Different Starch Sources Affect the Growth Performance and Hepatic Health Status of Largemouth Bass (Micropterus salmoides) in a High-Temperature Environment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Experimental Design and Management
2.3. Sample Collection
2.4. Analysis of Nutrient Composition, Plasma Biochemical Indices, and Hepatic Antioxidant Indices
2.5. Histological Analysis
2.6. RNA Extraction and qRT-PCR Assay
2.7. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Whole-Body Composition
3.3. Plasma Biochemical Indices
3.4. Hepatic Antioxidant Indices
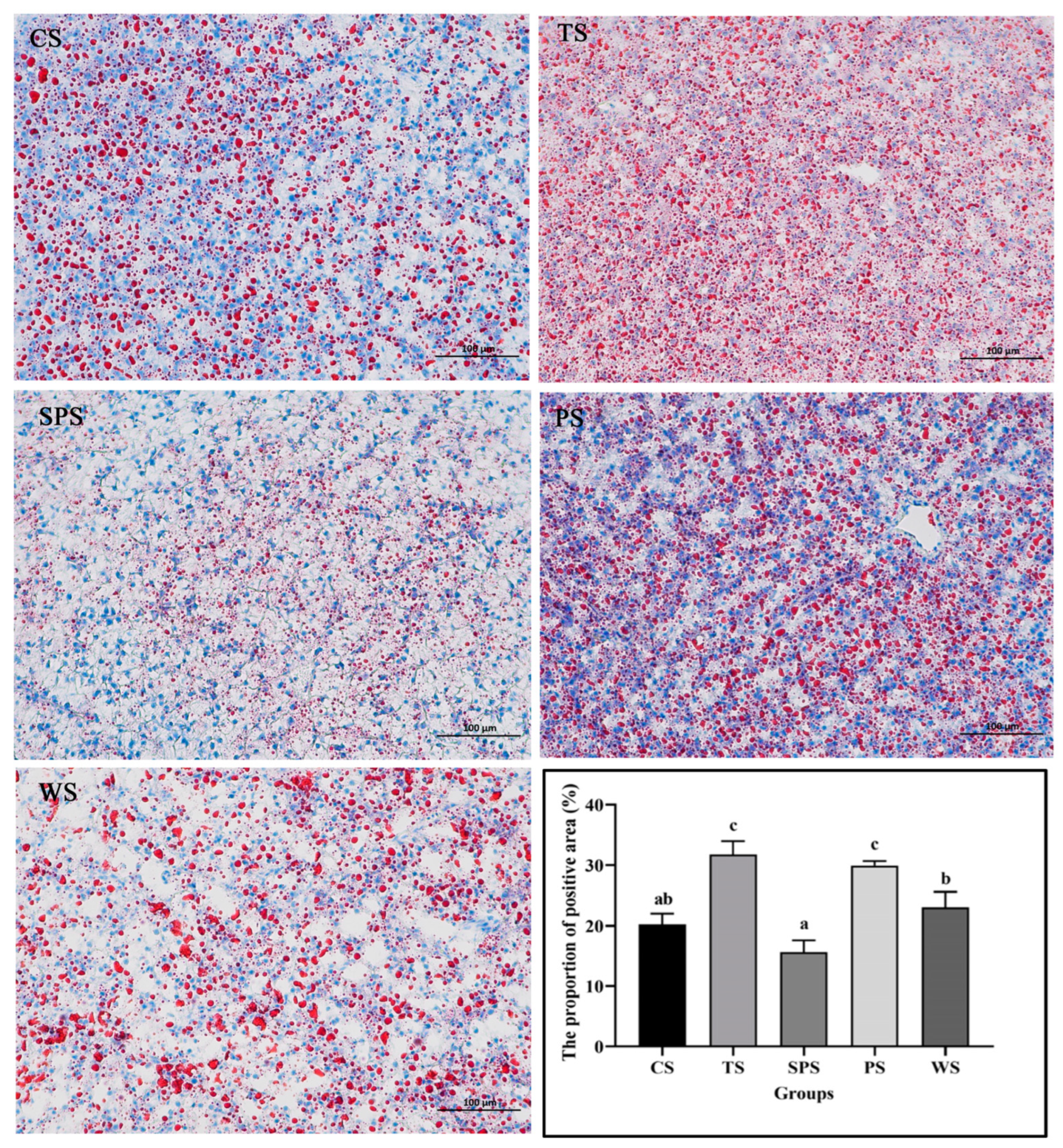
3.5. Liver Pathology Analysis
3.6. qRT-PCR Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Intergovernmental Panel on Climate Change. In Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. Available online: https://www.ipcc.ch/report/ar5/syr/ (accessed on 12 December 2014).
