Exploring Sound Emission of the Lizard Pristidactylus valeriae
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sound Analyses
2.2. Statistical Analysis
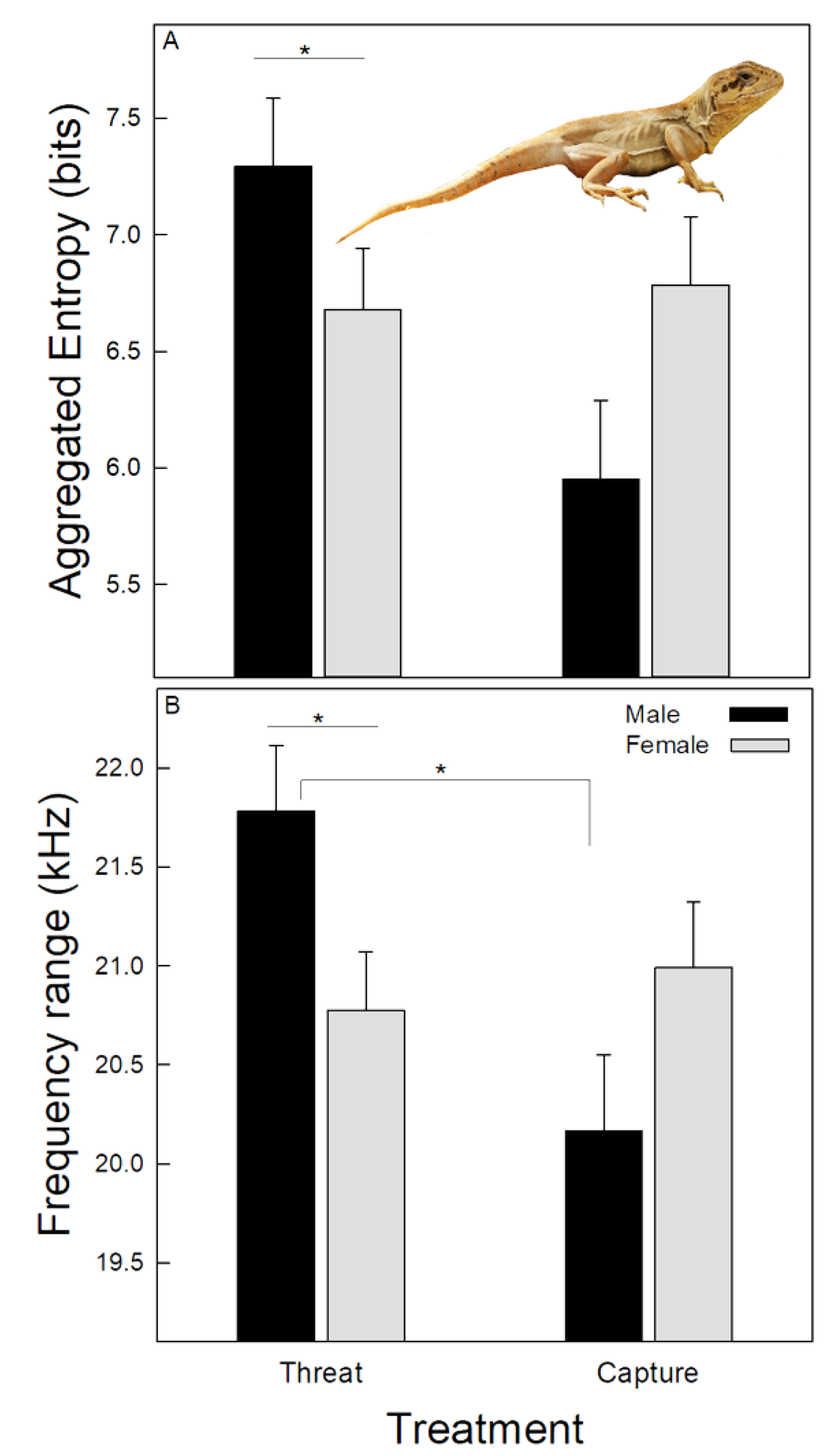
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bradbury, J.W.; Vehrencamp, S.L. Principles of Animal Communication; Sinauer Associated: Sunderland, MA, USA, 2011. [Google Scholar]
- Rohtla, E.A., Jr.; Russell, A.P.; Bauer, A.M. Sounding off: Relationships between call properties, body size, phylogeny, and laryngotracheal form of geckos. Herpetologica 2019, 75, 175–197. [Google Scholar] [CrossRef]
- Bustard, H.R. Observations on the life history and behavior of Chamaeleo hohnelii (Steindachner). Copeia 1965, 1965, 401–410. [Google Scholar] [CrossRef]
- Milton, T.H.; Jenssen, T.A. Description and significance of vocalizations by Anolis grahami (Sauria, Iguanidae). Copeia 1979, 1979, 481–489. [Google Scholar] [CrossRef]
- Böhme, W.; Hutterer, R.; Bings, W. Die stimme der Lacertidae, speziell der Kanareneidechsen (Reptilia: Sauria). Bonn. Zool. Beitr 1985, 36, 337–354. [Google Scholar]
- Frýdlová, P.; Šimková, O.; Janovská, V.; Velenský, P.; Frynta, D. Offenders tend to be heavier: Experimental encounters in mangrove-dwelling monitor lizards (Varanus indicus). Acta Ethol. 2017, 20, 37–45. [Google Scholar] [CrossRef]
- Baeckens, S.; Llusia, D.; García-Roa, R.; Martín, J. Lizard calls convey honest information on body size and bite performance: A role in predator deterrence? Behav. Ecol. Sociobiol. 2019, 73, 87. [Google Scholar] [CrossRef]
- Fernandes, D.C.; Passos, D.C. The voices of an alleged mute: Sound emissions in a Tropidurus lizard. Behaviour 2021, 158, 819–828. [Google Scholar] [CrossRef]
- De La Rosa, E.; Labra, A.; Hernández-Gallegos, O. Description of the vocalizations from the endemic Mexican lizard, Aspidoscelis costatus costatus (Balsas Basin Whiptail). Herpetol. Conserv. Biol. 2023, 18, 1–8. [Google Scholar]
- Russell, A.P.; Bauer, A.M. Vocalization by extant non-avian reptiles: A synthetic overview of phonation and the vocal apparatus. Anat. Rec. 2020, 2020, 1478–1528. [Google Scholar]
- Reyes-Olivares, C.; Labra, A. Emisión de sonidos en lagartos nativos de Chile: El estado del arte. Bol. Chileno Herpetol. 2017, 4, 1–9. [Google Scholar]
- Capshaw, G.; Willis, K.L.; Han, D.; Bierman, H.S. Reptile sound production and perception. In Neuroendocrine Regulation of Animal Vocalization; Rosenfeld, C.S., Hoffmann, F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 101–118. [Google Scholar]
