.06 ± 0.27 b | 9.35 ± 0.16 c |
| Treatment | I | II | III | IV | V |
|---|---|---|---|---|---|
| Leu | 14.86 ± 1.04 a | 14.53 ± 0.77 a | 13.39 ± 1.02 ab | 11.76 ± 1.47 bc | 10.38 ± 0.93 c |
| Lys | 15.69 ± 0.09 | 15.20 ± 0.56 | 14.80 ± 0.50 | 14.28 ± 0.76 | 15.22 ± 0.43 |
| Val | 7.22 ± 0.57 a | 6.81 ± 0.27 a | 6.09 ± 0.06 b | 5.64 ± 0.10 b | 4.85 ± 0.09 c |
| Ile | 8.04 ± 0.08 a | 8.24 ± 0.31 a | 7.46 ± 0.09 b | 7.13 ± 0.08 b | 6.74 ± 0.06 c |
| Phe | 9.84 ± 0.09 a | 9.59 ± 0.31 ab | 9.30 ± 0.17 b | 9.14 ± 0.08 bc | 8.74 ± 0.17 c |
| Met | 0.48 ± 0.03 | 0.48 ± 0.02 | 0.45 ± 0.03 | 0.42 ± 0.03 | 0.43 ± 0.03 |
| Arg | 12.85 ± 1.09 | 12.24 ± 0.82 | 11.83 ± 0.09 | 11.38 ± 0.59 | 11.00 ± 0.62 |
| His | 6.30 ± 0.14 | 6.17 ± 0.16 | 6.08 ± 0.16 | 6.24 ± 0.07 | 6.04 ± 0.26 |
| Thr | 7.56 ± 0.11 | 7.17 ± 0.07 | 7.13 ± 0.42 | 7.18 ± 0.47 | 7.67 ± 0.15 |
| EAA | 74.95 ± 0.80 a | 72.44 ± 1.38 a | 69.40 ± 0.78 b | 66.25 ± 1.88 c | 62.65 ± 0.99 d |
| Glu | 20.95 ± 0.11 | 21.02 ± 1.31 | 21.00 ± 1.20 | 20.40 ± 0.68 | 19.67 ± 0.94 |
| Asp | 17.58 ± 0.33 | 17.19 ± 0.20 | 17.47 ± 0.15 | 17.10 ± 0.20 | 16.92 ± 0.44 |
| Gly | 9.92 ± 0.10 a | 9.73 ± 0.18 a | 9.29 ± 0.15 a | 9.03 ± 0.09 b | 8.92 ± 0.19 b |
| Ala | 11.52 ± 0.45 a | 11.14 ± 0.30 a | 10.10 ± 0.24 b | 9.16 ± 0.15 c | 8.44 ± 0.17 d |
| Ser | 8.67 ± 0.26 a | 8.51 ± 0.29 a | 7.76 ± 0.06 b | 6.95 ± 0.08 c | 6.22 ± 0.09 d |
| Cys | 1.27 ± 0.07 | 1.25 ± 0.13 | 1.10 ± 0.11 | 1.07 ± 0.12 | 1.11 ± 0.09 |
| Pro | 8.72 ± 0.10 a | 8.43 ± 0.48 a | 7.76 ± 0.90 ab | 7.22 ± 0.11 bc | 6.44 ± 0.10 c |
| Tyr | 7.26 ± 0.05 a | 7.27 ± 0.20 a | 7.08 ± 0.12 ab | 6.88 ± 0.17 b | 6.87 ± 0.08 b |
| NEAAs | 85.96 ± 0.81 a | 83.35 ± 1.63 ab | 81.42 ± 1.47 bc | 77.82 ± 0.73 c | 74.55 ± 1.52 d |
| EAAs/NEAAs | 0.87 ± 0.01 | 0.87 ± 0.03 | 0.85 ± 0.02 | 0.85 ± 0.02 | 0.84 ± 0.01 |
| TAAs | 160.92 ± 1.14 a | 155.79 ± 1.66 b | 150.82 ± 1.24 c | 144.07 ± 2.40 d | 137.20 ± 2.33 e |
| Fatty Acid Type | I | II | III | IV | V |
|---|---|---|---|---|---|
| C12:0 | 0.14 ± 0.01 | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.02 | 0.15 ± 0.01 |
| C14:0 | 0.77 ± 0.03 | 0.74 ± 0.05 | 0.77 ± 0.04 | 0.77 ± 0.05 | 0.76 ± 0.05 |
| C16:0 | 27.27 ± 0.61 a | 26.87 ± 0.60 a | 25.27 ± 0.47 b | 22.78 ± 0.64 c | 20.37 ± 0.55 d |
| C18:0 | 6.71 ± 0.16 a | 6.47 ± 0.26 a | 5.93 ± 0.09 b | 5.62 ± 0.11 c | 4.91 ± 0.15 d |
| C20:0 | 0.61 ± 0.02 | 0.60 ± 0.02 | 0.63 ± 0.01 | 0.61 ± 0.02 | 0.61 ± 0.04 |
| ∑SFA | 35.50 ± 0.04 a | 34.84 ± 0.80 a | 32.73 ± 0.53 b | 29.93 ± 0.49 c | 26.79 ± 0.48 d |
| C16:1 | 0.38 ± 0.21 | 0.33 ± 0.04 | 0.36 ± 0.03 | 0.35 ± 0.04 | 0.36 ± 0.02 |
| C16:1n-7 | 1.56 ± 0.11 | 1.64 ± 0.10 | 1.60 ± 0.13 | 1.74 ± 0.09 | 1.72 ± 0.12 |
| C18:1n-9 | 22.17 ± 0.49 a | 21.16 ± 0.35 b | 20.29 ± 0.57 c | 19.08 ± 0.22 d | 18.28 ± 0.25 e |
| C18:1n-7 | 2.56 ± 0.12 | 2.71 ± 0.16 | 2.58 ± 0.14 | 2.57 ± 0.22 | 2.62 ± 0.19 |
| C20:1n-9 | 1.80 ± 0.06 | 1.77 ± 0.10 | 1.74 ± 0.09 | 1.78 ± 0.10 | 1.73 ± 0.06 |
| C20:1n-7 | 0.42 ± 0.07 | 0.46 ± 0.06 | 0.44 ± 0.05 | 0.47 ± 0.03 | 0.45 ± 0.03 |