- Shi, C.; Jiang, Z.; Zhu, L.; Zhang, X.; Yao, Y.; Laurent, L. Risks of temperature extremes over China under 1.5 °C and 2 °C global warming. J. Adv. Clim. Chang. Res. 2020, 11, 172–184. [Google Scholar] [CrossRef]
- Tuttle, J.T.; Smith, M.A.; Roy, L.A.; Jones, M.; Lochmann, R.; Kelly, A.M. Effects of different feeding regimes on growth rates and fatty acid composition of largemouth bass Micropterus nigricans at high water temperatures. Animals 2022, 12, 2797. [Google Scholar] [CrossRef]
- Fantini, L.E.; Smith, M.A.; Jones, M.; Roy, L.A.; Lochmann, R.; Kelly, A.M. Growth parameters in northern largemouth bass Micropterus salmoides raised near their upper thermal tolerance for 28 days. Aquac. Rep. 2021, 21, 100845. [Google Scholar] [CrossRef]
- Pereira, L.A.L.; Amanajás, R.D.; de Oliveira, A.M.; da Silva, M.D.N.P.; Val, A.L. Health of the Amazonian fish tambaqui (Colossoma macropomum): Effects of prolonged photoperiod and high temperature. Aquaculture 2021, 541, 736836. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Berdal, B.; Blust, R.; Brix, O.; Colosimo, A.; De Wachter, B.; Giuliani, A.; Johansen, T.; Fischer, T.; Knust, R.; et al. Climate induced temperature effects on growth performance, fecundity and recruitment in marine fish: Developing a hypothesis for cause and effect relationships in Atlantic cod (Gadus morhua) and common eelpout (Zoarces viviparus). Cont. Shelf Res. 2001, 21, 1975–1997. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Liu, Z.; Kang, Y.; Wang, J. Transcriptomic responses to heat stress in rainbow trout Oncorhynchus mykiss head kidney. Fish Shellfish Immunol. 2018, 82, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, X.; Li, J.; Shen, Y. Transcriptomic analysis of the liver and brain in grass carp (Ctenopharyngodon idella) under heat stress. Mar. Biotechnol. 2022, 24, 856–870. [Google Scholar] [CrossRef]
- Scaion, D.; Belhomme, M.; Sébert, P. Pressure and temperature interactions on aerobic metabolism of migrating European silver eel. Respir. Physiol. Neurobiol. 2008, 164, 319–322. [Google Scholar] [CrossRef]
- August, S.M.; Hicks, B.J. Water temperature and upstream migration of glass eels in New Zealand: Implications of climate change. Environ. Biol. Fishes 2008, 81, 195–205. [Google Scholar] [CrossRef]
- Stone, D.A. Dietary carbohydrate utilization by fish. Rev. Fish. Sci. 2003, 11, 337–369. [Google Scholar] [CrossRef]
- Wilson, R.P. Utilization of dietary carbohydrate by fish. Aquaculture 1994, 124, 67–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, X.F.; He, S.; Wang, J.; Li, L.; Zhang, Z.; Li, J.; Chen, X.; Li, L.; Alam, M.S. Metabolic responses of Chinese perch (Siniperca chuatsi) to different levels of dietary carbohydrate. Fish Physiol. Biochem. 2021, 47, 1449–1465. [Google Scholar] [CrossRef]
- Xu, T.; Liu, X.; Huang, W.; Li, G.; Zhang, Y.; Xu, D.; Wang, G. Effects of dietary carbohydrate levels on growth, metabolic enzyme activities and oxidative status of hybrid snakehead (Channa maculata ♀ × Channa argus ♂). Aquaculture 2023, 563, 738960. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Fish and Shrimp; National Academy Press: Washington, DC, USA, 2011. [Google Scholar]