- Labra, A.; Silva, G.; Norambuena, F.; Velásquez, N.; Penna, M. Acoustic features of the weeping lizard’s distress call. Copeia 2013, 2013, 206–212. [Google Scholar] [CrossRef]
- Hoare, M.; Labra, A. Searching for the audience of the weeping lizard’s distress call. Ethology 2013, 119, 860–868. [Google Scholar] [CrossRef]
- Labra, A.; Reyes-Olivares, C.; Weymann, M. Asymmetric response to heterotypic distress calls in the lizard Liolaemus chiliensis. Ethology 2016, 122, 758–768. [Google Scholar] [CrossRef]
- Ruiz-Monachesi, M.R.; Labra, A. Complex distress calls sound frightening: The case of the weeping lizard. Anim. Behav. 2020, 165, 71–77. [Google Scholar] [CrossRef]
- Borgia, G.; Coleman, S.W. Co–option of male courtship signals from aggressive display in bowerbirds. Proc. R. Soc. London. Ser. B Biol. 2000, 267, 1735–1740. [Google Scholar] [CrossRef]
- Donoso-Barros, R. Reptiles de Chile; Universidad de Chile: Santiago, Chile, 1966. [Google Scholar]
- Young, B.; Abishahin, G.; Bruther, M.; Kinney, C.; Sgroi, J. Acoustic analysis of the defensive sounds of Varanus salvator with notes on sound production in other varanid species. Hamadryas-Madras 1998, 23, 1–14. [Google Scholar]
- Laspiur, A.; Sanabria, E.; Acosta, J.C. Primeros datos sobre vocalización en Leiosaurus catamarcensis (Koslowsky, 1898) y Pristidactylus scapulatus Burmeister, 1861 (Iguania, Leiosauridae) de San Juan, Argentina. Rev. Peruana Biol. 2007, 14, 217–220. [Google Scholar] [CrossRef]
- Garcia-Roa, R.; Llusia, D.; López, P.; Martín, J. First evidence of sound production in the genus Iberolacerta Arribas, 1997 (Squamata: Sauria: Lacertidae). Herpetozoa 2017, 29, 175–181. [Google Scholar]
- Johnson, M.A.; Cook, E.G.; Kircher, B.K. Phylogeny and ontogeny of display behavior. In Behavior of Lizards: Evolutionary and Mechanistic Perspectives; Bels, V.L., Russell, A.P., Eds.; CRC Press: Boca Raton, FL, USA, 2019; p. 259. [Google Scholar]
- Cei, J.M.; Scolaro, J.A.; Videla, F. An updated biosystematic approach to the leiosaurid genus Pristidactylus. Bollett. Museo Reg. di Scienze Nat. Torino 2004, 21, 159–192. [Google Scholar]
- Garin, C.; Lobos, G.; Yamil Hussein, E. Gruñidores de Chile; SEREMI del Medio Ambiente de la Región Metropolitana de Santiago y Ecodiversidad Consultores: Santiago, Chile, 2020; Available online: https://mma.gob.cl/wp-content/uploads/2020/08/grunidores_Version-jun_-2020.pdf (accessed on 1 November 2023).
