Combination of CT-Guided Microwave Ablation and Cementoplasty as a Minimally Invasive Limb-Sparing Approach in a Dog with Appendicular Osteosarcoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Owners’ Informed Consent
2.2. Case Description
2.3. Microwave Ablation
2.4. Cementoplasty
2.5. Adjuvant Treatments and Analgesia
2.6. Evaluation of the Treatment Response and Quality of Life
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehrhart, N.P.; Christensen, N.I.; Fan, T.M. Tumors of the skeletal system. In Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 524–564. [Google Scholar]
- Simpson, S.; Rizvanov, A.A.; Jeyapalan, J.N.; de Brot, S.; Rutland, C.S. Canine osteosarcoma in comparative oncology: Molecular mechanisms through to treatment discovery. Front. Vet. Sci. 2022, 9, 965391. [Google Scholar] [CrossRef]
- Martin, T.W.; Griffin, L.; Custis, J.; Ryan, S.D.; Lafferty, M.; Boss, M.K.; Regan, D.; Rao, S.; Leary, D.; Withrow, S.J.; et al. Outcome and prognosis for canine appendicular osteosarcoma treated with stereotactic body radiation therapy in 123 dogs. Vet. Comp. Oncol. 2021, 19, 284–294. [Google Scholar] [CrossRef]
- Hans, E.C.; Pinard, C.; van Nimwegen, S.A.; Kirpensteijn, J.; Singh, A.; MacEachern, S.; Naber, S.; Dudley, R.M. Effect of surgical site infection on survival after limb amputation in the curative-intent treatment of canine appendicular osteosarcoma: A Veterinary Society of Surgical Oncology retrospective study. Vet. Surg. 2018, 47, E88–E96, Erratum in: Vet Surg. 2019, 48, 112. [Google Scholar] [CrossRef] [PubMed]
- Spodnick, G.J.; Berg, J.; Rand, W.M.; Schelling, S.H.; Couto, G.; Harvey, H.J.; Henderson, R.A.; MacEwen, G.; Mauldin, N.; McCaw, D.L.; et al. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978–1988). J. Am. Vet. Med. Assoc. 1992, 200, 995–999. [Google Scholar]
- Yu, Z.; Geng, J.; Zhang, M.; Zhou, Y.; Fan, Q.; Chen, J. Treatment of osteosarcoma with microwave thermal ablation to induce immunogenic cell death. Oncotarget 2014, 5, 6526–6539. [Google Scholar] [CrossRef] [PubMed]