- Hemre, G.I.; Mommsen, T.P.; Krogdahl, A. Carbohydrates in fish nutrition: Effects on growth, glucose metabolism and hepatic enzymes. Aquac. Nutr. 2002, 8, 175–194. [Google Scholar] [CrossRef]
- Kamalam, B.S.; Medale, F.; Panserat, S. Utilisation of dietary carbohydrates in farmed fishes: New insights on influencing factors, biological limitations and future strategies. Aquaculture 2017, 467, 3–27. [Google Scholar] [CrossRef]
- Limbu, S.M.; Zhang, H.; Luo, Y.; Chen, L.Q.; Zhang, M.; Du, Z.Y. High carbohydrate diet partially protects Nile tilapia (Oreochromis niloticus) from oxytetracycline-induced side effects. Environ. Pollut. 2020, 256, 113508. [Google Scholar] [CrossRef] [PubMed]
- Svihus, B.; Uhlen, A.K.; Harstad, O.M. Effect of starch granule structure, associated components and processing on nutritive value of cereal starch: A review. Anim. Feed Sci. Technol. 2005, 122, 303–320. [Google Scholar] [CrossRef]
- Pfeffer, E.; Beckmann-Toussaint, J.; Henrichfreise, B.; Jansen, H.D. Effect of extrusion on efficiency of utilization of maize starch by rainbow trout (Oncorhynchus mykiss). Aquaculture 1991, 96, 293–303. [Google Scholar] [CrossRef]
- Enes, P.; Panserat, S.; Kaushik, S.; Oliva-Teles, A.A. Effect of normal and waxy maize starch on growth, food utilization and hepatic glucose metabolism in European sea bass (Dicentrarchus labrax) juveniles. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 143, 89–96. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Jia, S.; Song, F.; Zhou, C.; Wu, G. Oxidation of energy substrates in tissues of largemouth bass (Micropterus salmoides). Amino Acids 2020, 52, 1017–1032. [Google Scholar] [CrossRef]
- Coutant, C.C. Compilation of temperature preference data. J. Fish. Res. Board Can. 1977, 34, 739–745. [Google Scholar] [CrossRef]
- Díaz, F.; Re, A.D.; Gonz’alez, R.A.; Sanchez, L.N.; Leyva, G.; Valenzuela, F. Temperature preference and oxygen consumption of the largemouth bass Micropterus salmoides (Lacépede) acclimated to different temperatures. Aquac. Res. 2007, 38, 1387–1394. [Google Scholar] [CrossRef]
- Vanlandeghem, M.M.; Wahl, D.H.; Suski, C.D. Physiological responses of largemouth bass to acute temperature and oxygen stressors. Fish. Manag. Ecol. 2010, 17, 414–425. [Google Scholar] [CrossRef]
- Landsman, S.J.; Gingerich, A.J.; Philipp, D.P.; Suski, C.D. The effects of temperature change on the hatching success and larval survival of largemouth bass Micropterus salmoides and smallmouth bass Micropterus dolomieu. J. Fish Biol. 2011, 78, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhong, D.; Li, S.; Zhang, Z.; Mo, H.; Wang, L. Acute temperature stresses trigger liver transcriptome and microbial community remodeling in largemouth bass (Micropterus salmoides). Aquaculture 2023, 573, 739573. [Google Scholar] [CrossRef]
- Zhao, L.L.; Liao, L.; Tang, X.H.; Liang, J.; Liu, Q.; Luo, W.; Adam, A.A.; Luo, J.; Li, Z.Q.; Yang, S.; et al. High-carbohydrate diet altered conversion of metabolites, and deteriorated health in juvenile largemouth bass. Aquaculture 2022, 549, 737816. [Google Scholar] [CrossRef]