- Labra, A.; Sufán-Catalán, J.; Solis, R.; Penna, M. Hissing sounds by the lizard Pristidactylus volcanensis. Copeia 2007, 2007, 1019–1023. [Google Scholar] [CrossRef]
- Lamborot, M.; Díaz, N. A new species of Pristidactylus (Sauria:Iguanidae) from central Chile and comments on the speciation in the genus. J. Herpetol. 1987, 21, 29–37. [Google Scholar] [CrossRef]
- Castro, C.; Tobar-González, M. Nuevo registro geográfico del Gruñidor de Valeria Pristidactylus valeriae (Donoso-Barros, 1966) (Squamata, Leiosauridae) en Chile. Bol. Museo Nac. Hist. Nat. Chile 2014, 63, 61–64. [Google Scholar] [CrossRef]
- Súfan-Catalán, J.; Núñez, H. Estudios autoecológicos en Pristidactylus cf. valeriae (Squamata, Polychridae) en Chile central. Bol. Museo Nac. Hist. Nat. Chile 1993, 44, 115–130. [Google Scholar]
- Herrel, A.; Andrade Denis, V.; de Carvalho José, E.; Brito, A.; Abe, A.; Navas, C. Aggressive behavior and performance in the Tegu lizard Tupinambis merianae. Physiol. Biochem. Zool. 2009, 82, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Constanzo-Chávez, J.; Penna, M.; Labra, A. Comparing the antipredator behaviour of two sympatric, but not syntopic, Liolaemus lizards. Behav. Processes. 2018, 148, 34–40. [Google Scholar] [CrossRef]
- Crowley, S.R.; Pietruszka, R.D. Aggressiveness and vocalization in the leopard lizard (Gambelia wislizennii): The influence of temperature. Anim. Behav. 1983, 31, 1055–1060. [Google Scholar] [CrossRef]
- Jono, T.; Inui, Y. Secret calls from under the eaves: Acoustic behavior of the Japanese house gecko, Gecko japonicus. Copeia 2012, 2012, 145–149. [Google Scholar] [CrossRef]
- Yu, X.; Peng, Y.; Aowphol, A.; Ding, L.; Brauth, S.E.; Tang, Y.Z. Geographic variation in the advertisement calls of Gekko gecko in relation to variations in morphological features: Implications for regional population differentiation. Ethol. Ecol. Evol. 2011, 23, 211–228. [Google Scholar] [CrossRef]
- Hibbitts, T.J.; Whiting, M.J.; Stuart-Fox, D. Shouting the odds: Vocalization signals status in a lizard. Behav. Ecol. Sociobiol. 2007, 61, 1169–1176. [Google Scholar] [CrossRef]
- Brady, B.; Hedwig, D.; Trygonis, V.; Gerstein, E. Classification of Florida manatee (Trichechus manatus latirostris) vocalizations. J. Acoust. Soc. Am. 2020, 147, 1597–1606. [Google Scholar] [CrossRef]
- Hambálková, L.; Policht, R.; Horák, J.; Hart, V. Acoustic individuality in the hissing calls of the male black grouse (Lyrurus tetrix). PeerJ 2021, 9, e11837. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.M.; Penna, M.; Valenzuela-Sánchez, A.; Mendez, M.A.; Azat, C. Monomorphic call structure and dimorphic vocal phenology in a sex-role reversed frog. Behav. Ecol. Sociobiol. 2020, 74, 127. [Google Scholar] [CrossRef]
- Wever, E.G. The Reptile Ear: Its Structure and Function; Princeton University Press: Princeton, NJ, USA, 1978. [Google Scholar]
- Manley, G.A. Evolution of structure and function of the hearing organ of lizards. J. Neurobiol. 2002, 53, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Labra, A.; Reyes-Olivares, C.; Moreno-Gómez, F.; Velásquez, N.A.; Penna, M.; Delano, P.; Narins, P.M. Geographic variation in the matching between call characteristics and tympanic sensitivity in the Weeping lizard. Ecolo. Evol. 2021, 11, 18633–18650. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.A.; Kraus, J.E.M. Exceptional high-frequency hearing and matched vocalizations in Australian pygopod geckos. J. Exp. Biol. 2010, 213, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Zuberbühler, K.; Jenny, D.; Bshary, R. The predator deterrence function of primate alarm calls. Ethology 1999, 105, 477–490. [Google Scholar] [CrossRef]