- Wustefeld-Janssens, B.G.; Séguin, B.; Ehrhart, N.P.; Worley, D.R. Analysis of outcome in dogs that undergo secondary amputation as an end-point for managing complications related to limb salvage surgery for treatment of appendicular osteosarcoma. Vet. Comp. Oncol. 2020, 18, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, F.; Farouil, G.; de Baere, T. Ablation percutanée des lésions tumorales de l’os. J. Radiol. Diagn. Interv. 2014, 4333, 637–752. [Google Scholar] [CrossRef]
- Zheng, K.; Yu, X.; Hu, Y.; Zhang, Y.; Wang, Z.; Wu, S.; Shen, J.; Ye, Z.; Tu, C.; Zhang, Y.; et al. Clinical Guideline for Microwave Ablation of Bone Tumors in Extremities. Orthop. Surg. 2020, 12, 1036–1044. [Google Scholar] [CrossRef]
- Han, K.; Dang, P.; Bian, N.; Chen, X.; Yang, T.; Fan, Q.; Zhou, Y.; Zhao, T.; Wang, P. Is Limb Salvage With Microwave-induced Hyperthermia Better Than Amputation for Osteosarcoma of the Distal Tibia? Clin. Orthop. Relat. Res. 2017, 475, 1668–1677. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z.; Wang, Z.; Fan, H.; Fu, J. Does Microwave Ablation of the Tumor Edge Allow for Joint-sparing Surgery in Patients With Osteosarcoma of the Proximal Tibia? Clin. Orthop. Relat. Res. 2015, 473, 3204–3211. [Google Scholar] [CrossRef]
- Sgalambro, F.; Zugaro, L.; Bruno, F.; Palumbo, P.; Salducca, N.; Zoccali, C.; Barile, A.; Masciocchi, C.; Arrigoni, F. Interventional Radiology in the Management of Metastases and Bone Tumors. J. Clin. Med. 2022, 11, 3265. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Yu, X.C.; Xu, M.; Wang, J.M. Conservative surgery with microwave ablation for recurrent bone tumor in the extremities: A single-center study. BMC Cancer 2022, 22, 1122. [Google Scholar] [CrossRef] [PubMed]
- Frayssinet, P.; Mathon, D.; Simonet, S.; Trouillet, J.L.; Mathon, V.; Rouquet, N. Treatment of canine osteosarcoma using autologous active immunotherapy with or without surgery. In A Pesquisa Nos Diferentes Campos da Medicina Veterinária 3; Pereira, A.M., Reis, S.S., Pereira, W.M.R., Grossa, P., Eds.; Atena: Ponta Grossa, Brazil, 2020. [Google Scholar]
- Testoni, I.; De Vincenzo, C.; Campigli, M.; Caregnato Manzatti, A.; Ronconi, L.; Uccheddu, S. Validation of the HHHHHMM Scale in the Italian Context: Assessing Pets’ Quality of Life and Qualitatively Exploring Owners’ Grief. Animals 2023, 13, 1049. [Google Scholar] [CrossRef]