- Zhao, L.L.; Liang, J.; Chen, F.K.; Tang, X.H.; Liao, L.; Liu, Q.; Luo, J.; Du, Z.J.; Li, Z.Q.; Luo, W.; et al. High carbohydrate diet induced endoplasmic reticulum stress and oxidative stress, promoted inflammation and apoptosis, impaired intestinal barrier of juvenile largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2021, 119, 308–317. [Google Scholar] [CrossRef]
- Ren, M.; Habte-Tsion, H.M.; Xie, J.; Liu, B.; Zhou, Q.; Ge, X.; Pan, L.; Chen, R. Effects of dietary carbohydrate source on growth performance, diet digestibility and liver glucose enzyme activity in blunt snout bream, Megalobrama amblycephala. Aquaculture 2015, 438, 75–81. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists Inc.: Arlington, TX, USA, 2003. [Google Scholar]
- Liang, H.; Xu, G.; Xu, P.; Zhu, J.; Li, S.; Ren, M. Dietary Histidine Supplementation Maintained Amino Acid Homeostasis and Reduced Hepatic Lipid Accumulation of Juvenile Largemouth Bass, Micropterus salmoides. Aquac. Nutr. 2022, 2022, 4034922. [Google Scholar] [CrossRef]
- Gu, J.Z.; Liang, H.L.; Ge, X.P.; Xia, D.; Pan, L.K.; Mi, H.F.; Ren, M.C. A study of the potential effect of yellow mealworm (Tenebrio molitor) substitution for fish meal on growth, immune and antioxidant capacity in juvenile largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2022, 120, 214–221. [Google Scholar] [CrossRef]
- Yang, P.; Wang, W.; Chi, S.; Mai, K.; Song, F.; Wang, L. Effects of dietary lysine on regulating GH-IGF system, intermediate metabolism and immune response in largemouth bass (Micropterus salmoides). Aquacult. Rep. 2020, 17, 100323. [Google Scholar] [CrossRef]
- Hofmann, N.; Fischer, P. Impact of temperature on food intake and growth in juvenile burbot. J. Fish Biol. 2003, 63, 1295–1305. [Google Scholar] [CrossRef]
- Weerd, J.D.; Komen, J. The effects of chronic stress on growth in fish: A critical appraisal. Comp. Biochem. Physiol. Part A 1999, 54, 556–572. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, C.; Guo, C.; Xie, S.; Luo, J.; Zhu, T.; Ye, Y.; Jin, M.; Zhou, Q. Effect of dietary carbohydrate sources on the growth, glucose metabolism and insulin pathway for swimming crab, Portunus trituberculatus. Aquac. Rep. 2021, 21, 100967. [Google Scholar] [CrossRef]
- Kumar, S.; Sahu, N.P.; Pal, A.K.; Kerepeczki, E.; Sinha, A.K.; Gal, D. Metabolic fitness and growth performance in tropical freshwater fish Labeo rohita are modulated in response to dietary starch type (gelatinized versus non-gelatinized) and water temperature. Aquac. Nutr. 2016, 22, 966–975. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, H.; Zhai, S.W.; Newton, R.J.; Shepherd, B.; Tian, J.; Lofald, A.G.; Teh, S.; Binkowski, F.P.; Deng, D.F. Nutritional quality of different starches in feed fed to juvenile yellow perch, Perca flavescens. Aquac. Nutr. 2020, 26, 671–682. [Google Scholar] [CrossRef]
- Frías-Quintana, C.; Álvarez-González, C.; Tovar-Ramírez, D.; Martínez-García, R.; Susana, C.C.; Emyr, P. Use of potato starch in diets of tropical gar (Atractosteus tropicus, gill 1863) larvae. Fishes 2017, 2, 3. [Google Scholar] [CrossRef]