- Koenig, W.D.; Stanback, M.T.; Hooge, P.N.; Mumme, R.L. Distress calls in the acorn woodpecker. Condor 1991, 93, 637–643. [Google Scholar] [CrossRef]
- Ramírez-Jaramillo, S.M.; Allan-Miranda, N.A.; Salazar, M.; Jácome-Chiriboga, N.B.; Robayo, J.; Marcayata, A.; Reyes-Puig, J.P.; Yánez-Muñoz, M.H. Revisión de las presas vertebradas consumidas por Falco sparverius en América del sur y nuevos registros para Ecuador. El Hornero 2018, 33, 51–57. [Google Scholar] [CrossRef]
- Figueroa, R.A.; Alvarado, S.; Bravo, C.; Corales, E.S.; González, B.A.; Ibarra-Vidal, H. Características de las presas del peuquito (Accipiter chilensis) en el bosque templado austral. El Hornero 2004, 19, 77–82. [Google Scholar] [CrossRef]
- Medel, R.G.; Jaksic, F.M. Ecología de los cánidos sudamericanos: Una revisión. Rev. Chil. Hist. Nat. 1988, 61, 67–79. [Google Scholar]
- Greene, H.W.; Jaksić, F.M. The feeding behavior and natural history of two Chilean snakes, Philodryas chamissonis and Tachymenis chiliensis (Colubridae). Rev. Chil. Hist. Nat. 1992, 65, 485–493. [Google Scholar]
- Dooling, R. Avian Hearing and the Avoidance of Wind Turbines Technical Report NREL/TP-500-30844; National Renewable Energy Laboratory: Golden, CO, USA, 2002. [Google Scholar]
- Malkemper, E.P.; Topinka, V.; Burda, H. A behavioral audiogram of the red fox (Vulpes vulpes). Hearing Res. 2015, 320, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, A.L.; Thomas, J.A.; Stalf, C.E.; Murphy, L.D.; Lombardi, D.; Carpenter, J.; Mueller, T. Behavioral audiogram of two Arctic foxes (Vulpes lagopus). Polar Biol. 2014, 37, 417–422. [Google Scholar] [CrossRef]
- Young, B.A. Snake bioacoustics: Toward a richer understanding of the behavioral ecology of snakes. Quart. Rev. Biol. 2003, 78, 303–325. [Google Scholar] [CrossRef]
- Young, B.A.; Nejman, N.; Meltzer, K.; Marvin, J. The mechanics of sound production in the puff adder Bitis arietans (Serpentes: Viperidae) and the information content of the snake hiss. J. Exp. Biol. 1999, 202, 2281–2289. [Google Scholar] [CrossRef]
- Leal, M. Honest signalling during prey–predator interactions in the lizard Anolis cristatellus. Anim. Behav. 1999, 58, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Bohórquez-Alonso, M.L.; Cotrina, J.M.; Pardo, D.A.; Font, E.; Molina-Borja, M. Sex differences in antipredator tail-waving displays of the diurnal yellow-headed gecko Gonatodes albogularis from tropical forests of Colombia. J. Zool. 2010, 28, 305–311. [Google Scholar] [CrossRef]
- Telemeco, R.S.; Baird, T.A.; Shine, R. Tail waving in a lizard (Bassiana duperreyi) functions to deflect attacks rather than as a pursuit-deterrent signal. Anim. Behav. 2011, 82, 369–375. [Google Scholar] [CrossRef]
- Manser, M.B. The acoustic structure of suricates’ alarm calls varies with predator type and the level of response urgency. Proc. R. Soc. London. Ser. B Biol. 2001, 268, 2315–2324. [Google Scholar] [CrossRef]