- Nguyen, S.M.; Thamm, D.H.; Vail, D.M.; London, C.A. Response evaluation criteria for solid tumors in dogs (v1.0): A Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet. Comp. Oncol. 2015, 13, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Kalamaras, A.B.; Wavreille, V.; Jones, S.C.; Litsky, A.S.; Selmic, L. Impact of microwave ablation treatment on the biomechanical properties of the distal radius in the dog: A cadaveric study. Vet. Surg. 2020, 49, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Salyer, S.A.; Wavreille, V.A.; Fenger, J.M.; Jennings, R.N.; Selmic, L.E. Evaluation of microwave ablation for local treatment of dogs with distal radial osteosarcoma: A pilot study. Vet. Surg. 2020, 49, 1396–1405. [Google Scholar] [CrossRef]
- Dornbusch, J.A.; Wavreille, V.A.; Dent, B.; Fuerst, J.A.; Green, E.M.; Selmic, L.E. Percutaneous microwave ablation of solitary presumptive pulmonary metastases in two dogs with appendicular osteosarcoma. Vet. Surg. 2020, 49, 1174–1182. [Google Scholar] [CrossRef]
- Mazzaccari, K.; Boston, S.E.; Toskich, B.B.; Bowles, K.; Case, J.B. Video-assisted microwave ablation for the treatment of a metastatic lung lesion in a dog with appendicular osteosarcoma and hypertrophic osteopathy. Vet. Surg. 2017, 46, 1161–1165. [Google Scholar] [CrossRef]
- Böttcher, P.; Krastel, D.; Hierholzer, J.; Westphalen, K.; Florian, S.; Hildebrandt, G.; Vera, G.; Oechtering, G. Percutaneous cementoplasty in the palliative, multimodal treatment of primary bone tumors of the distal aspect of the radius in four dogs. Vet. Surg. 2009, 38, 888–901. [Google Scholar] [CrossRef]
- Villamonte Chevalier, A.; Molle, C.; Carabalona, J.; Klajer, A.; Letesson, J.; Ragetly, G.; Vedrine, B.; Blondiau, J.; Gauthier, O. Percutaneous cementoplasty as a palliative treatment for dogs with osteosarcoma using a new self-setting bone substitute. In ESVONC Annual Congress Proceedings, Siracusa, Italy, 26–28 May 2022; ESVONC: Maastricht, The Netherlands, 2022. [Google Scholar]
- Turner, T.M.; Urban, R.M.; Singh, K.; Hall, D.J.; Renner, S.M.; Lim, T.H.; Tomlinson, M.J.; An, H.S. Vertebroplasty comparing injectable calcium phosphate cement compared with polymethylmethacrylate in a unique canine vertebral body large defect model. Spine J. 2008, 8, 482–487. [Google Scholar] [CrossRef]