- Frías-Quintana, C.A.; Domínguez-Lorenzo, J.; Álvarez-González, C.A.; Tovar-Ramírez, D.; Martínez-García, R. Using cornstarch in microparticulate diets for larvicultured tropical gar (Atractosteus tropicus). Fish Physiol. Biochem. 2016, 42, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.Y.; Huang, J.F. Effect of environmental temperature and protein level on growth and metabolism of fish. J. Northeast Agric. Univ. 2011, 42, 1–8. [Google Scholar] [CrossRef]
- Rawles, S.; Lochmann, R. Effects of amylopectin/amylose starch ratio on growth, body composition and glycemic response of sunshine bass Morone chrysops × M. saxatilis. J. World Aquac. Soc. 2003, 34, 278–288. [Google Scholar] [CrossRef]
- Liu, X.H.; Ye, C.X.; Ye, J.D.; Shen, B.D.; Wang, C.Y.; Wang, A.L. Effects of dietary amylose/amylopectin ratio on growth performance, feed utilization, digestive enzymes, and postprandial metabolic responses in juvenile obscure puffer Takifugu obscurus. Fish Physiol. Biochem. 2014, 40, 1423–1436. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Q.; Shi, C.M.; Lin, S.M.; Chen, Y.J.; Shen, H.M.; Luo, L. Effect of starch sources on growth, hepatic glucose metabolism and antioxidant capacity in juvenile largemouth bass, Micropterus salmoides. Aquaculture 2018, 490, 355–361. [Google Scholar] [CrossRef]
- Dang, J.Y.; Cai, Y.W.; Zhang, C.Y.; Cao, K.L.; Li, X.Q.; Leng, X.J. Different effects of various dietary starches on the growth and metabolism of rainbow trout juveniles. Acta Hydrobiol. Sin. 2022, 46, 8. [Google Scholar] [CrossRef]
- Hilton, J.W.; Atkinson, J.L. Response of rainbow trout (Salmo gairdneri) to increased levels of available carbohydrate in practical trout diets. Br. J. Nutr. 1982, 47, 597–607. [Google Scholar] [CrossRef]
- Kumar, S.; Sahu, N.P.; Pal, A.K.; Choudhury, D.; Yengkokpam, S.; Mukherjee, S.C. Effect of dietary carbohydrate on haematology, respiratory burst activity and histological changes in L. rohita juveniles. Fish Shellfish Immunol. 2005, 19, 331–344. [Google Scholar] [CrossRef]
- Du, Z.Y. Causes of fatty liver in farmed fish: A review and new perspectives. J. Fish. China 2014, 38, 1628–1638. [Google Scholar]
- Sheikh, Z.A.; Ahmed, I. Impact of environmental changes on plasma biochemistry and hematological parameters of Himalayan snow trout, Schizothorax plagiostomus. Comp. Clin. Pathol. 2019, 28, 793–804. [Google Scholar] [CrossRef]
- Cheng, C.H.; Guo, Z.X.; Luo, S.W.; Wang, A.L. Effects of high temperature on biochemical parameters, oxidative stress, DNA damage and apoptosis of pufferfish (Takifugu obscurus). Ecotoxicol. Environ. Saf. 2018, 150, 190–198. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; Von Knethen, A. Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef]
- Timme-Laragy, A.R.; Karchner, S.I.; Franks, D.G.; Jenny, M.J.; Harbeitner, R.C.; Goldstone, J.V.; McArthur, A.G.; Hahn, M.E. Nrf2b, novel zebrafish paralog of oxidant-responsive transcription factor NF-E2-related factor 2 (NRF2). J. Biol. Chem. 2012, 287, 4609–4627. [Google Scholar] [CrossRef]
- Wang, H.H.; Shen, Z.J.; Xiao, H.; Liu, X.Q.; Ai, Y.; Zhang, Y.S. Effects of heat stress on Keap1-Nrf2-ARE signal pathway of liver in dairy cows. J. Nanjing Agric. Univ. 2017, 40, 151–156. [Google Scholar] [CrossRef]