- Blumstein, D.T.; Récapet, C. The sound of arousal: The addition of novel non-linearities increases responsiveness in marmot alarm calls. Ethology 2009, 115, 1074–1081. [Google Scholar] [CrossRef]
- Blesdoe, E.K.; Blumstein, D.T. What is the sound of fear? Behavioral responses of white-crowned sparrows Zonotrichia leucophrys to synthesized nonlinear acoustic phenomena. Curr. Zool. 2014, 60, 534–541. [Google Scholar] [CrossRef]
- Townsend, S.W.; Manser, M.B. The function of nonlinear phenomena in meerkat alarm calls. Biol. Lett. 2011, 7, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Caro, T.M. Antipredator Defenses in Birds and Mammals; The University of Chicago Press: London, UK, 2005. [Google Scholar]
- Young, B.A.; Sheft, S.; Yost, W. Sound production in Pituophis melanoleucus (Serpentes: Colubridae) with the first description of a vocal cord in snakes. J. Exp. Biol. 1995, 273, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Lingle, S.; Wyman, M.T.; Kotrba, R.; Teichroeb, L.J.; Romanow, C.A. What makes a cry a cry? A review of infant distress vocalizations. Curr. Zool. 2012, 58, 698–726. [Google Scholar] [CrossRef]
- Jurisevic, M.A.; Sanderson, K.J. A comparative analysis of distress call structure in Australian Passerine and Non-Passerine species: Influence of size and phylogeny. J. Avian Biol. 1998, 29, 61–71. [Google Scholar] [CrossRef]
- Valdez-Ovallez, F.M.; Erostarbe, A.V.; Cocilio, R.N.; Gomez-Ales, R.; Fernández, R.; Acosta, R.; Blanco, G.; Acosta, J.C.; Corrales, L. Microhabitat use and selection by Pristidactylus scapulatus (Squamata Leiosauridae) in the Puna region of the Central Andes in Argentina. Ethol. Ecol. Evol. 2022, 35, 488–502. [Google Scholar] [CrossRef]
- Morton, E.S. Ecological sources of selection on avian sounds. Am. Nat. 1975, 109, 17–34. [Google Scholar] [CrossRef]
- Boncoraglio, G.; Saino, N. Habitat structure and the evolution of bird song: A meta-analysis of the evidence for the acoustic adaptation hypothesis. Funct. Ecol. 2007, 21, 134–142. [Google Scholar] [CrossRef]
- Ey, E.; Fischer, J. The “acoustic adaptation hypothesis”—A review of the evidence from birds, anurans and mammals. Bioacoustics 2009, 19, 21–48. [Google Scholar] [CrossRef]
- Mikula, P.; Valcu, M.; Brumm, H.; Bulla, M.; Forstmeier, W.; Petrusková, T.; Kempenaers, B.; Albrecht, T. A global analysis of song frequency in passerines provides no support for the acoustic adaptation hypothesis but suggests a role for sexual selection. Ecol. Lett. 2021, 24, 477–486. [Google Scholar] [CrossRef]
- Goutte, S.; Dubois, A.; Howard, S.; Márquez, R.; Rowley, J.; Dehling, J.; Grandcolas, P.; Xiong, R.; Legendre, F. How the environment shapes animal signals: A test of the acoustic adaptation hypothesis in frogs. J. Evol. Biol. 2018, 31, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.L.; Laspiur, A.; Klaczko, J.; Rivas, L.R.; Rodrigues, M.T.; Sena, M.A.d.; Céspedes, R. Total evidence phylogenetic analysis of Leiosauridae (Squamata) with focus on the ‘para-anoles’ and description of a new Urostrophus species from the Bolivian Andes. Syst. Biodivers. 2023, 21, 2200306. [Google Scholar] [CrossRef]