- Kinne, R.W.; Gunnella, F.; Kunisch, E.; Heinemann, S.; Nies, B.; Maenz, S.; Horbert, V.; Illerhaus, B.; Huber, R.; Firkowska-Boden, I.; et al. Performance of Calcium Phosphate Cements in the Augmentation of Sheep Vertebrae—An Ex Vivo Study. Materials 2021, 14, 3873. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.-M. A review of calcium phosphate cements and acrylic bone cements as injectable materials for bone repair and implant fixation. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019872594. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, M.A.; Kerrigan, S.M. Quality of Life Measurement in Prospective Studies of Cancer Treatments in Dogs and Cats. J. Vet. Intern. Med. 2014, 28, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
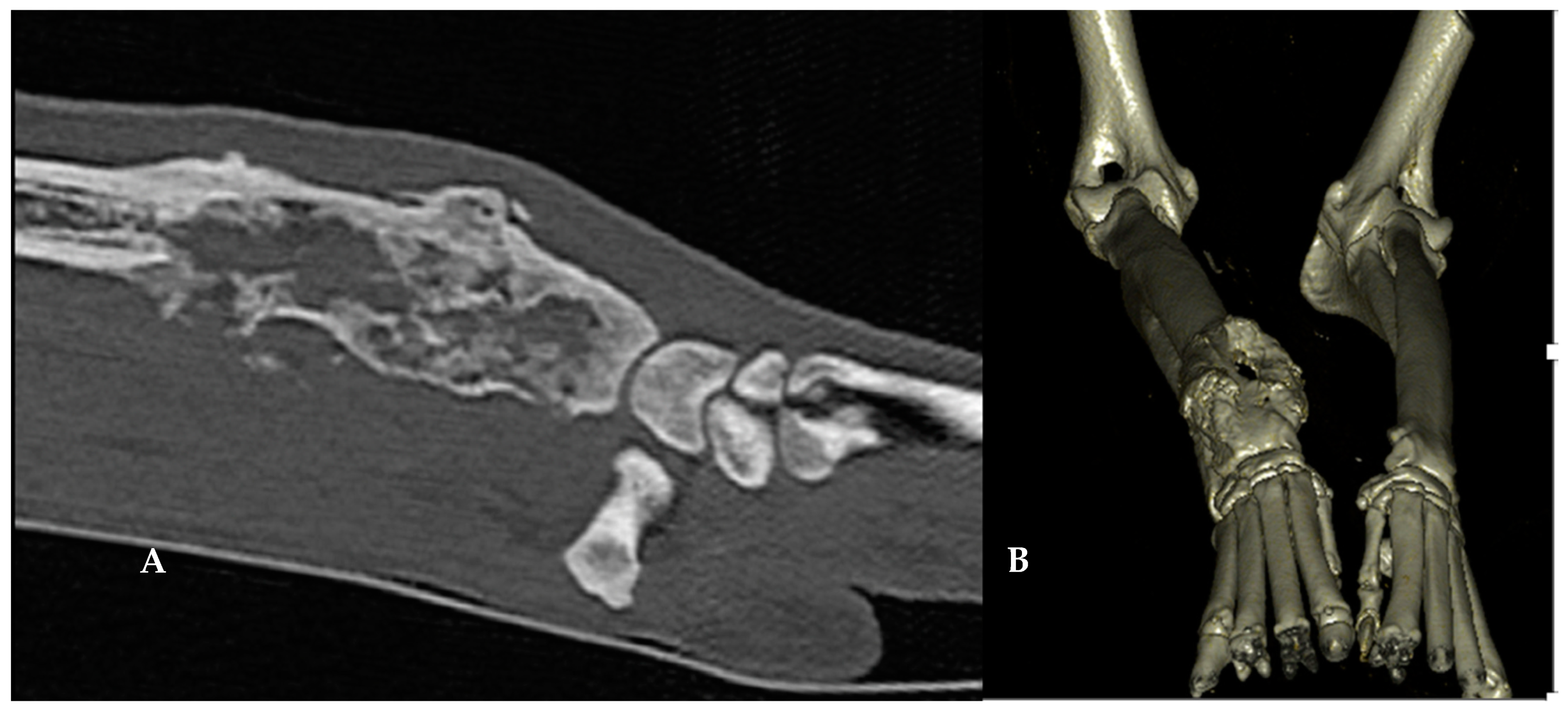


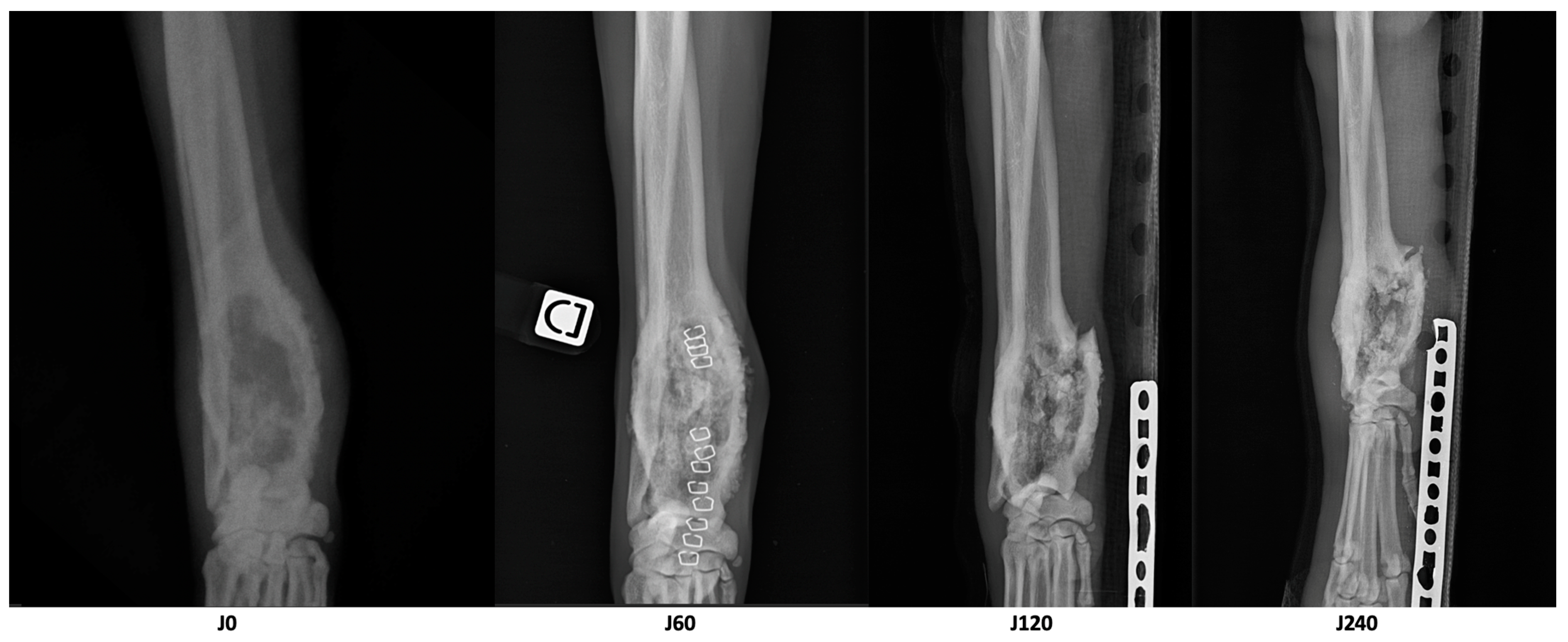
| Parameter | D0 | D60 | D210 | Variation D60/D0 | Variation D210/D0 |
|---|---|---|---|---|---|
| Longest diameter of the osteolytic area (cm) | 8.1 | 6.7 | 6.1 | −17% | −25% |
| Volume of dense tissue in the osteolytic area (cm3) | 18.8 | 21.1 | 17.5 | +12% | −7% |
| Volume of the cortical bone of the area of interest (cm3) | 8.2 | 10.6 | 7.7 | +29% | −6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayag, D.; Jacques, D.; Thierry, F.; Castell, Y.; Aumann, M.; Gauthier, O.; Wavreille, V.; Tselikas, L. Combination of CT-Guided Microwave Ablation and Cementoplasty as a Minimally Invasive Limb-Sparing Approach in a Dog with Appendicular Osteosarcoma. Animals 2023, 13, 3804. https://doi.org/10.3390/ani13243804
Sayag D, Jacques D, Thierry F, Castell Y, Aumann M, Gauthier O, Wavreille V, Tselikas L. Combination of CT-Guided Microwave Ablation and Cementoplasty as a Minimally Invasive Limb-Sparing Approach in a Dog with Appendicular Osteosarcoma. Animals. 2023; 13(24):3804. https://doi.org/10.3390/ani13243804
Chicago/Turabian StyleSayag, David, David Jacques, Florence Thierry, Yoann Castell, Marcel Aumann, Olivier Gauthier, Vincent Wavreille, and Lambros Tselikas. 2023. "Combination of CT-Guided Microwave Ablation and Cementoplasty as a Minimally Invasive Limb-Sparing Approach in a Dog with Appendicular Osteosarcoma" Animals 13, no. 24: 3804. https://doi.org/10.3390/ani13243804