- Kong, Y.Q.; Ding, Z.L.; Zhang, Y.X.; Zhou, P.X.; Wu, C.B.; Zhu, M.H.; Ye, J.Y. Types of carbohydrate in feed affect the growth performance, antioxidant capacity, immunity, and activity of digestive and carbohydrate metabolism enzymes in juvenile Macrobrachium nipponense. Aquaculture 2019, 512, 734282. [Google Scholar] [CrossRef]
- Liu, X.P.; Dong, W.J.; Huang, H.; Liao, R.S.; Chen, Y.J.; Tan, B.P.; Lin, S.M. Effects of dietary high amylose on intestinal health of Micropterus salmoides. J. Fish. China 2023, 47, 109601. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Shu-Man, L.; Ling, L.W.; Mahyar, K.; Sohrab, N.; Farzaneh, S.H.; Kieffer, D.A.; Adams, S.H.; Martin, R.J.; Sands, J.M. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS ONE 2014, 9, e114881. [Google Scholar] [CrossRef]
- Downs, C.A.; Fauth, J.E.; Woodley, C.M. Assessing the health of grass shrimp (Palaeomonetes pugio) exposed to natural and anthropogenic stressors: A molecular biomarker system. Mar. Biotechnol. 2001, 3, 380–397. [Google Scholar] [CrossRef] [PubMed]
- Uner, N.; Tamer, L. Comparison of Na (+) K (+)-ATPase activities and malondialdehyde contents in liver tissue for three fish species exposed to azinphosmethyl. Bull. Environ. Contam. Toxicol. 2002, 69, 271–277. [Google Scholar] [CrossRef]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.S.; Yu, S.; Kong, A.N. Activation of Nrf2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Demartino, P.; Cockburn, D.W. Resistant starch: Impact on the gut microbiome and health. Curr. Opin. Biotechnol. 2020, 61, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Tortosa, M.C.; Ramirez-Tortosa, C.L.; Mesa, M.D.; Granados, S.; Gil, A.; Quiles, J.L. Curcumin ameliorates rabbits’s steatohepatitis via respiratory chain, oxidative stress, and TNF-α. Free Radic. Biol. Med. 2009, 47, 924–931. [Google Scholar] [CrossRef]
- Yoshida, N.; Sasaki, K.; Sasaki, D.; Yamashita, T.; Fukuda, H.; Hayashi, T.; Tabata, T.; Osawa, R.; Hirata, K.; Kondo, A. Effect of resistant starch on the gut microbiota and its metabolites in patients with coronary artery disease. J. Atheroscler. Thromb. 2019, 26, 705–719. [Google Scholar] [CrossRef]
- Jeong, S.M.; SanazLee, S.Y.; Kim, K.W.; Lee, B.J.; Lee, S.M. Evaluation of the three different sources of dietary starch in an extruded feed for juvenile olive flounder, Paralichthys olivaceus. Aquaculture 2021, 533, 736242. [Google Scholar] [CrossRef]
- Yildirim-Aksoy, M.; Lim, C.; Shelby, R.; Klesius, P.H. Increasing fish oil levels in commercial diets influences hematological and immunological responses of channel catfish, Ictalurus punctatus. J. World Aquac. Soc. 2009, 40, 76–86. [Google Scholar] [CrossRef]
- Jha, A.K.; Pal, A.K.; Sahu, N.P.; Kumar, S.; Mukherjee, S.C. Haemato-immunological responses to dietary yeast RNA, ω-3 fatty acid and β-carotene in Catla catla juveniles. Fish Shellfish Immunol. 2007, 23, 917–927. [Google Scholar] [CrossRef] [PubMed]


| Diet | CS | TS | SPS | PS | WS |
|---|---|---|---|---|---|
| Fish meal 1 | 46.00 | 46.00 | 46.00 | 46.00 | 46.00 |