- Rautenberg, R.; Laps, R.R. Natural history of the lizard Enyalius iheringii (Squamata, Leiosauridae) in southern Brazilian Atlantic forest. Iheringia Ser. Zool. 2010, 100, 287–290. [Google Scholar] [CrossRef]

| Variable | Description |
|---|---|
| Duration (s) | Time from the beginning to the end of the sound |
| Low frequency (kHz) | Lower frequency limit of the sound |
| Center frequency (kHz) | The frequency that divides the sound range frequency into two intervals of equal energy |
| High frequency (kHz) | The upper-frequency limit of the sound |
| Peak frequency (kHz) | The frequency at which the maximum power occurs in the sound |
| Delta frequency (kHz) | Difference between the upper and lower frequency limits of the sound (frequency range) |
| Aggregate entropy (bits) | Measurement of the disorder of the sound, which analyzes the energy distribution in the sound |
| Capture (n = 15) | Threat (n = 9) | |
|---|---|---|
| Duration (ms) | 124.85 ± 16.22 (52–268) | 265.01 ± 46.00 (51.2–434.14) |
| Low Frequency (kHz) | 3.13 ± 0.27 (0.90–4.55) | 2.69 ± 0.24 (1.70–4.16) |
| Center frequency (kHz) | 9.06 ± 0.59 (4.88–13.22) | 9.67 ± 0.32 (7.97–10.76) |
| High frequency (kHz) | 23.72 ± 0.18 (21.43–24.0) | 23.91 ± 0.07 (23.37–24.0) |
| Peak frequency (kHz) | 7.58 ± 0.57 (4.78–12.38) | 8.92 ± 0.68 (5.91–13.03) |
| Delta frequency (kHz) | 20.59 ± 0.25 (19.00–22.53) | 21.22 ± 0.24 (19.84–22.30) |
| Aggregate entropy (bits) | 6.45 ± 0.19 (4.73–7.28) | 6.95 ± 0.12 (6.54–7.48) |
| Repeated Measures (n = 8; df = 1,8) | Non-Repeated Measures (n = 7 Captured, 9 Threatened; 9 ♀, 7 ♂; df = 1,12) | |||||
|---|---|---|---|---|---|---|
| Variable | Treatment | Sex | Treatment × Sex | Treatment | Sex | Treatment × Sex |
| Duration (ms) | 8.092 (0.029) | 1.004 (0.355) | 0.078 (0.789) | 5.531 (0.037) | 0.0000 (0.988) | 0.273 (0.611) |
| Low Frequency (kHz) | 4.604 (0.076) | 0.997 (0.357) | 2.111 (0.196) | 0.472 (0.505) | 0.733 (0.409) | 0.633 (0.441) |
| Center frequency (kHz) | 0.386 (0.557) | 0.260 (0.628) | 0.009 (0.926) | 0.804 (0.387) | 2.826 (0.119) | 3.003 (0.109) |
| High frequency (kHz) | 0.600 (0.470) | 7.600 (0.033) | 0.400 (0.546) | 1.200 (0.295) | 1.200 (0.295) | 2.560 (0.136) |
| Peak frequency (kHz) | 0.138 (0.723) | 1.009 (0.354) | 0.088 (0.777) | 5.117 (0.043) | 1.453 (0.251) | 0.078 (0.785) |
| Delta frequency (kHz) | 2.621 (0.157) | 2.582 (0.159) | 0.406 (0.548) | 4.270 (0.061) | 0.070 (0.793) | 7.330 (0.019) |
| Aggregate entropy (bits) | 4.530 (0.077) | 4.586 (0.076) | 0.281 (0.615) | 4.316(0.060) | 0.139 (0.716) | 5.920 (0.032) |
| Species | Duration (ms) | Center Frequency | Delta Frequency | Reference |
|---|---|---|---|---|
| P. scapulatus | 455 * | - | <4 kHz | Laspiur et al. [20] |
| P. valeriae | 270 | >8 kHz | >20 kHz | This study |
| P. volcanensis | 429 | <4 kHz | <4 kHz | Labra et al. [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, S.; Labra, A. Exploring Sound Emission of the Lizard Pristidactylus valeriae. Animals 2023, 13, 3813. https://doi.org/10.3390/ani13243813
Díaz S, Labra A. Exploring Sound Emission of the Lizard Pristidactylus valeriae. Animals. 2023; 13(24):3813. https://doi.org/10.3390/ani13243813
Chicago/Turabian StyleDíaz, Sebastián, and Antonieta Labra. 2023. "Exploring Sound Emission of the Lizard Pristidactylus valeriae" Animals 13, no. 24: 3813. https://doi.org/10.3390/ani13243813