| C22:1n-9 | 2.08 ± 0.04 | 2.12 ± 0.22 | 2.11 ± 0.14 | 2.17 ± 0.25 | 2.19 ± 0.12 |
| ∑MUFA | 30.98 ± 0.51 a | 30.19 ± 0.09 a | 29.11 ± 0.57 b | 28.16 ± 0.51 c | 27.36 ± 0.43 c |
| C18:2n-6 | 2.95 ± 0.10 | 2.78 ± 0.16 | 2.92 ± 0.15 | 2.97 ± 0.12 | 2.90 ± 0.24 |
| C18:3n-6 | 0.31 ± 0.02 | 0.33 ± 0.06 | 0.35 ± 0.04 | 0.35 ± 0.03 | 0.34 ± 0.04 |
| C18:3n-3 | 2.25 ± 0.10 | 2.46 ± 0.08 | 2.95 ± 0.12 | 3.32 ± 0.10 | 3.65 ± 0.17 |
| C18:4n-3 | 1.03 ± 0.09 | 1.05 ± 0.11 | 0.97 ± 0.18 | 1.02 ± 0.13 | 1.04 ± 0.11 |
| C20:3n-6 | 0.79 ± 0.08 | 0.83 ± 0.10 | 0.83 ± 0.13 | 0.85 ± 0.12 | 0.87 ± 0.06 |
| C20:3n-3 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 |
| C20:4n-6 | 2.32 ± 0.03 a | 2.22 ± 0.18 a | 2.19 ± 0.22 a | 1.89 ± 0.08 b | 1.51 ± 0.04 b |
| C20:5n-3 | 4.51 ± 0.16 | 4.28 ± 0.27 | 4.48 ± 0.33 | 4.50 ± 0.13 | 4.51 ± 0.24 |
| C22:5n-3 | 1.63 ± 0.14 | 1.90 ± 0.15 | 1.93 ± 0.15 | 1.76 ± 0.12 | 1.89 ± 0.16 |
| C22:6n-3 | 27.28 ± 0.29 a | 27.04 ± 0.07 a | 27.11 ± 0.51 a | 26.47 ± 0.15 b | 22.54 ± 0.01 b |
| ∑PUFA | 43.16 ± 0.23 a | 42.97 ± 0.12 a | 43.82 ± 0.95 a | 43.21 ± 0.26 a | 39.34 ± 0.34 b |
| n-3 | 36.79 ± 0.24 a | 36.81 ± 0.40 a | 37.52 ± 0.82 a | 37.15 ± 0.30 a | 33.71 ± 0.23 b |
| n-6 | 6.37 ± 0.16 a | 6.16 ± 0.38 a | 6.30 ± 0.15 a | 6.06 ± 0.08 a | 5.63 ± 0.29 b |
| n-3/n-6 | 5.78 ± 0.16 | 5.99 ± 0.45 | 5.96 ± 0.07 | 6.13 ± 0.11 | 6.00 ± 0.32 |
| DHA/EPA | 6.05 ± 0.21 a | 6.34 ± 0.40 a | 6.07 ± 0.36 a | 5.88 ± 0.15 a | 5.00 ± 0.27 b |
| EPA/ARA | 1.95 ± 0.05 a | 1.94 ± 0.27 a | 2.06 ± 0.34 ab | 2.39 ± 0.17 b | 2.98 ± 0.12 c |
| DHA+EPA | 31.79 ± 0.35 a | 31.32 ± 0.31 a | 31.59 ± 0.78 a | 30.97 ± 0.23 a | 27.05 ± 0.23 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Cao, S.; Chen, R.; Fei, F.; Li, W.; Zhang, X.; Zhu, Z.; Liu, B. A Comprehensive Biochemical Characterization of Hybrid Grouper Larvae (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂) during Yolk-Sac Larval Development. Animals 2023, 13, 3801. https://doi.org/10.3390/ani13243801
Gao X, Cao S, Chen R, Fei F, Li W, Zhang X, Zhu Z, Liu B. A Comprehensive Biochemical Characterization of Hybrid Grouper Larvae (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂) during Yolk-Sac Larval Development. Animals. 2023; 13(24):3801. https://doi.org/10.3390/ani13243801
Chicago/Turabian StyleGao, Xiaoqiang, Shuquan Cao, Rongjie Chen, Fan Fei, Wenyang Li, Xianhong Zhang, Zhiwen Zhu, and Baoliang Liu. 2023. "A Comprehensive Biochemical Characterization of Hybrid Grouper Larvae (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂) during Yolk-Sac Larval Development" Animals 13, no. 24: 3801. https://doi.org/10.3390/ani13243801
APA StyleGao, X., Cao, S., Chen, R., Fei, F., Li, W., Zhang, X., Zhu, Z., & Liu, B. (2023). A Comprehensive Biochemical Characterization of Hybrid Grouper Larvae (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂) during Yolk-Sac Larval Development. Animals, 13(24), 3801. https://doi.org/10.3390/ani13243801