| Blood meal 1 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Soybean meal 1 | 14.00 | 14.00 | 14.00 | 14.00 | 14.00 |
| Corn gluten meal 1 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Enzymatic hydrolysis of poultry by-products 1 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Rice bran | 8.35 | 8.35 | 8.35 | 8.35 | 8.35 |
| Shrimp paste | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Fish oil | 3.90 | 3.90 | 3.90 | 3.90 | 3.90 |
| Vitamin premix 3 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mineral premix 3 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Calcium dihydrogen phosphate | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 |
| Vitamin C | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Choline chloride | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Corn starch 2 | 10.00 | ||||
| Tapioca starch 2 | 10.00 | ||||
| Sweet potato starch 2 | 10.00 | ||||
| Potato starch 2 | 10.00 | ||||
| Wheat starch 2 | 10.00 | ||||
| Analyzed proximate composition | |||||
| Crude protein (%) | 48.50 | 49.06 | 48.73 | 48.96 | 48.99 |
| Crude lipid (%) | 10.44 | 10.01 | 10.41 | 10.57 | 10.52 |
| Items | Methods | Assay Kits/Testing Equipment |
|---|---|---|
| Plasma biochemistry parameters | ||
| TP | International Federation of Clinical Chemistry recommended | Assay kits purchased from Mindray Medical International Ltd. (Shenzhen, China); Mindray BS-400 automatic biochemical analyzer (Mindray Medical International Ltd., Shenzhen, China). |
| ALB | ||
| ALT | ||
| AST | ||
| Enzyme activity parameters | ||
| MDA | TBA method | Assay kits purchased from Jian Cheng Bioengineering Institute (Nanjing, China); Spectrophotometer (Thermo Fisher Multiskan GO, Shanghai, China). |
| CAT | Ammonium molybdenum acid method | |
| SOD | WST-1 method | |
| GSH | Microplate method | |
| Genes | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Reference |
|---|---|---|---|
| nrf2 | AGAGACATTCGCCGTAGA | TCGCAGTAGAGCAATCCT | NM_212855.2 |
| keap1 | CGTACGTCCAGGCCTTACTC | TGACGGAAATAACCCCCTGC | XP_018520553.1 |
| cat | CTATGGCTCTCACACCTTC | TCCTCTACTGGCAGATTCT | MK614708.1 |
| sod | TGGCAAGAACAAGAACCACA | CCTCTGATTTCTCCTGTCACC | Gu et al., 2022 [33] |
| gpx | GAAGGTGGATGTGAATGGA | CCAACCAGGAACTTCTCAA | MK614713.1 |
| nfκB | CCACTCAGGTGTTGGAGCTT | TCCAGAGCACGACACACTTC | XP_027136364.1 |
| tnf-α | CTTCGTCTACAGCCAGGCATCG | TTTGGCACACCGACCTCACC | Gu et al., 2022 [33] |
| il-8 | TCGGTCCTCCTGGGTGAAAA | GTGCTCCTTCCTGCTGATGTA | ASK51661.1 |
| il-10 | CGGCACAGAAATCCCAGAGC | CAGCAGGCTCACAAAATAAACATCT | Yang et al., 2020 [34] |
| β-actin | CCACCTTCAACAGCATCA | AGCCTCCAATCCATACAGA | MH018565.1 |
| Groups | IBW (g) 1 | FBW (g) 2 | WGR (%) 3 | SGR (%/Day) 4 | FCR 5 | SR (%) 6 |
|---|---|---|---|---|---|---|
| Corn starch | 198.89 ± 0.59 | 254.85 ± 6.08 ab | 28.12 ± 2.77 ab | 0.55 ± 0.05 ab | 1.53 ± 0.05 bc | 71.1 ± 2.22 |
| Tapioca starch | 198.89 ± 0.89 | 243.64 ± 7.44 a | 22.53 ± 4.21 a | 0.45 ± 0.08 a | 1.69 ± 0.13 c | 84.4 ± 8.01 |
| Sweet potato starch | 200.44 ± 0.80 | 250.75 ± 1.50 ab | 25.10 ± 1.09 ab | 0.50 ± 0.02 ab | 1.39 ± 0.12 abc | 68.9 ± 2.22 |
| Potato starch | 199.56 ± 0.97 | 271.19 ± 1.91 b | 35.89 ± 0.34 b | 0.68 ± 0.01 b | 1.20 ± 0.02 ab | 73.3 ± 3.85 |
| Wheat starch | 200.22 ± 1.60 | 272.85 ± 7.85 b | 36.26 ± 3.55 b | 0.69 ± 0.06 b | 1.13 ± 0.04 a | 84.4 ± 8.01 |
| p-value | 0.738 | 0.016 | 0.018 | 0.022 | 0.005 | 0.202 |
| Groups | Moisture (%) | Crude Protein (%) | Crude Lipid (%) | Crude Ash (%) |
|---|---|---|---|---|
| Corn starch | 67.97 ± 0.16 | 16.88 ± 0.17 | 8.56 ± 0.29 | 4.94 ± 0.07 |
| Tapioca starch | 67.35 ± 0.94 | 16.52 ± 0.46 | 8.71 ± 0.08 | 4.76 ± 0.25 |
| Sweet potato starch | 66.65 ± 0.67 | 16.72 ± 0.37 | 8.87 ± 0.47 | 5.06 ± 0.25 |
| Potato starch | 67.54 ± 0.58 | 17.35 ± 0.38 | 8.10 ± 0.30 | 5.07 ± 0.09 |
| Wheat starch | 67.29 ± 0.18 | 17.29 ± 0.17 | 8.33 ± 0.29 | 4.83 ± 0.09 |
| p-value | 0.635 | 0.384 | 0.475 | 0.645 |
| Groups | TP (g/L) | ALB (g/L) | ALT (U/L) | AST (U/L) |
|---|---|---|---|---|
| Corn starch | 43.06 ± 1.06 ab | 15.96 ± 0.64 | 1.78 ± 0.25 b | 15.46 ± 1.28 |
| Tapioca starch | 40.11 ± 1.21 a | 14.83 ± 0.49 | 1.64 ± 0.17 b | 17.48 ± 2.67 |
| Sweet potato starch | 43.74 ± 1.34 b | 15.78 ± 0.36 | 1.35 ± 0.19 ab | 14.58 ± 2.48 |
| Potato starch | 45.05 ± 1.01 b | 14.94 ± 0.42 | 0.80 ± 0.15 a | 13.35 ± 0.98 |
| Wheat starch | 44.48 ± 0.85 b | 14.93 ± 0.79 | 1.03 ± 0.23 a | 15.92 ± 1.37 |
| p-value | 0.049 | 0.456 | 0.005 | 0.455 |
| Groups | CAT (U/mg Prot) | SOD (U/mg Prot) | MDA (nmol/mL) | GSH (μmol/g Prot) |
|---|---|---|---|---|
| Corn starch | 0.95 ± 9.12 a | 0.31 ± 0.03 a | 0.20 ± 0.03 a | 1.56 ± 0.22 ab |
| Tapioca starch | 1.15 ± 0.19 ab | 0.30 ± 0.05 a | 0.37 ± 0.07 ab | 1.59 ± 0.28 ab |
| Sweet potato starch | 1.18 ± 0.06 ab | 0.35 ± 0.03 a | 0.50 ± 0.11 b | 0.91 ± 0.12 a |
| Potato starch | 1.32 ± 0.13 ab | 0.36 ± 0.02 ab | 0.35 ± 0.04 ab | 1.46 ± 0.50 ab |
| Wheat starch | 1.41 ± 0.11 b | 0.44 ± 0.01 b | 0.25 ± 0.06 a | 2.16 ± 0.27 b |
| p-value | 0.008 | 0.009 | 0.035 | 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, D.; Gu, J.; Xue, C.; Zhang, L.; Chen, X.; Wang, Y.; Liang, H.; Ren, M. Different Starch Sources Affect the Growth Performance and Hepatic Health Status of Largemouth Bass (Micropterus salmoides) in a High-Temperature Environment. Animals 2023, 13, 3808. https://doi.org/10.3390/ani13243808
Huang D, Gu J, Xue C, Zhang L, Chen X, Wang Y, Liang H, Ren M. Different Starch Sources Affect the Growth Performance and Hepatic Health Status of Largemouth Bass (Micropterus salmoides) in a High-Temperature Environment. Animals. 2023; 13(24):3808. https://doi.org/10.3390/ani13243808
Chicago/Turabian StyleHuang, Dongyu, Jiaze Gu, Chunyu Xue, Lu Zhang, Xiaoru Chen, Yongli Wang, Hualiang Liang, and Mingchun Ren. 2023. "Different Starch Sources Affect the Growth Performance and Hepatic Health Status of Largemouth Bass (Micropterus salmoides) in a High-Temperature Environment" Animals 13, no. 24: 3808. https://doi.org/10.3390/ani13243808
APA StyleHuang, D., Gu, J., Xue, C., Zhang, L., Chen, X., Wang, Y., Liang, H., & Ren, M. (2023). Different Starch Sources Affect the Growth Performance and Hepatic Health Status of Largemouth Bass (Micropterus salmoides) in a High-Temperature Environment. Animals, 13(24), 3808. https://doi.org/10.3390/ani13243808







